Abstract
Bax is a pro-apoptotic member of the Bcl-2 protein family that resides in the outer mitochondrial membrane. It is controversial whether Bax promotes cell death directly through its putative function as a channel protein versus indirectly by inhibiting cellular regulators of the cell death proteases (caspases). We show here that addition of submicromolar amounts of recombinant Bax protein to isolated mitochondria can induce cytochrome c (Cyt c) release, whereas a peptide representing the Bax BH3 domain was inactive. When placed into purified cytosol, neither mitochondria nor Bax individually induced proteolytic processing and activation of caspases. In contrast, the combination of Bax and mitochondria triggered release of Cyt c from mitochondria and induced caspase activation in cytosols. Supernatants from Bax-treated mitochondria also induced caspase processing and activation. Recombinant Bcl-XL protein abrogated Bax-induced release of Cyt c from isolated mitochondria and prevented caspase activation. In contrast, the broad-specificity caspase inhibitor benzyloxycarbonyl-valinyl-alaninyl-aspartyl-(0-methyl)-fluoromethylketone (zVAD-fmk) and the caspase-inhibiting protein X-IAP had no effect on Bax-induced release of Cyt c from mitochondria in vitro but prevented the subsequent activation of caspases in cytosolic extracts. Unlike Ca2+, a classical inducer of mitochondrial permeability transition, Bax did not induce swelling of mitochondria in vitro. Because the organellar swelling caused by permeability transition causes outer membrane rupture, the findings, therefore, dissociate these two events, implying that Bax uses an alternative mechanism for triggering release of Cyt c from mitochondria.
Bcl-2 family proteins play a pivotal role in controlling cell life and death, with some members such as Bcl-2 and Bcl-XL inhibiting apoptosis and others such as Bax inducing cell death (1). Many Bcl-2 family proteins are anchored in the outer membrane of mitochondria by a C-terminal hydrophobic stretch of amino acids (2, 3). Moreover, Bcl-2 family proteins can have profound influences on mitochondrial alterations associated with apoptosis. For example, during apoptosis, several mitochondrial events typically occur, including loss of the electrochemical gradient (ΔΨ) across the inner membrane, resulting in uncoupling of oxidative phosphorylation, generation of superoxide free radicals, and dumping of matrix-associated Ca2+ into the cytosol (reviewed in ref. 4). In addition, cytochrome c (Cyt c) and possibly other proteins are released from mitochondria into the cytosol (5, 6). Upon entering the cytosol, Cyt c promotes the assembly of a multiprotein complex that induces proteolytic processing and activation of cell death proteases known as caspases (7, 8). Overexpression of Bcl-2 in cells has been reported to prevent the loss of ΔΨ, release of Cyt c, and activation of caspases, whereas Bax induces these changes (5, 6, 9–11).
How Bax induces and Bcl-2 inhibits these mitochondrial alterations is currently controversial, but two prevailing theories have been advanced (reviewed in ref. 11). One view relates to the structural similarity of some Bcl-2 family proteins to the pore-forming domains of certain bacterial toxins, such as diphtheria toxin and the colicins that form channels in biomembranes for transport of proteins and ions, respectively (12). In this regard, both anti-apoptotic (Bcl-2, Bcl-XL) and pro-apoptotic (Bax) members of the Bcl-2 family have been demonstrated to form ion channels in synthetic membranes in vitro (13–16). Moreover, under some in vitro circumstances, Bcl-2 can evidently prevent the formation of Bax channels in liposomes. The dissipation of the mitochondrial ΔΨ during apoptosis has been attributed to the opening of the mitochondrial megapore, a cyclosporine-inhibitable high-conductance channel that appears to be comprised of multiple proteins, including inner and outer membrane proteins that come into contact at the junctional complexes of this organelle (17, 18). Conceivably, Bcl-2 and Bax could participate in the formation or regulation of this large channel. Alternatively, because some studies have suggested that release of Cyt c from mitochondria precedes loss of ΔΨ (5, 6), it is possible that Bax creates pores in the outer membrane which are large enough to allow escape of Cyt c, resulting secondarily in megapore opening from either failed electron chain transport or because of Cyt c-mediated activation of caspases which then cleave mitochondrial proteins (19).
An opposing view has been deduced from studies of the cell death proteins discovered in the nematode Caenorhabditis elegans. The worm homolog of Bcl-2 (CED-9) has been shown to interact with a caspase (CED-3) via a bridging protein CED-4 that binds simultaneously to CED-9 and CED-3 (20–22). CED-9 prevents CED-4 from inducing proteolytic processing and activation of CED-3 (23). Bcl-XL can also bind to the worm CED-4 protein and alter its location in cells, pulling it from the cytosol to the intracellular membranes where Bcl-2 family proteins often reside (21, 22). However, heterodimerization of Bax-like proteins with Bcl-XL abrogates binding of Bcl-XL to CED-4, presumably freeing it to activate caspases (22). Because caspases have been shown to induce mitochondrial permeability transition (PT) (19), it has been argued that Bax may indirectly induce release of Cyt c and loss of mitochondrial ΔΨ by interfering with the suppressive effects of Bcl-XL and similar anti-apoptotic Bcl-2 family proteins on CED-4-like proteins, thus allowing caspase activation to occur and thereby secondarily triggering megapore opening.
By using isolated mitochondria and recombinant Bax protein, we present evidence that Bax can directly induce Cyt c release from mitochondria without apparent requirement for caspases. Moreover, this Bax-mediated release of Cyt c is not accompanied by permeability transition and the attendant mitochondrial swelling that is known to rupture the outer membrane.
MATERIALS AND METHODS
Expression and Purification of Recombinant Proteins.
Murine Bax or human Bcl-XL were expressed recombinantly as described (24). X-IAP protein was prepared as described (25). Proteins (0.2–0.4 mg/ml) were dialyzed into 20 mM Hepes (pH 7.5), 10 mM KCl, 20 mM MgCl2, and 1 mM EDTA before use in all experiments.
Isolation of Mitochondria.
Female rats were killed by decapitation, and mitochondria were purified from the liver by differential centrifugation and separation on a sucrose gradient (26). Mitochondria were suspended at 10 mg protein/ml in MSB (400 mM mannitol/50 mM Tris⋅HCl, pH 7.2/5 mg/ml BSA/10 mM KH2PO4) and kept on ice for up to 4 hr.
Cyt c Release Assays.
Mitochondria (50 μg protein) were incubated with recombinant proteins (1 μM Bax; 1–12 μM Bcl-XL; 0.2 μM X-IAP) or 50 μM HPLC-purified Bax-BH3 peptide (NH2-KKLSESLKRIGDELDS-amide) (residues 61–76 of human Bax) (Chiron) with or without 10 μM CsA (Novartis, Basel, Switzerland) or 10 μM benzyloxycarbonyl-valinyl-alaninyl-aspartyl-(0-methyl)-fluoromethylketone (zVAD-fmk; Enzyme Systems Products, Livermore, CA) in 50 μl of MSB at 30°C for 1 hr. Mitochondria were pelleted by centrifugation at 4,000 × g for 5 min, solubilized in RIPA buffer, and analyzed by immunoblotting with antibodies to F1-β-ATPase (gift of W. Neupert, Munich, Germany) or Bax (27). The resulting supernatants were employed either for caspase activity assays or analyzed by SDS/PAGE immunoblotting with either 10–20% gradient gels or 15% gels with high Tris (75 mM). Proteins were transferred to nitrocellulose (pore size, 0.1 μm), and the blots were incubated with mAb 7H8.2C12 (PharMingen, or gift of R. Jemmerson, University of Minnesota, Minneapolis, MN) (28, 29) followed by ECL-based detection (30). Similar results were obtained by using a variety of buffer conditions (31, 32).
Assay for Mitochondrial Permeability Transition.
The in vitro swelling of mitochondria caused by induction of PT was assayed by using mitochondria resuspended in CFS (220 mM mannitol/68 mM sucrose/2 mM NaCl/5 mM KH2PO4/2 mM MgCl2/10 mM Hepes-NaOH, pH 7.4/5 mM succinate/2 mM ATP/50 μg/ml creatine phosphokinase/10 mM phosphocreatine) with 2 μM rotenone (9, 33–35). Mitochondria were treated with 1 μM Bax or 150 μM Ca2+, in the presence or absence of 10 μM CsA, and the absorbance variation caused by swelling was measured by using a double-beam spectrophotometer at 520 nm.
Assay for Mitochondria-Dependent Caspase Activation.
Cytosolic extracts were prepared from 293 embryonic kidney cells as described (25). Supernatants (10 μl) from Bax-treated or control mitochondria were incubated with 10 μl of cytosolic extract at 30°C for 1 hr. Aliquots were utilized for caspase assays, initiating caspase processing and activation by addition of 1 mM dATP (7). Alternatively, purified mitochondria (50 μg protein in 5 μl MSB) were incubated in 30 μl of cytosolic extracts, with or without various recombinant proteins and compounds as described above. After incubation at 30°C for 1 hr, mitochondria were removed by centrifugation, and the resulting supernatants were analyzed for caspase activity or used for immunoblot assays with polyclonal antisera to caspase-3 (25).
Caspase Assay.
Caspase activity was assayed by release of 7-amino-4-trifluoromethyl-coumarin (AFC) from DEVD containing synthetic peptides using continuous-reading instruments as described (25, 36). Typically, 1 μl of final cytosol-containing solution was added to 99 μl caspase buffer [50 mM Hepes/0.1 M NaCl/1 mM EDTA/0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS)/10% sucrose/5 mM DTT] and reactions were initiated by addition of 100 μM Ac-DEVD-AFC (Enzyme Systems Products).
Transfections and Subcellular Fractionations.
293T cells were seeded into 10-cm dishes and transfected with pcDNA3 control, pcDNA Bax (5 μg), pGFP (3 μg), or various combinations of these plasmids (normalized for total 25 μg DNA) by a CaPO4 precipitation method (37). Transfection efficiency was 20–50%, as monitored by green fluorescent protein (GFP) fluorescence. Cells were lysed after 24 hr in HEB (50 mM Pipes/50 mM KCl/5 mM EGTA/2 mM MgCl2/10 μM Cytochalasin/1 mM DTT) for 20 min on ice. The samples were adjusted to 220 mM mannitol and 68 mM sucrose (final concentration) and were dounced briefly before pelleting organelles by centrifugation at 16,000 × g for 0.5 hr. The resulting supernatants (cytosol) and pellets were mixed with RIPA buffer and analyzed by SDS/PAGE immunoblotting (25 μg per lane) as described above.
RESULTS
Bax Induces Cyt c Release from Isolated Mitochondria.
To examine the effects of Bax on mitochondria, purified recombinant Bax protein was added to mitochondria that had been isolated from rat liver. For solubility purposes, Bax protein was produced without its C terminal last 20 amino acids, which constitutes a membrane-anchoring domain. Previously we showed that when expressed in mammalian cells, this truncated Bax protein associates with mitochondria and induces apoptosis (37). After coincubation of mitochondria with either Bax or diluent control for 1 hr, the mitochondria were pelleted by centrifugation and the resulting supernatants were analyzed for the presence of Cyt c by immunoblotting.
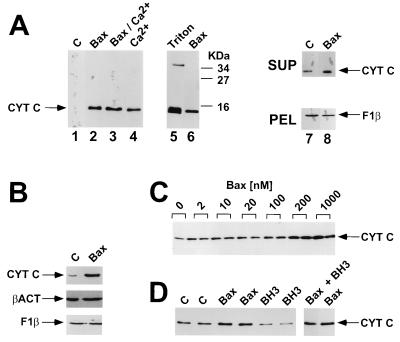
As shown in Fig. 1A, the supernatants from Bax-treated mitochondria contained higher amounts of Cyt c compared with the diluent control. Though the amounts of spontaneous Cyt c release varied among preparations of mitochondria, Bax consistently triggered further release (typically ≥5-fold above background; n > 12 experiments). The relative proportion of Cyt c release from mitochondria following Bax treatment was similar to that stimulated by 150 μM Ca2+, a known inducer of mitochondrial permeability transition (Fig. 1A). Bax and Ca2+ appeared to induce release of ≈20% of the total Cyt c from mitochondria under the conditions of these assays, based on comparisons with Triton-X 100. Immunoblot analysis with an antibody to the inner membrane protein F1-β-ATPase confirmed equivalent amounts of input mitochondria. Overexpression of Bax in 293 cells also induced Cyt c release from mitochondria, indicating that this phenomenon also occurs in intact cells (Fig. 1B).
Figure 1.
Bax induces Cyt c release from isolated mitochondria. In A, mitochondria were incubated for 1 hr at 30°C with 1 μM Bax, 150 μM Ca2+, both, or neither of these reagents. Mitochondria were then pelleted by centrifugation, and the resulting supernatants were subjected to SDS/PAGE immunoblot analysis by using an anti-Cyt c antibody. Complete pelleting of mitochondria and input of equivalent amount of mitochondria was verified in every experiment by immunoblot analysis of the pellets and supernatant fractions by using an antibody to the integral inner membrane protein F1-β-ATPase (lanes 7 and 8 and data not shown). As a control, mitochondria were also treated with 0.2% (vol/vol) Triton X-100, which released all Cyt c from these organelles. Data are representative of over 12 experiments performed with 3 independent preparations of Bax protein. In B, 293T cells were transfected with 5 μg of pcDNA3 or pcDNA3-Bax. Cells were harvested after 1 day, processed to obtain cytosolic and organellar (pellet) subfractions, and aliquots (25 μg total protein) were analyzed by SDS/PAGE by using antibodies specific for Cyt c, β-actin (cytosolic protein) and F1-β-ATPase (mitochondrial matrix protein). No F1-β-ATPase was detected in the cytosolic fraction (data not shown). In C, mitochondria were similarly incubated with various concentrations of recombinant Bax protein and Cyt c release was measured by immunoblotting (representative of two experiments). In D, mitochondria were incubated with 1 μM Bax protein, 50 μM BH3 peptide, both, or neither of these reagents. Cyt c release was measured 1 hr later as above (n = 2).
The Bax-induced release of Cyt c from mitochondria was dependent on the concentration of Bax protein added, reaching a plateau at 200 μM (Fig. 1C). Similar observations were made by using at least five different preparations of Bax protein, including Bax in which the glutathione S-transferase domain was not removed (data not shown). These concentrations of Bax are within physiological ranges, because basal amounts of Bax protein in a variety of human tumor cell lines can be as high as ≈0.1 μM (unpublished data) and because Bax protein levels can rise in some tumor cells by over 10-fold following exposure to chemotherapeutic drugs or γ-radiation (38). Moreover, immunoblot experiments indicated that at 0.1–1 μM, only 10–20% of the total input Bax protein bound mitochondrial in vitro (data not shown).
Previous studies have suggested that the BH3 domain of Bak, a close homolog Bax, is sufficient to bind anti-apoptotic Bcl-2 family proteins and induce apoptosis (39). We therefore also examined the effects of a synthetic 16-amino acid peptide representing the BH3 domain of Bax, because mitochondria isolated from rat liver might have anti-apoptotic Bcl-2 family proteins integrated into their outer membranes that perhaps could be neutralized with BH3 peptides (40). In contrast to recombinant Bax protein, addition of 50 μM BH3 peptide failed to induce release of Cyt c release from mitochondria in vitro (Fig. 1D). At these or lower concentrations, however, the same BH3 peptide completely prevented binding of Bax to Bcl-XL as determined by surface plasmon resonance (BIAcore) experiments (24), thus confirming its ability to bind Bcl-2 family proteins.
Bax-Treated Mitochondria Release Factors that Activate Caspases in Vitro.
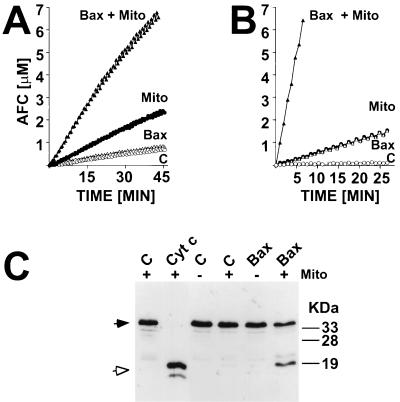
Cyt c has been shown to induce processing and activation of caspase-3 and some other caspases that cleave the peptide DEVD (7, 25). To investigate whether Bax-treated mitochondria could release factors that induce activation of DEVD-cleaving caspases, isolated mitochondria were treated in vitro for 1 hr with either Bax protein or diluent control, followed by removal of the mitochondria by centrifugation and collection of the resulting supernatants. These supernatants and control supernatants were then added to cytosolic fractions derived from human cell lines, and caspase activity was measured 0.5 hr later by spectrofluorimetric assays with DEVD-AFC as a substrate. As shown in Fig. 2A, Bax by itself failed to induce increases in caspase activity in cytosols. Addition of supernatants derived from control-treated mitochondria caused only a modest elevation in DEVD-cleaving activity. In contrast, supernatants from Bax-treated mitochondria consistently resulted in striking increases in caspase activity.
Figure 2.
Bax induces release of mitochondrial factors that trigger processing and activation of cytosolic caspases. In A, supernatants from mitochondria that had been incubated for 1 hr with either 1 μM Bax or diluent control (C) were added to purified cytosol. Alternatively, 1 μM Bax or diluent control were added directly to cytosol. After incubation at 30°C for 0.5 hr, caspase activity was measured by hydrolysis of DEVD-AFC. Data represent micromolar amount of AFC released per time, where samples are normalized for total cytosolic protein content. In B, mitochondria were added to cytosolic extracts with or without 1 μM Bax. After incubation at 30°C for 1 hr, the mitochondria were pelleted by centrifugation and caspase activity was measured in the resulting supernatant. In C, an aliquot of cytosolic fractions was subjected to SDS/PAGE immunoblot assay by using anti-Cyt c antibody. As an additional control (Left), cytosol was treated with 10 μM Cyt c, as described (25). Closed and open arrows indicate the unprocessed zymogen of caspase-3 and the processed form associated with active enzyme, respectively.
Similar results were obtained when mitochondria were added directly to cytosolic fractions. The combination of Bax plus mitochondria resulted in marked increases in caspase activity, whereas neither Bax nor mitochondria individually stimulated substantial elevations in caspase activity (Fig. 2B). Immunoblot analysis of these same cytosolic extracts demonstrated that the combination of Bax plus mitochondria induced proteolytic processing of caspase-3, whereas addition of neither Bax nor mitochondria alone was sufficient for triggering processing of this DEVD-cleaving caspase (Fig. 2C).
Bax Does Not Induce Mitochondrial Swelling.
The swelling of mitochondria is a colloidosmotic process that is observed during induction of PT in vitro (17, 33). To determine whether Bax triggers opening of the mitochondrial megapore leading to PT, mitochondria were exposed to Bax protein under conditions previously shown to be conducive for swelling induced by Ca2+ and other activators of mitochondrial PT. As expected, addition of Ca2+ to isolated mitochondria induced rapid swelling, which was measured as a decrease in OD at 520 nm (Fig. 3). This Ca2+-induced swelling of mitochondria was completely abrogated by prior addition of cyclosporin A (CsA), which is known to block mitochondrial PT by inhibiting a mitochondrial cyclophilin which regulates the megapore (17, 33–35). In contrast to Ca2+, Bax did not induce mitochondrial swelling under these same conditions. Electron microscopy studies confirmed massive swelling of Ca2+-treated mitochondria with loss of cristae and outer membrane rupture, whereas Bax-treated mitochondria retained essentially normal size and morphology (data not shown). Thus, Bax-induced Cyt c release occurs by a different mechanism than that involved with Ca2+.
Figure 3.
Bax does not induce mitochondrial permeability transition. Mitochondria were placed into a cuvette and absorbance was measured by using a double-beam spectrophotometer at 520 nm. In some cases, samples were pretreated with 10 μM CsA. Either 1 μM Bax or 150 μM Ca2+ was added (arrow) and optical density was monitored for the ensuing 75 min (data representative of five experiments).
Bcl-XL Prevents Bax-Induced Release of Cyt c from Mitochondria.
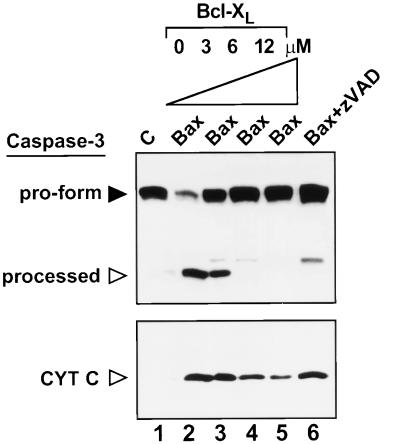
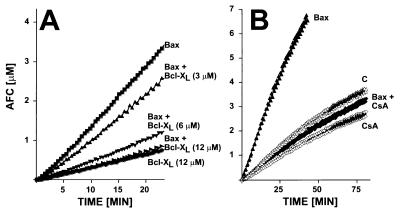
When coexpressed in cells, Bcl-XL can abrogate the pro-apoptotic effects of Bax. We therefore examined the impact of recombinant Bcl-XL protein on Bax-induced release of Cyt c from mitochondria in vitro. Addition of a 12-fold molar excess of Bcl-XL inhibited almost completely Bax-induced Cyt c release from mitochondria (Fig. 4A). Recombinant Bcl-XL protein also suppressed in a concentration-dependent manner the proteolytic processing of caspase-3 that is induced by coaddition of Bax protein and mitochondria to cytosolic extracts (Fig. 5). Bcl-XL also inhibited pro-caspase-3 processing induced by Bax-treated mitochondria (Fig. 5). Moreover, the levels of active DEVD-cleaving caspases in these cytosols were also lower when Bcl-XL was added along with Bax and mitochondria to cytosolic extracts (Fig. 6A). In contrast, when exogenously added Cyt c was used to induce processing of caspase-3 and activation of DEVD-cleaving caspases in cytosolic extracts, recombinant Bcl-XL protein failed to inhibit (data not shown), suggesting that Bcl-XL operates upstream of Cyt c and cannot provide protection once Cyt c has been released into the cytosol.
Figure 4.
Effects of Bcl-XL, CsA, and zVAD-fmk on Bax-induced release of Cyt c from mitochondria. Mitochondria were treated (sometimes in duplicate) with 1 μM Bax or diluent control (C), with or without other reagents, and Cyt c release into supernatants was measured 1 hr later by immunoblotting. In A, 12 μM Bcl-XL was added to some samples simultaneously with Bax, as indicated. In B, 10 μM CsA or 10 μM zVAD-fmk was added 5 min prior to Bax. In C, the mitochondrial pellets were analyzed in addition to the supernatants (25 μg). Bax binding to mitochondria was determined by SDS/PAGE immunoblot analysis by using an anti-Bax antibody.
Figure 5.
Bcl-XL inhibits processing of caspase-3 induced by Bax plus mitochondria. Mitochondria were added to cytosolic extracts followed by addition of 1 μM Bax or diluent control (C), with or without various concentrations of Bcl-XL protein or zVAD-fmk. After removing mitochondria by centrifugation, aliquots of the cytosol were analyzed by SDS/PAGE immunoblotting with antibodies specific for either caspase-3 (Upper) or Cyt c (Lower). The positions of the unprocessed pro-form of caspase-3 (filled arrow) and processed ≈17-kDa large subunit of caspase-3 (open arrow) are shown. The slightly slower migrating band represents a partially processed form of the large subunit of caspase-3 (25, 51).
Figure 6.
Effects of Bcl-XL and CsA on caspase activation induced by Bax plus mitochondria. Cytosol was treated at 30°C for 1 hr with mitochondria, 1 μM Bax, diluent control (C), or various combinations of these in the presence or absence of (A) 3–12 μM Bcl-XL or (B) 10 μM CsA. Caspase activity was measured by DEVD-AFC hydrolysis.
CsA Inhibits Bax-Induced Release of Cyt c from Mitochondria.
Although Bax did not induce mitochondria swelling, we investigated whether CsA affected Bax-induced Cyt c release. As shown in Fig. 4B, incubation of mitochondria with Bax in the presence of CsA prevented the release of Cyt c. CsA also completely prevented the activation of caspases that is normally induced by addition of Bax protein and mitochondria to cytosolic extracts (Fig. 6B). In contrast, the diluent in which CsA was dissolved had no effect on Bax-induced Cyt c release. Similar results were obtained with a nonimmunosuppressive cyclosporine analogue (data not shown). CsA did not prevent the binding of Bax to mitochondria (Fig. 4C), excluding this as a trivial explanation for the observed suppression of Bax-induced Cyt c release.
Caspases Are Not Required for Bax-Induced Release of Cyt c from Mitochondria.
The effects of Bax on Cyt c release from mitochondria could be an indirect consequence of Bax-induced activation of caspases that subsequently cleave mitochondrial proteins and liberate Cyt c from these organelles (19). To explore this possibility, zVAD-fmk, an irreversible broad-specificity peptidyl inhibitor of caspases, was added to isolated mitochondria prior to Bax. As shown in Fig. 4B, zVAD-fmk did not inhibit Bax-induced release of Cyt c from isolated mitochondria. However, when zVAD-fmk was added with mitochondria to cytosolic extracts, Bax-induced processing of caspase-3 was suppressed by zVAD-fmk, and only small amounts of a partially processed form of caspase-3 appeared (Fig. 5). Similar results were obtained when zVAD-fmk was replaced with ≈0.2 μM recombinant X-IAP protein (data not shown), an anti-apoptotic protein that directly binds to and inhibits caspases within the Cyt c pathway (27). The addition of zVAD-fmk or X-IAP protein to cytosolic extracts prior to Bax and mitochondria also inhibited caspase activity as measured by DEVD hydrolysis, confirming that these caspase inhibitors were active in these assays (data not shown). Taken together, these data suggest that Bax directly induces release of Cyt c from mitochondria without requirement for caspases. Moreover, the findings indicate that Cyt c release precedes processing of caspase-3.
DISCUSSION
Elevations in Bax protein levels are induced in several clinically relevant settings where cell death occurs, including tumor cells during responses to chemotherapy and radiation (38, 41), neurons following cerebral ischemia (27), and myocardiocytes following acute myocardial infarction (42). However, the mechanisms by which Bax promotes apoptosis have been difficult to determine. In many other areas of cell biology and metabolism, in vitro reconstitution of complicated pathways has greatly assisted analysis of the cause-and-effect relations among the various components. Here we describe a step toward this goal, demonstrating that addition of recombinant purified Bax protein to isolated mitochondria can induce Cyt c release.
A critical issue in understanding the mechanism by which Bax induces dissipation of the mitochondrial ΔΨ and caspase activation in cells is whether these events are direct effects of Bax, perhaps related to its putative function as a channel protein, versus indirect consequences of Bax-mediated effects on anti-apoptotic members of the Bcl-2 family that might prevent caspase activation by inhibiting CED-4-like proteins. The data presented here suggest that Bax can directly induce release of Cyt c, without apparent requirement for caspases. Of course, the caveat must be raised that undiscovered caspases may exist that are not inhibited by zVAD-fmk, even at the high concentrations employed here (100 μM). Our data, therefore, cast doubts on models that envision Bax as merely a trans-dominant inhibitor of anti-apoptotic Bcl-2 family proteins that triggers the release of CED-4-like proteins upon heterodimerizing with Bcl-2 or Bcl-XL.
If Bax does not rely on caspases for inducing release of Cyt c from mitochondria, then how does it accomplish this? One possibility is that recombinant Bax protein heterodimerizes with anti-apoptotic proteins such as Bcl-2 and Bcl-XL that might be present endogenously in the outer membranes of mitochondria isolated from rat liver. Previous studies have demonstrated the dependence of the BH3 domain of Bax for its homo- and heterodimerization with itself and other anti-apoptotic Bcl-2 family proteins, respectively (43). Moreover, expression in mammalian cells of Bak fragments that contain essentially only the BH3 domain reportedly induces apoptosis (39). However, with isolated mitochondria, a peptide representing the BH3 domain of Bax failed to induce Cyt c release, implying that Bax does not necessarily trigger Cyt c release merely by inserting its BH3 domain into the hydrophobic binding pocket recently revealed on the surface of Bcl-XL and predicted for Bcl-2 as well (12, 44). Thus, these data provide further evidence that Bax may directly induce release of Cyt c.
Because Bax has been shown to form channels in synthetic membranes, and may even create very high conductance channels under some circumstances (17), it is conceivable that Bax forms a pore in the outer membrane of mitochondria that allows Cyt c to leak out. Alternatively, Bax might indirectly alter the permeability of the outer membrane through interactions with other proteins. In this regard, recent attempts to purify the mitochondria megapore complex and reconstitute it in artificial membranes have demonstrated a copurification and enrichment of Bax (45). Thus, Bax may be intimately associated with the megapore. However, addition of recombinant Bax to isolated mitochondria did not induce the permeability change in the inner membrane that causes mitochondria swelling in vitro, thus indicating that Bax does not cause release of Cyt c as a secondary consequence of mitochondrial PT-induced rupture of the outer membrane.
Nevertheless, the finding that CsA potently inhibits Bax-induced Cyt c release from mitochondria suggests the possibility of communication between components of the megapore and Bax. Alternatively, the CsA-inhibitable cyclophilin located in the mitochondrial matrix may alter the conformations of other proteins not associated with the megapore but which collaborate with Bax to induce Cyt c release. Moreover, these effects of CsA on Bax function could be very indirect, for example, influencing the environment of mitochondria so that the Bax protein fails to integrate into membranes and form functional channels. In particular, because the Bax channel has been reported to be pH-dependent and voltage-gated, CsA-mediated alterations in inner membrane proteins that control the pH gradient and voltage potential across this membrane conceivably could impact Bax even in the outer membrane, given that the outer and inner membranes of mitochondria come into close apposition at the junctional complexes. An example of one candidate protein complex is the F0F1-ATPase/proton-pump of the inner membrane, which is required for optimal Bax-induced cell death (46). Regardless of the actual mechanism, these findings with CsA illustrate an advantage of using a cell-free system for evaluating the effects of Bax on mitochondrial function in that this approach permits some experiments that are impossible in cells. For example, micromolar concentrations of CsA are required to inhibit the megapore of mitochondria but generally only nanomolar concentrations of this drug are tolerated by cells. Moreover, CsA affects other cellular proteins, complicating interpretation of the results derived from intact cells. In contrast, mitochondria contain only one CsA-binding protein (10, 17, 47, 48).
The data reported here are consistent with evidence that gene transfer-mediated overexpression of Bax in whole cells can induce mitochondrial ΔΨ even in the presence of the broad-specificity caspase inhibitor zVAD-fmk (49), suggesting that Bax can have effects on mitochondria even when caspases are inhibited. However, our findings go further to demonstrate that Bax directly induces release of Cyt c from mitochondria without necessarily triggering mitochondrial PT. Thus, we can deduce that Bax-induced release of Cyt c does not necessarily occur as a secondary consequence of PT-induced rupture of the mitochondrial outer membrane. This finding does not discount the possibility that stimuli which use the megapore as their primary target, such as elevated cytosolic Ca2+, can induce Cyt c release by a PT-dependent mechanism. Thus, more than one mechanism for releasing Cyt c probably exists, with the Bax-mediated route occurring without concomitant swelling of mitochondria and subsequent rupture of the outer membrane but other stimuli relying upon it.
Though it could be argued that Bax-induced release of Cyt c from mitochondria should have secondarily induced mitochondrial PT because of failed electron chain transport and subsequent generation of reactive oxygen species, we noted that Bax typically induced release of only ≈20% of the total Cyt c from mitochondria. Thus, under these conditions, presumably sufficient Cyt c remains associated with the inner membrane to sustain electron chain transport. However, this small proportion of Cyt c released is evidently capable of potently triggering caspase activation. Of note, it has been reported that even with isolated mitoplasts in which the outer membrane has been selectively solubilized, Cyt c remains tightly bound to the inner membrane through its interactions with proteins such as Cyt c oxidase, typically requiring high salt concentrations to efficiently release it (50). Thus, by releasing only a small proportion of the total Cyt c initially, Bax may favor induction of a cell death process that involves rapid activation of caspases causing apoptosis, as opposed to the oxidative stress type of cell death that generally befalls mitochondrial PT and which can lead to necrosis.
Acknowledgments
We thank S. Schendel for peptide purification, G. Kroemer for helpful comments, W. Neupert and R. Jemmerson for antibodies, T. Brown for manuscript preparation, J. Wade for technical assistance, and the California Breast Cancer Research Program (BCRP-1RB-0093) and CaP-CURE, Inc. for their generous support. J.M.J. is a recipient of a fellowship from the Deutsche Forschungsgemeinschaft.
Footnotes
This paper was submitted directly (Track II) to the Proceedings office.
Abbreviations: Cyt c, cytochrome c; PT, permeability transition; zVAD-fmk, benzyloxycarbonyl-valinyl-alaninyl-aspartyl-(0-methyl)-fluoromethylketone; AFC, 7-amino-4-trifluoromethyl-coumarin; DEVD, Asp-Glu-Val-Asp; CsA, cyclosporin A.
References
- 1.Reed J C. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krajewski S, Tanaka S, Takayama S, Schibler M J, Fenton W, Reed J C. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- 3.González-Garcia M, Pérez-Ballestero R, Ding L, Duan L, Boise L, Thompson C, Nœñez G. Development (Cambridge, UK) 1994;120:3033–3042. doi: 10.1242/dev.120.10.3033. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch T, Marzo I, Kroemer G. Biosci Rep. 1997;17:67–76. doi: 10.1023/a:1027339418683. [DOI] [PubMed] [Google Scholar]
- 5.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng I-I, Jones D P, Wang X. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 6.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 8.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 9.Zamzami N, Susin A, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, Kroemer G. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolli A, Basso E, Petronilli V, Wenger R M, Bernardi P. J Biol Chem. 1996;271:2185–2192. doi: 10.1074/jbc.271.4.2185. [DOI] [PubMed] [Google Scholar]
- 11.Reed J C. Nature (London) 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 12.Muchmore S W, Sattler M, Liang H, Meadows R P, Harlan J E, et al. Nature (London) 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 13.Minn A J, Velez P, Schendel S L, Liang H, Muchmore S W, Fesik S W, Fill M, Thompson C B. Nature (London) 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 14.Schendel S L, Xie Z, Montal M O, Matsuyama S, Montal M, Reed J C. Proc Natl Acad Sci USA. 1997;94:5113–5118. doi: 10.1073/pnas.94.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, et al. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 16.Schlesinger P, Gross A, Yin X-M, Yamamoto K, Saito M, Waksman G, Korsmeyer S. Proc Natl Acad Sci USA. 1997;94:11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernardi P, Broekemeier K M, Pfeiffer D R. J Bioenerg Biomembr. 1994;26:509–517. doi: 10.1007/BF00762735. [DOI] [PubMed] [Google Scholar]
- 18.Petit P X, Susin S-A, Zamzami N, Mignotte B, Kroemer G. FEBS Lett. 1996;396:7–13. doi: 10.1016/0014-5793(96)00988-x. [DOI] [PubMed] [Google Scholar]
- 19.Susin S, Zamzami N, Castedo M, Daugas E, Wang H-G, Geley S, Fassy F, Reed J, Kroemer G. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spector M S, Desnoyers S, Heoppner D J, Hengartner M O. Nature (London) 1997;385:653–656. doi: 10.1038/385653a0. [DOI] [PubMed] [Google Scholar]
- 21.Wu D, Wallen H D, Nunez G. Science. 1997;275:1126–1129. doi: 10.1126/science.275.5303.1126. [DOI] [PubMed] [Google Scholar]
- 22.Chinnaiyan A M, O’Rourke K, Lane B R, Dixit V M. Science. 1997;275:1122–1126. doi: 10.1126/science.275.5303.1122. [DOI] [PubMed] [Google Scholar]
- 23.Seshagiri S, Miller L. Curr Biol. 1997;7:455–460. doi: 10.1016/s0960-9822(06)00216-8. [DOI] [PubMed] [Google Scholar]
- 24.Xie, Z., Schendel, S., Matsuyama, S. & Reed, J. C. (1998) Biochemistry, in press. [DOI] [PubMed]
- 25.Deveraux Q, Takahashi R, Salvesen G S, Reed J C. Nature (London) 1997;388:300–303. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 26.Boutry M, Briquet M. Eur J Biochem. 1982;127:129–135. doi: 10.1111/j.1432-1033.1982.tb06846.x. [DOI] [PubMed] [Google Scholar]
- 27.Krajewski S, Mai J K, Krajewska M, Sikorska M, Mossakowski M J, Reed J C. J Neurosci. 1995;15:6364–6376. doi: 10.1523/JNEUROSCI.15-10-06364.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jemmerson R, Johnson J G, Burrell E, Taylor P S, Jenkins M K. Eur J Immunol. 1991;21:143–151. doi: 10.1002/eji.1830210122. [DOI] [PubMed] [Google Scholar]
- 29.Jemmerson R, Johnson J G. Proc Natl Acad Sci USA. 1991;88:4428–4432. doi: 10.1073/pnas.88.10.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jürgensmeier J M, Krajewski S, Armstrong R, Wilson G M, Oltersdorf T, Fritz L C, Reed J C, Ottilie S. Mol Biol Cell. 1997;8:325–339. doi: 10.1091/mbc.8.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Susin S A, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. J Exp Med. 1996;184:1331–1342. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy A N, Bredesen D E, Cotopassi G, Wang E, Fiskum G. Neurobiology. 1996;93:9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zoratti M, Szabo I. J Bioenerg Biomembr. 1994;26:543–553. doi: 10.1007/BF00762739. [DOI] [PubMed] [Google Scholar]
- 34.Petronilli V, Cola C, Massari S, Colonna R, Bernardi P. J Biol Chem. 1993;268:21939–21945. [PubMed] [Google Scholar]
- 35.Pfeiffer D R, Gudz T I, Novgorodov S A, Erdahl W L. J Biol Chem. 1995;270:4923–4932. doi: 10.1074/jbc.270.9.4923. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Q, Snipas S, Orth K, Muzio M, Dixit V M, Salvesen G S. J Biol Chem. 1997;272:7797–7800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]
- 37.Zha H, Fisk H A, Yaffe M P, Mahajan N, Herman B, Reed J C. Mol Cell Biol. 1996;16:6494–6508. doi: 10.1128/mcb.16.11.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhan Q, Fan S, Bae I, Guillouf C, Liebermann D A, O’Connor P M, Fornace A J., Jr Oncogene. 1994;9:3743–3751. [PubMed] [Google Scholar]
- 39.Chittenden T, Flemington C, Houghton A B, Ebb R G, Gallo G J, Elangovan B, Chinnadurai G, Lutz R J. EMBO J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaz J-L, Oltersdorf T, Horne W, McConnell M, Wilson G, Weeks S, Garcia T, Fritz L C. J Biol Chem. 1997;272:11350–11355. doi: 10.1074/jbc.272.17.11350. [DOI] [PubMed] [Google Scholar]
- 41.Miyashita T, Reed J C. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 42.Cheng W, Kajstura J, Nitahara J A, Li B, Reiss K, et al. Exp Cell Res. 1996;226:316–327. doi: 10.1006/excr.1996.0232. [DOI] [PubMed] [Google Scholar]
- 43.Zha H, Aime-Sempe C, Sato T, Reed J C. J Biol Chem. 1996;271:7440–7444. doi: 10.1074/jbc.271.13.7440. [DOI] [PubMed] [Google Scholar]
- 44.Sattler M, Liang H, Nettesheim D, Meadows R P, Harlan J E, et al. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 45.Marzo, I., Brenner, C., Zamzami, N., Susin, S., Beutner, G., Brdiczka, D., Xie, Z., Reed, J. & Kroemer, G. (1997) J. Exp. Med., in press. [DOI] [PMC free article] [PubMed]
- 46.Matsuyama S, Xu Q, Velours J, Reed J C. Mol Cell. 1998;1:1–20. doi: 10.1016/s1097-2765(00)80033-7. [DOI] [PubMed] [Google Scholar]
- 47.Connern C P, Halestrap A P. Biochem J. 1992;284:381–385. doi: 10.1042/bj2840381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foxwell B M, Woerly G, Husi H, Mackie A, Quesniaux V F, Hiestand P C, Wenger R M, Ryffel B. Biochim Biophys Acta. 1992;1138:115–121. doi: 10.1016/0925-4439(92)90050-w. [DOI] [PubMed] [Google Scholar]
- 49.Xiang J, Chao D T, Korsmeyer S J. Proc Natl Acad Sci USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobs E E, Sanadi D R. J Biol Chem. 1960;235:531–534. [PubMed] [Google Scholar]
- 51.Martin S J, Newmeyer D D, Mathias S, Farschon D M, Wang H G, Reed J C, Kolesnick R N, Green D R. EMBO J. 1995;14:5191–5200. doi: 10.1002/j.1460-2075.1995.tb00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]