Abstract
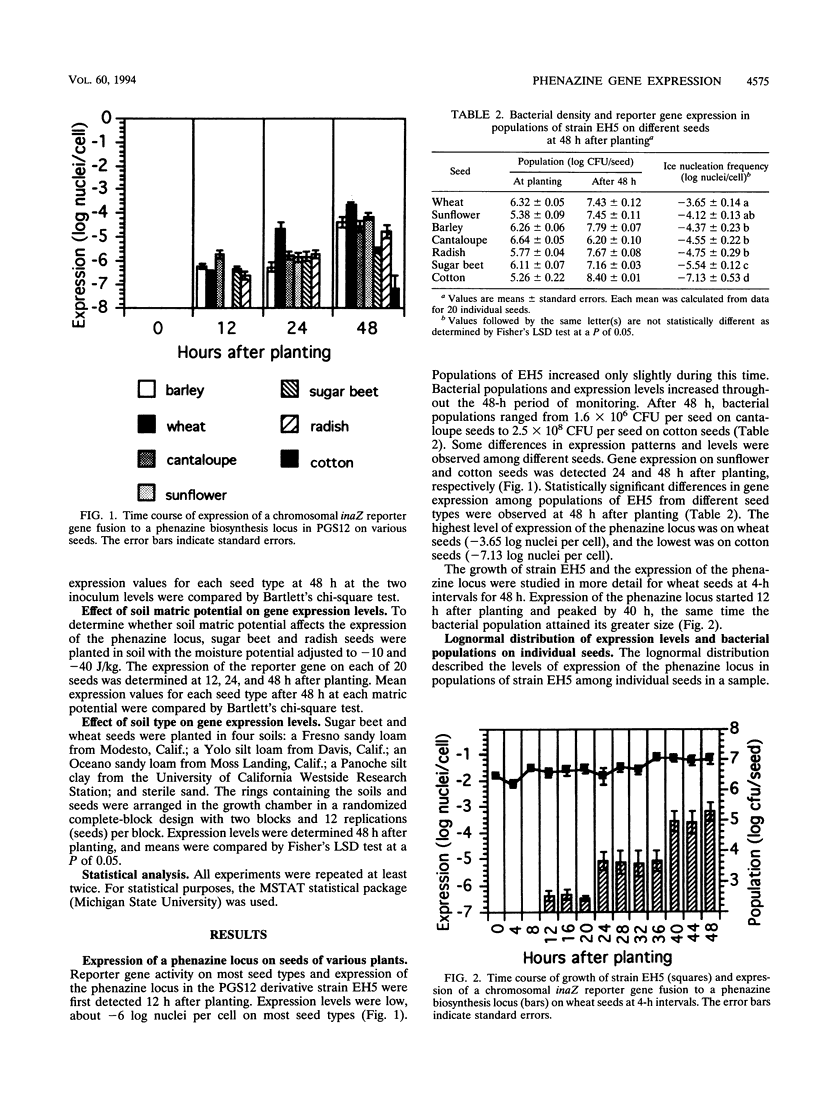
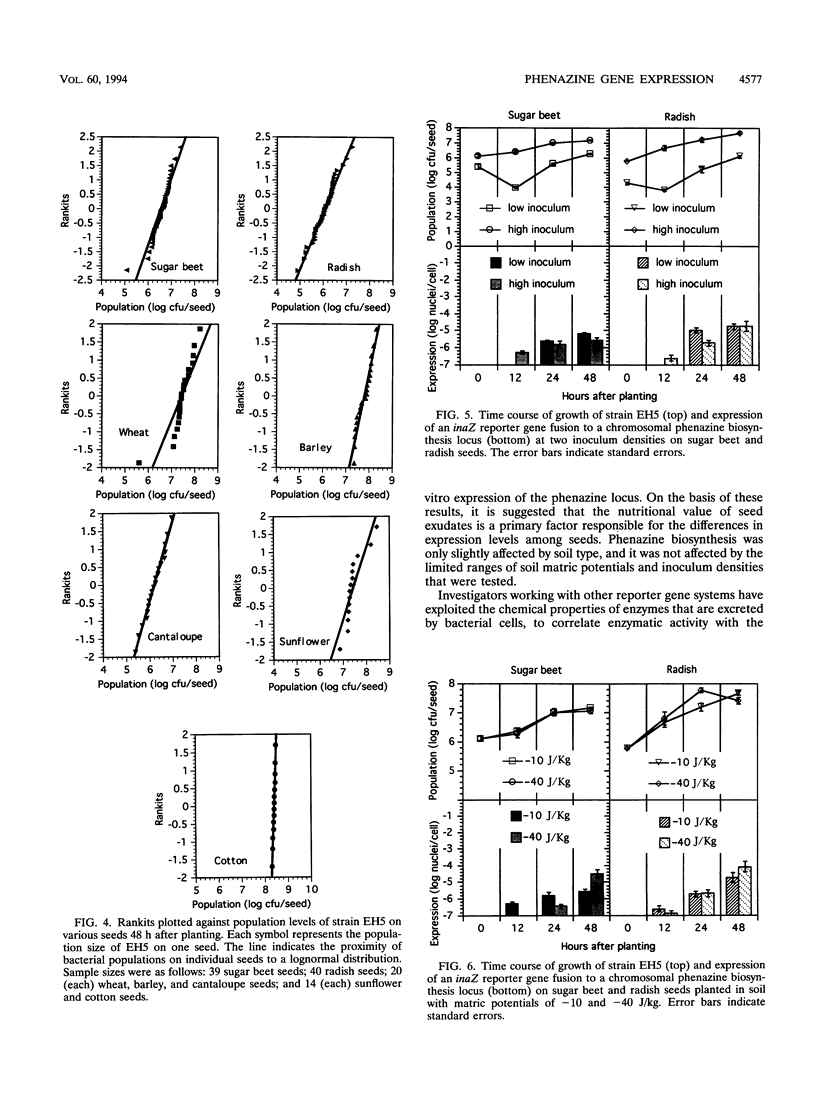
A derivative of Pseudomonas aureofaciens PGS12 expressing a promoterless ice nucleation gene under the control of a phenazine biosynthesis locus was used to study the expression of a phenazine antibiotic locus (Phz) during bacterial seed colonization. Seeds of various plants were inoculated with wild-type PGS12 and a PGS12 ice nucleation-active phz:inaZ marker exchange derivative and planted in soil, and the expression of the reporter gene was monitored at different intervals for 48 h during seed germination. phz gene expression was first detected 12 h after planting, and the expression increased during the next 36-h period. Significant differences in expression of bacterial populations on different seeds were measured at 48 h. The highest expression level was recorded for wheat seeds (one ice nucleus per 4,000 cells), and the lowest expression level was recorded for cotton seeds (one ice nucleus per 12,000,000 cells). These values indicate that a small proportion of bacteria in a seed population expressed phenazine biosynthesis. Reporter gene expression levels and populations on individual seeds in a sample were lognormally distributed. There was greater variability in reporter gene expression than in population size among individual seeds in a sample. Expression on sugar beet and radish seeds was not affected by different inoculum levels or soil matric potentials of -10 and -40 J/kg; only small differences in expression on wheat and sugar beet seeds were detected when the seeds were planted in various soils. It is suggested that the nutrient level in seed exudates is the primary reason for the differences observed among seeds. The lognormal distribution of phenazine expression on seeds and the timing and difference in expression of phenazine biosynthesis on seeds have implications for the potential efficacy of biocontrol microorganisms against plant pathogens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- James D. W., Jr, Gutterson N. I. Multiple antibiotics produced by Pseudomonas fluorescens HV37a and their differential regulation by glucose. Appl Environ Microbiol. 1986 Nov;52(5):1183–1189. doi: 10.1128/aem.52.5.1183-1189.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Lindgren P. B., Frederick R., Govindarajan A. G., Panopoulos N. J., Staskawicz B. J., Lindow S. E. An ice nucleation reporter gene system: identification of inducible pathogenicity genes in Pseudomonas syringae pv. phaseolicola. EMBO J. 1989 May;8(5):1291–1301. doi: 10.1002/j.1460-2075.1989.tb03508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahme L. G., Mindrinos M. N., Panopoulos N. J. Genetic and transcriptional organization of the hrp cluster of Pseudomonas syringae pv. phaseolicola. J Bacteriol. 1991 Jan;173(2):575–586. doi: 10.1128/jb.173.2.575-586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan P., O'sullivan D. J., Simpson P., Glennon J. D., O'gara F. Isolation of 2,4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl Environ Microbiol. 1992 Jan;58(1):353–358. doi: 10.1128/aem.58.1.353-358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow L. S., Weller D. M. Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J Bacteriol. 1988 Aug;170(8):3499–3508. doi: 10.1128/jb.170.8.3499-3508.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. M., Messenger A. J. Occurrence, biochemistry and physiology of phenazine pigment production. Adv Microb Physiol. 1986;27:211–275. doi: 10.1016/s0065-2911(08)60306-9. [DOI] [PubMed] [Google Scholar]



