Abstract
Enzymes IIa and IIb, which catalyze the conversion of epichlorohydrin (ECH) to 3-chloro-1,2-propanediol (MCP), were purified from Corynebacterium sp. strain N-1074, which catalyzes the formation of (R)-MCP from prochiral 1,3-dichloro-2-propanol via ECH. The specific activity of enzyme IIa for the formation of MCP from ECH was about 6.4-fold higher than that of enzyme IIb. Both enzymes catalyzed the conversion of 1,2-epoxides to the corresponding diol, although they differed in several enzymatic properties.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. H., Jakoby W. B. Tartaric acid metabolism. IX. Synthesis with tartrate epoxidase. J Biol Chem. 1969 Apr 25;244(8):2078–2084. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Jacobs M. H., Van den Wijngaard A. J., Pentenga M., Janssen D. B. Characterization of the epoxide hydrolase from an epichlorohydrin-degrading Pseudomonas sp. Eur J Biochem. 1991 Dec 18;202(3):1217–1222. doi: 10.1111/j.1432-1033.1991.tb16493.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Michaels B. C., Ruettinger R. T., Fulco A. J. Hydration of 9,10-epoxypalmitic acid by a soluble enzyme from Bacillus megaterium. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1189–1195. doi: 10.1016/0006-291x(80)90412-x. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nagasawa T., Yu F., Watanabe I., Yamada H. Characterization of a novel enantioselective halohydrin hydrogen-halide-lyase. Appl Environ Microbiol. 1994 Apr;60(4):1297–1301. doi: 10.1128/aem.60.4.1297-1301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
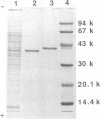
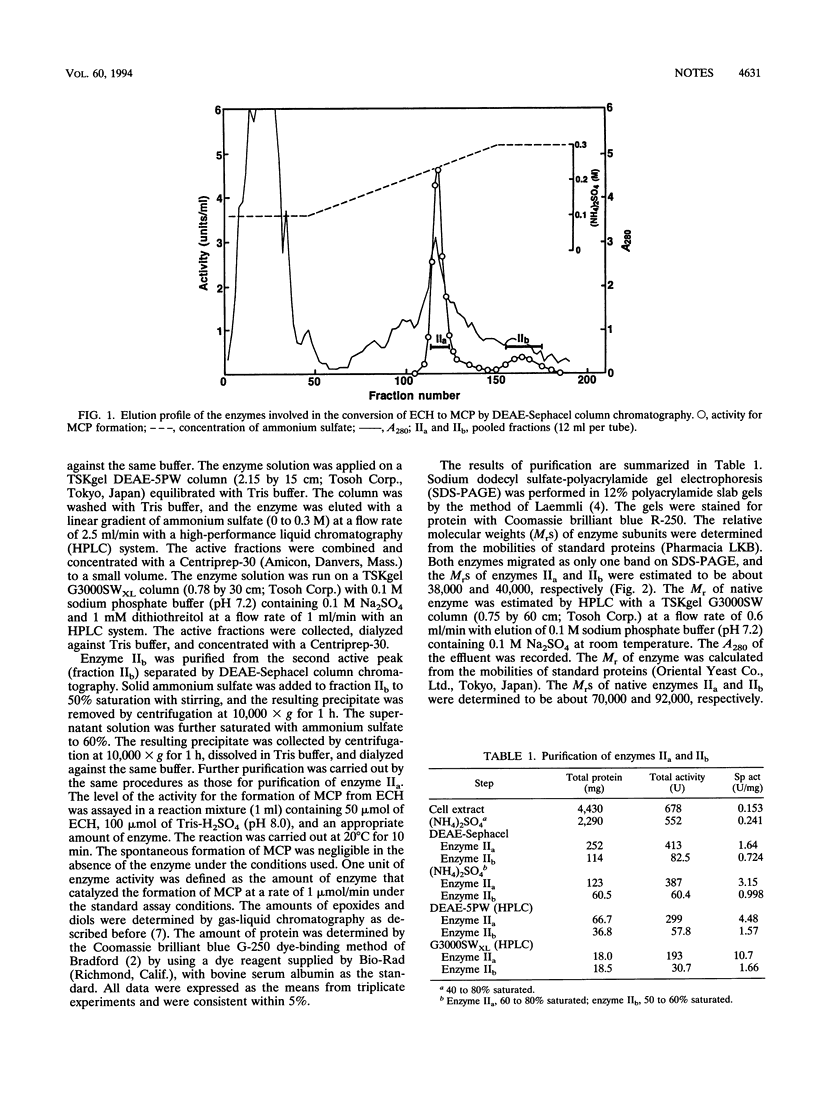
- Nakamura T., Nagasawa T., Yu F., Watanabe I., Yamada H. Resolution and some properties of enzymes involved in enantioselective transformation of 1,3-dichloro-2-propanol to (R)-3-chloro-1,2-propanediol by Corynebacterium sp. strain N-1074. J Bacteriol. 1992 Dec;174(23):7613–7619. doi: 10.1128/jb.174.23.7613-7619.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Yu F., Mizunashi W., Watanabe I. Production of (R)-3-Chloro-1,2-Propanediol from Prochiral 1,3-Dichloro-2-Propanol by Corynebacterium sp. Strain N-1074. Appl Environ Microbiol. 1993 Jan;59(1):227–230. doi: 10.1128/aem.59.1.227-230.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidegård J., DePierre J. W. Microsomal epoxide hydrolase. Properties, regulation and function. Biochim Biophys Acta. 1983 Dec 29;695(3-4):251–270. doi: 10.1016/0304-419x(83)90014-8. [DOI] [PubMed] [Google Scholar]
- Yu F., Nakamura T., Mizunashi W., Watanabe I. Cloning of two halohydrin hydrogen-halide-lyase genes of Corynebacterium sp. strain N-1074 and structural comparison of the genes and gene products. Biosci Biotechnol Biochem. 1994 Aug;58(8):1451–1457. doi: 10.1271/bbb.58.1451. [DOI] [PubMed] [Google Scholar]