Cryptosporidium spp. are ubiquitous waterborne parasites responsible for outbreaks of diarrheal disease worldwide [1]. The infection is self-limiting in immunocompetent individuals, but in immunocompromised individuals the infection can develop into a chronic, debilitating, and sometimes life-threatening disease [2]. In malnourished children, cryptosporidiosis is associated with growth and developmental delays [1]. Currently, there are no vaccines to prevent cryptosporidiosis, and nitazoxanide, the only approved drug in the US [3] is not effective in AIDS patients [4]. Because the parasite cannot be propagated in vitro or genetically manipulated, data that would permit targeted drug design or vaccine development are sorely lacking.
Ingested Cryptosporidium oocysts excyst in the intestine, releasing sporozoites that attach to and invade intestinal epithelial cells, initiating the lifecycle [5]. The parasite undergoes two rounds of asexual reproduction, in each cycle producing merozoites that invade new epithelial cells, before entering the sexual cycle and producing oocysts. One research focus has been to identify the antigens on the invasive zoite stages that allow the parasite to attach to and invade the epithelial cell with the goal of preventing these parasite-host cell interactions. The best characterized of the zoite antigens is Cpgp40/15, (also called Cp17, gp15/45/60 or S60) [6–10], a mucin like glycoprotein antigen that is synthesized as a single precursor protein and proteolytically cleaved into the mature glycoproteins, gp40 and gp15 [11]. gp15 is anchored in the sporozoite membrane by a glycosylphosphatidyl inositol (GPI) moiety [12], while the gp40 glycoprotein does not contain any predicted transmembrane domains or GPI anchors and is predicted to be soluble [7]. However, gp40 has been shown to bind intestinal epithelial cells in a dose dependent and saturable manner [7] suggesting that this antigen recognizes a host cell receptor. If this interaction is important for zoite attachment and invasion, then gp40 must have some mechanism of associating with the parasite membrane. In these studies we explore the possibility that gp40 and gp15 associate to form a protein complex capable of linking zoite and host cell surfaces.
To generate gp40 specific antiserum, recombinant (r)gp40 fusion protein [7] was purified via the His tag with Talon metal affinity resin (Clontech, Mountain View, CA) and resolved on preparative SDS-PAGE gels. Mice were immunized as described [7] and the specificity of the antiserum confirmed by western blot. To determine if gp40 is associated with the sporozoite membrane, we subjected excysted oocysts (C. parvum Iowa strain) to Triton X-114 extraction and phase separation [13] and analyzed aqueous and detergent phase antigens by Western blotting (Figure 1A). gp15 partitioned predominantly into the detergent phase as expected for a GPI-anchored antigen (Figure 1A, panel 1, lane 2). While some gp40 partitioned into the soluble phase (Figure 1A panel 2, lane 1), most of the gp40 was found in the detergent phase (Figure 1A panel 2, lane 2), suggesting that this antigen does associate with the sporozoite membrane. To corroborate this observation, we performed live cell immunofluorescence assays on C. parvum GCH1 sporozoites following the procedure described by Riggs et al [14]. The anti-gp40 serum reacted with the surface of live sporozoites and could be observed being shed off the surface in trails (Figure 1B). Both these experiments strongly suggest that, despite being soluble, gp40 is associated with the surface of sporozoites.
Figure 1. Soluble gp40 associates with the membrane of sporozoites.
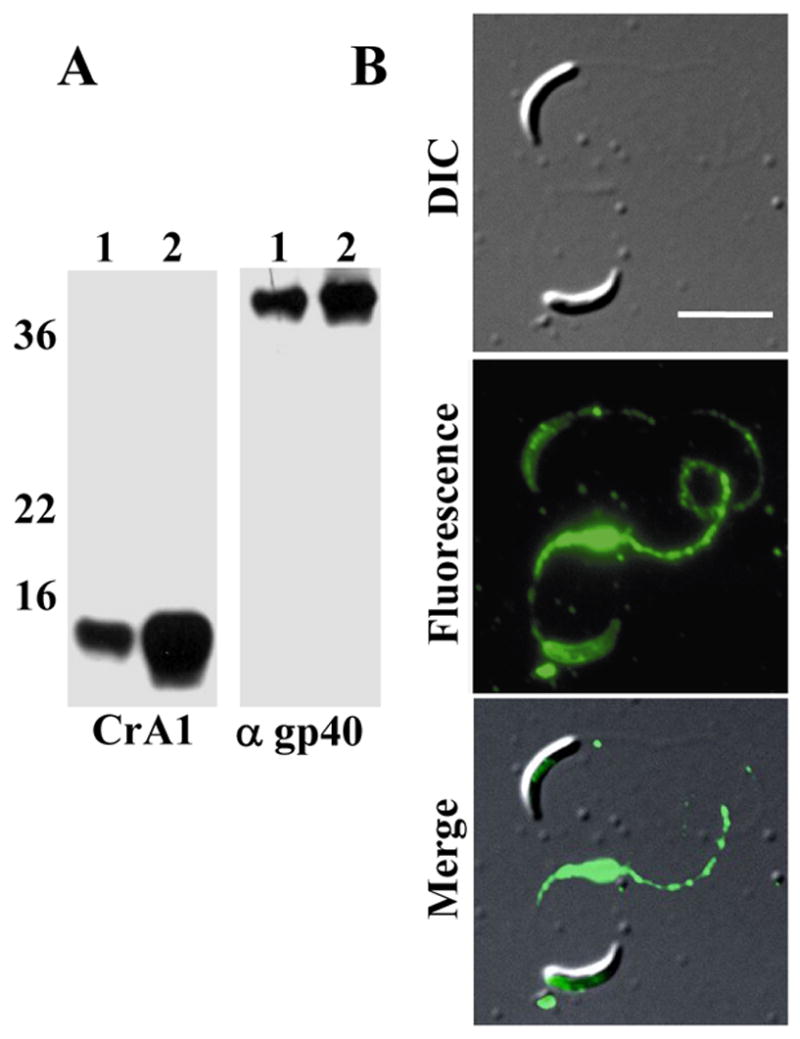
A. Triton X-114 extraction: Oocysts were excysted, lysed in 2% TX-114 and proteins partitioning into the aqueous (lanes 1) and detergent (lanes 2) phases following phase separation were analyzed by Western blot. The blots were probed with anti-gp15 mAb CrA2 (left panel) and mouse anti-rgp40 (right panel). B. Live IFA: Oocysts were excysted and live sporozoites reacted with mouse anti-rgp40 serum, and FITC-conjugated goat anti-mouse IgG. The labeled sporozoites were placed on slides and images captured using a Zeiss Axioplan 2 fluorescent microscope and OpenLab software (Improvision, Lexington, MA). Scale bar=5 μm.
To determine if gp40 associates with the sporozoite membrane by forming a complex with gp15, we performed co-immunoprecipitations using a rat polyclonal antibody raised against native gp40 (a gift from Dr Richard Nelson, University of California, San Francisco, CA [8]). Control immunoprecipitations were run in parallel using normal rat serum. Western blots of the captured proteins were probed with the mouse IgM anti-gp40 mAb 4E9 [6], and the mouse IgA anti-gp15 mAb CrA2, (mAb CrA2 was a gift from Dr. Marian Neutra, Harvard Medical School, Boston, MA [7]). Detection with these reagents allowed identification of the precipitated antigens without interference from reactions with the precipitating antibody. Figure 2A shows that both gp40 (Figure 2A, panel 1, lane 3) and gp15 (Figure 2A, panel 2, lane 3) were precipitated by the gp40 specific antisera, but not by normal rat serum (Figure 2A, panels 1 and 2, lane 2). Since the rat anti-gp40 serum does not react with gp15 [8], gp15 was precipitated via its interaction with gp40, suggesting that the two antigens associate on the sporozoite surface to form a heteroprotein complex.
Figure 2. gp40 localizes to the sporozoite membrane by association with gp15.
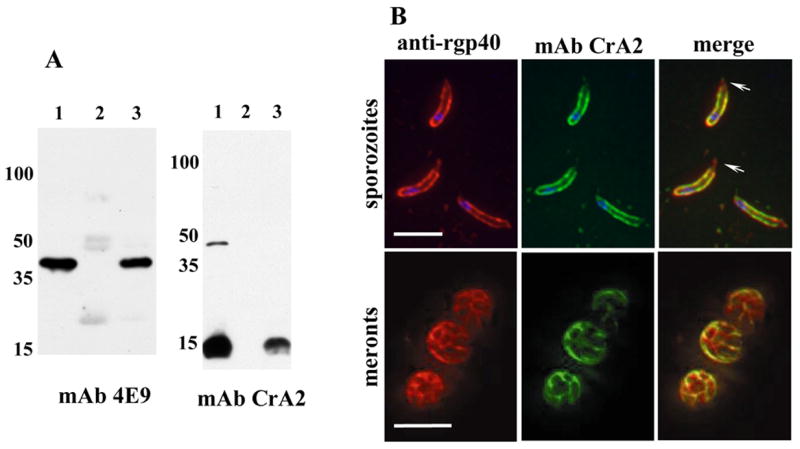
A. Co-immunoprecipitations: C. parvum GCH1 oocysts were excysted, lysed in low salt lysis buffer (50mM Tris, pH 7.5, 10mM NaCl, 1% TX-100, 1X protease inhibitor cocktail) and proteins precipitated with either rat anti-native gp40 serum or normal rat serum and protein G sepharose beads. After extensive washing, the beads were boiled and the captured proteins analyzed by Western blotting with anti-gp40 mAb 4E9 and anti-gp15 mAb CrA2. Lane 1: total lysate, lane 2: precipitate using normal rat serum, lane 3: precipitate using rat anti-gp40 serum. The high molecular weight band recognized by CrA2 in total lysates is elongation factor EF1α [8, 21]. B. Fixed IFA: C. parvum Iowa isolate sporozoites (top panel) and HCT-8 cells infected with oocysts for 20 hours (meront stage, lower panel) were fixed, reacted with rabbit anti-rgp40 serum and anti-gp15 IgA mAb CrA2, and the primary antibodies co-localized with Alexaflour 594-conjugated goat anti rabbit IgG (Invitrogen, Carlsbad, CA) and FITC-conjugated goat anti mouse IgA (Southern Biotech, Birmingham, AL). Z-stacks with 0.1mm spacing were captured using a Zeiss Axioimager fluorescent microscope and the images deconvolved and merged using Volocity’s iterative restoration program (Improvision, Lexington, MA). Blue fluorescence is nuclei stained with DAPI. Scale bars=5 μm. Arrowheads indicate apical localization of gp40.
To confirm that gp40 and gp15 associate on zoite membranes, we co-localized the antigens in sporozoites and meronts. Because all gp40 and gp15 specific reagents were murine, and there was insufficient amounts of the rat anti-gp40 serum, we generated rabbit anti-rgp40 serum for these experiments Antigen was prepared and rabbits immunized as described (Harlan Bioproducts for Science, Indianapolis IN) [7], and the titers monitored by Western blot. The sera specifically recognized gp40 in lysates of C. parvum oocysts (not shown). Using rabbit anti-gp40 serum and mAb CrA2, we performed immunofluorescence assays on sporozoites and intracellular stages. gp40 and gp15 both localized to the membrane of sporozoites (Figure 2B, upper panel), consistent with the two antigens associating with each other in a complex. However, in sporozoites, gp40 was found on both the sporozoite membrane and in the apical region of the sporozoite (Figure 2B arrowheads), whereas gp15 appeared to be associated only with the membrane. Both antigens were shed in trails. Similar localization on the intracellular stages (Figure 2B, lower panel) suggests that the gp40/15 complex is also present on the merozoite membrane. Biosynthetic labeling experiments using intracellular parasites have shown that the gp40/15 precursor is rapidly cleaved [11], suggesting that co-localization of gp40 and gp15 on the membrane of intracellular stages is unlikely to be attributable to the presence of significant amounts of the precursor.
Taken together these observations suggest that gp40 is found on the surface of sporozoites in association with the GPI anchored gp15. Although predominantly found in the detergent phase of the TX-114 extractions, some gp40 and a small amount of gp15 are also in the aqueous phase. This may be attributable to the gp40/gp15 complex being shed off the sporozoite surface during excystation (the mechanism by which this occurs is unknown), or it is possible that the amount of the gp40/gp15 complex is regulated by an equilibrium. In several aspects, the gp40/gp15 antigen complex is very similar to the Plasmodium merozoite surface protein 1 (MSP1). MSP1 is a GPI anchored 190kDa glycoprotein that is cleaved into several polypeptides that non-covalently associate with each other [15,16]. The 42 kDa C-terminal domain of MSP1 is a substrate for PfSUB2 [17], a serine protease that may be similar to the furin-like serine protease that cleaves gp40/15 [11]. Cleavage of the 42kDa fragment is necessary for merozoite invasion into erythrocytes [18]. The MSP1 42kDa fragment has been shown to be the target of protective antibodies [19] and is currently in Phase I trials as a vaccine candidate [20]. In comparison, little is known about the structure, processing and functions of the gp40/15 antigen complex. Additional investigations are needed to fully understand the role this antigen complex plays in host-parasite interactions, and to determine the feasibility of gp40/gp15 as a vaccine candidate for cryptosporidiosis.
Acknowledgments
This work was supported by NIH grants K01DK062816 (R. M. O.), RO1 AI05786 (H. D. W.) and the GRASP Digestive Diseases Center at Tufts-New England Medical Center (P30 DK34928-18). We would like to thank Delynn Moss and Michael Arrowood for Cryptosporidium antigen preparation. Use of trade names is for identification only and does not imply endorsement by the Public Health Service or by the U.S. Department of Health and Human Services. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang DB, White AC. An updated review on Cryptosporidium and Giardia. Gastroenterol Clin North Am. 2006;35:291–314. viii. doi: 10.1016/j.gtc.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Hunter PR, Nichols G. Epidemiology and clinical features of cryptosporidium infection in immunocompromised patients. Clin Microbiol Rev. 2002;15:145–54. doi: 10.1128/CMR.15.1.145-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bobak DA. Use of nitazoxanide for gastrointestinal tract infections: treatment of protozoan parasitic infection and beyond. Curr Infect Dis Rep. 2006;8:91–5. doi: 10.1007/s11908-006-0003-y. [DOI] [PubMed] [Google Scholar]
- 4.Abubakar I, Aliyu SH, Arumugam C, Hunter PR, Usman NK. Prevention and treatment of cryptosporidiosis in immunocompromised patients. Cochrane Database Syst Rev. 2007:CD004932. doi: 10.1002/14651858.CD004932.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Tzipori S, Ward H. Cryptosporidiosis: biology, pathogenesis and disease. Microbes Infect. 2002:4, 1047. doi: 10.1016/s1286-4579(02)01629-5. [DOI] [PubMed] [Google Scholar]
- 6.Cevallos AM, Bhat N, Verdon R, Hamer DH, Stein B, Tzipori S, Pereira ME, Keusch GT, Ward HD. Mediation of Cryptosporidium parvum infection in vitro by mucin-like glycoproteins defined by a neutralizing monoclonal antibody. Infect Immun. 2000;68:5167–75. doi: 10.1128/iai.68.9.5167-5175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cevallos AM, Zhang X, Waldor MK, Jaison S, Zhou X, Tzipori S, Neutra MR, Ward HD. Molecular cloning and expression of a gene encoding Cryptosporidium parvum glycoproteins gp40 and gp15. Infect Immun. 2000;68:4108–16. doi: 10.1128/iai.68.7.4108-4116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strong WB, Gut J, Nelson RG. Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15- and 45-kilodalton zoite surface antigen products. Infect Immun. 2000;68:4117–34. doi: 10.1128/iai.68.7.4117-4134.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Priest JW, Kwon JP, Arrowood MJ, Lammie PJ. Cloning of the immunodominant 17-kDa antigen from Cryptosporidium parvum. Mol Biochem Parasitol. 2000;106:261–71. doi: 10.1016/s0166-6851(99)00223-6. [DOI] [PubMed] [Google Scholar]
- 10.Winter G, Gooley AA, Williams KL, Slade MB. Characterization of a major sporozoite surface glycoprotein of Cryptosporidum parvum. Funct Integr Genomics. 2000;1:207–17. doi: 10.1007/s101420000028. [DOI] [PubMed] [Google Scholar]
- 11.Wanyiri JW, O’Connor R, Allison G, Kim K, Kane A, Qiu J, Plaut AG, Ward HD. Proteolytic processing of the Cryptosporidium spp. glycoprotein gp40/15 by human furin and by a parasite-derived furin-like protease activity. Infection and Immunity. 2007;75:184–192. doi: 10.1128/IAI.00944-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Priest JW, Xie LT, Arrowood MJ, Lammie PJ. The immunodominant 17-kDa antigen from Cryptosporidium parvum is glycosylphosphatidylinositol-anchored. Mol Biochem Parasitol. 2001;113:117–26. doi: 10.1016/s0166-6851(00)00386-8. [DOI] [PubMed] [Google Scholar]
- 13.Priest JW, Kwon JP, Moss DM, Roberts JM, Arrowood MJ, Dworkin MS, Juranek DD, Lammie PJ. Detection by enzyme immunoassay of serum immunoglobulin G antibodies that recognize specific Cryptosporidium parvum antigens. J Clin Microbiol. 1999;37:1385–92. doi: 10.1128/jcm.37.5.1385-1392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riggs MW, Perryman LE. Infectivity and neutralization of Cryptosporidium parvum sporozoites. Infect Immun. 1987;55:2081–7. doi: 10.1128/iai.55.9.2081-2087.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holder AA, Freeman RR. The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J Exp Med. 1984;160:624–9. doi: 10.1084/jem.160.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McBride JS, Heidrich HG. Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol Biochem Parasitol. 1987;23:71–84. doi: 10.1016/0166-6851(87)90189-7. [DOI] [PubMed] [Google Scholar]
- 17.Harris PK, Yeoh S, Dluzewski AR, O’Donnell RA, Withers-Martinez C, Hackett F, Bannister LH, Mitchell GH, Blackman MJ. Molecular identification of a malaria merozoite surface sheddase. PLoS Pathog. 2005;1:241–51. doi: 10.1371/journal.ppat.0010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackman MJ, Scott-Finnigan TJ, Shai S, Holder AA. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J Exp Med. 1994;180:389–93. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holder AA, Guevara Patino JA, Uthaipibull C, Syed SE, Ling IT, Scott-Finnigan T, Blackman MJ. Merozoite surface protein 1, immune evasion, and vaccines against asexual blood stage malaria. Parassitologia. 1999;41:409–14. [PubMed] [Google Scholar]
- 20.Malkin E, Long CA, Stowers AW, Zou L, Singh S, Macdonald NJ, Narum DL, Miles AP, Orcutt AC, Muratova O, Moretz SE, Zhou H, Diouf A, Fay M, Tierney E, Leese P, Mahanty S, Miller LH, Saul A, Martin LB. Phase 1 Study of Two Merozoite Surface Protein 1 (MSP1(42)) Vaccines for Plasmodium falciparum Malaria. PLoS Clin Trials. 2007;2:e12. doi: 10.1371/journal.pctr.0020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonafonte MT, Priest JW, Garmon D, Arrowood MJ, Mead JR. Isolation of the gene coding for elongation factor-1alpha in Cryptosporidium parvum. Biochim Biophys Acta. 1997;1351:256–60. doi: 10.1016/s0167-4781(97)00013-4. [DOI] [PubMed] [Google Scholar]


