Abstract
Objective
This study compared reflex responsiveness of the first dorsal interosseus muscle during two tasks that employ different strategies to stabilize the finger while exerting the same net muscle torque.
Methods
Healthy human subjects performed two motor tasks that involved either pushing up against a rigid restraint to exert a constant isometric force equal to 20% of maximum, or maintaining a constant angle at the metacarpophalangeal joint while supporting an equivalent inertial load. Each task consisted of six 40-s contractions during which electrical and mechanical stimuli were delivered.
Results
The amplitude of short and long latency reflex responses to mechanical stretch did not differ significantly between tasks. In contrast, reflexes evoked by electrical stimulation were significantly greater when supporting the inertial load.
Conclusions
Agonist motor neurons exhibited heightened reflex responsiveness to synaptic input from heteronymous afferents when controlling the position of an inertial load. Task differences in the reflex response to electrical stimulation were not reflected in the response to mechanical perturbation, indicating a difference in the efficacy of the pathways that mediate these effects.
Significance
Results from this study suggest that modulation of spinal reflex pathways may contribute to differences in the control of force and position during isometric contractions of the first dorsal interosseus muscle.
Keywords: spinal reflexes, isometric contraction, motor control, muscle spindle afferents, presynaptic inhibition
1. Introduction
Over a decade ago, Buchanan and Lloyd (1995) observed that activation of the elbow flexor muscles differs when subjects are instructed to maintain a constant elbow angle while supporting an inertial load (position control) compared with exerting an equivalent torque against a rigid restraint (force control). Subsequent studies have demonstrated that the control strategy used during isometric contractions can alter the recruitment and discharge behavior of single motor units both with (Tax et al., 1989; Tax et al., 1990; Rudroff et al., 2006) and without (Mottram et al., 2005) changes in the distribution of activity among accessory muscles. Furthermore, the duration that sustained (Hunter et al., 2002; Maluf et al., 2005; Rudroff et al., 2005) and intermittent (Sjogaard et al., 2000) contractions can be maintained before task failure is also influenced by the control strategy used to sustain the task. Maluf and Enoka (2005) postulated that these effects may be explained by changes in the sensitivity of spinal motor neurons to synaptic input from peripheral afferents for loads of similar magnitude that involve different stability demands.
Findings from several studies indicate that the magnitude of the stretch reflex increases when subjects are asked to maintain a constant limb position in the presence of a destabilizing load (Akazawa et al., 1983; Kanosue et al., 1983; De Serres and Milner, 1991; Doemges and Rack, 1992a; Doemges and Rack, 1992b; Dietz et al., 1994). These studies used a servo-controlled motor to simulate a variety of loading conditions with different stability requirements. However, it is unclear whether the changes in reflex responsiveness observed during simulated loading conditions translate to more functional tasks that are known to elicit changes in motor neuron activity (c.f. Buchanan and Lloyd, 1995; Hunter et al., 2002; Maluf et al., 2005; Rudroff et al., 2005). Furthermore, changes in the H-reflex during tasks involving force and position control have not been assessed in previous studies. The stretch reflex and the H-reflex both involve monosynaptic excitation of Ia afferents, yet the H-reflex bypasses the muscle spindle and has been used in combination with the stretch reflex to assess excitability of the fusimotor system. However, it is well known that transcutaneous nerve stimulation can activate oligosynaptic pathways and other types of afferents, such as Ib and cutaneous fibers, which may also contribute to the H-reflex waveform (reviewed in Schieppati, 1987). Furthermore, the H-reflex and the stretch reflex differ in their sensitivity to a variety of factors that can influence presynaptic inhibition of Ia terminals (reviewed in Schieppati, 1987 and Pierrot-Deseilligny and Burke, 2005). Such differences in the origin of the H-reflex and stretch reflex preclude a direct comparison of these reflexes to identify task-specific modulation of any single component of the monosynaptic stretch reflex pathway. However, concurrent examination of stretch and H-reflexes may provide insight into the neural mechanisms that underlie differences in the control of force and position during isometric contractions if interpreted in the context of known differences between electrically and mechanically evoked reflexes.
Despite known differences in motor unit activity and time to task failure for isometric tasks involving either force or position control, there is currently no direct evidence of reflex modulation for these two tasks. Therefore, the purpose of this preliminary investigation was to compare reflex responsiveness of the first dorsal interosseus muscle during two tasks that employ different strategies to stabilize the finger while exerting the same net muscle torque. Based on a review of previous research, we recently proposed a theoretical model to identify the primary reflex pathways that may contribute to observed differences in force and position control (Maluf and Enoka, 2005). This model emphasized the role of presynaptic mechanisms, including a reduction in the presynaptic inhibition of feedback transmitted by group Ia afferents when controlling limb position. Consistent with this model, we expected to observe heightened reflex responsiveness to both mechanical and electrical stimuli when supporting an inertial load compared with producing an equivalent force against a rigid restraint.
2. Methods
2.1. Subjects
Sixteen healthy adults provided informed consent in accordance with study procedures approved by the Human Subjects Committee at the University of Colorado. All subjects were right-hand dominant and reported being free from any neurological or orthopedic impairment that could interfere with hand function.
2.2. Apparatus and testing procedure
Subjects were seated upright with the left hand positioned in the custom apparatus as illustrated in Figure 1. To facilitate normalization of tonic EMG activity, maximal voluntary contractions (MVC) of the first dorsal interosseus (agonist), second palmar interosseus (antagonist), and abductor pollicis brevis (accessory) muscles were performed at the beginning of each session. Reflex responses to mechanical and electrical stimuli were then assessed during the force and position tasks using the same electrode location and stimulus intensity for each task. Force and position tasks were performed with the index finger as described in detail by Maluf et al. (2005). Briefly, subjects were required to match either a target force equal to 20% of their maximal force by pushing up against a rigid bar (force task), or a target position corresponding to 0 degrees abduction of the metacarpophalangeal joint while supporting an equivalent load suspended from the index finger (position task). Visual feedback was provided on a monitor during both tasks at a gain equal to 2.5% / cm of the maximal performance range, operationally defined as MVC force for the force task and full range of motion about the metacarpophalangeal joint for the position task (Maluf et al., 2005). Subjects performed one block of 6 trials for each of the two tasks, with each trial lasting approximately 40 s and separated by 2 minutes rest. The order of the tasks was randomized, and the maximal abduction force of the index finger was reassessed prior to each task. Four mechanical and 4 electrical stimuli were delivered in random order during each trial using the procedures described below, yielding a total of 24 reflex responses for each type of stimulus in the two tasks. The interval between successive stimuli was randomly varied between 3-5 s. To minimize the influence of transient fluctuations in task mechanics on the reflex response, stimuli were delivered only when force and position signals were within 5% and 2 degrees of their respective targets. These criteria were chosen to reflect the typical range of fluctuations observed prior to the beginning of task failure in previous experiments (Maluf et al., 2005). Custom software was written in LabView v.7.1 (National Instruments, Austin, TX) to trigger the delivery of electrical and mechanical stimuli based on the parameters defined above using a National Instruments PCI-6052E multifunction IO card.
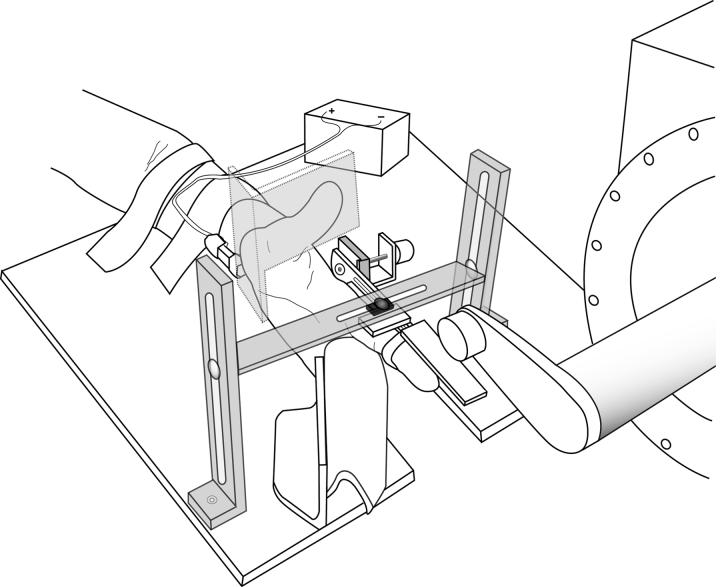
Fig 1.
Experimental arrangement for the assessment of mechanically and electrically evoked reflexes in the first dorsal interosseus muscle during the force and position tasks. The thumb was restrained in extension and the index finger was placed in a splint attached to a low-friction hinge that allowed only abduction-adduction movements about the metacarpophalangeal joint. The position of the index finger was maintained at 0 degrees of abduction by a rigid metal bar during the force task and subjects produced a target force equal to 20% of MVC using visual feedback from a force transducer located at the proximal interphalangeal joint. The bar was removed during the position task and an equivalent mass was hung from the finger brace at the proximal interphalangeal joint. Subjects maintained the position of the index finger at 0 degrees of abduction using visual feedback from a potentiometer that was aligned with the metacarpophalangeal joint. Heteronymous reflexes were evoked by electrical stimuli applied to the median nerve at the wrist. A hammer attached to the shaft of a servo-controlled torque motor was used to rapidly adduct the index finger by striking the distal end of the finger splint, thereby evoking a stretch reflex.
2.3. Delivery of mechanical and electrical stimuli
To avoid mechanical coupling between the finger and the motor shaft that could alter the inertial characteristics and stability demands of the position task, the first dorsal interosseus muscle was stretched by striking the distal end of a finger splint with a hammer that was attached to the shaft of a servo-controlled torque motor (PMA44Q, Pacific Scientific, Rockford, IL & PCI-7352, National Instruments, Austin, TX). Subjects were instructed not to resist or assist displacement of the index finger, and to resume the target force or position as quickly as possible after each stretch. The major advantage of this approach was that it closely replicated the conditions under which the nervous system must respond to sudden, external disturbances of finger position during tonic postural contractions. A potential disadvantage was that the amplitude and velocity of the perturbation could not be precisely controlled due to differences in load mechanics between the two tasks. Therefore, pilot experiments were conducted in which the displacement and velocity of the torque motor shaft were varied to determine the effects on finger kinematics and reflex responses for the two tasks. A range of stimulus parameters (∼10-30 degrees and ∼250-600 degrees/s of index finger displacement and velocity, respectively) comparable to those used in previous studies (Tarkka, 1986; Balestra et al., 1992; Duchateau and Hainaut, 1993) were found to be associated with a similar pattern of reflex responses for the two tasks. The parameters selected from within this range resulted in a similar magnitude of finger displacement (11.0 ± 3.2 and 10.5 ± 3.0 degrees; paired t-test P = 0.27) and velocity (333 ± 104 and 316 ± 96 degrees/s; paired t-test P = 0.24) during the force and position tasks performed in subsequent experimental sessions.
Heteronymous reflexes in the first dorsal interosseus muscle were assessed by delivering an electrical stimulus (1-ms rectangular pulse) to the median nerve at the wrist as described by others (Duchateau and Hainaut, 1993). The first dorsal and second palmar interosseus muscles are both innervated by the ulnar nerve, therefore, median nerve stimulation permitted the assessment of agonist responses to feedback transmitted by low-threshold afferents without concurrent activation of the antagonist muscle. This approach also avoided contamination of the H-reflex by F waves evoked by antidromic activation of homonymous motor axons (Fisher, 1992). The sensitivity of monosynaptic reflexes to a variety of facilitatory and inhibitory effects is greatest for reflexes of moderate amplitude (Crone et al., 1990). To maximize our ability to detect these modulatory effects, the stimulus intensity was adjusted to produce a short latency (∼30 ms) response in the first dorsal interosseus with an amplitude equal to half of its maximal value for each subject. This intensity was typically just below the threshold necessary to produce a direct motor response in the homonymous abductor pollicis brevis muscle (Duchateau and Hainaut, 1993).
The magnitude of the reflex response has been shown to increase in proportion to the level of preexisting tonic muscle activation (Gottlieb and Agarwal, 1979; Al-Falahe and Vallbo, 1988), which is often greater for novel tasks involving unstable loads due to the coactivation of agonist and antagonist muscles (De Serres and Milner, 1991; Osu et al., 2002). In an attempt to minimize the effects of coactivation on tonic activity of the first dorsal interosseus muscle, all subjects practiced the MVC, force, and position tasks during a familiarization session 1-3 days prior to the experiment. Subjects were provided with specific instructions to avoid coactivation of hand and forearm muscles during both the familiarization and experimental sessions. In addition, agonist EMG activity was monitored throughout the experiment, and subjects were provided with verbal feedback regarding the consistency of muscle activation across the two tasks.
2.4. Data processing and statistical analyses
Interference EMG signals from the left first dorsal interosseus, abductor pollicis brevis, biceps brachii, and extensor digitorum muscle groups were recorded using bipolar surface electrodes (silver-silver chloride; 4- or 8-mm electrode diameter; 12-mm interelectrode distance). The electrodes for first dorsal interosseus were placed parallel to the radial border of the second metacarpal, just proximal to the junction between the muscle and distal tendon (Zijdewind et al. 1995). Electrodes were placed over accessory muscle groups (abductor pollicis brevis, biceps brachii, and extensor digitorum) according to previously published recommendations (Hermens et al. 1999; Basmajian and Blusmenstein, 1983). The EMG activity of accessory muscles was monitored to detect any task differences in accessory muscle activity that may have contributed to reflex modulation of the primary agonist. EMG signals from the antagonist muscle, second palmar interosseus, were recorded with a bipolar intramuscular electrode consisting of two Formvar-insulated stainless steel wires (50 μm diameter), with 2 mm of insulation removed from the distal tip of one wire to increase the recording volume of the electrode. Reference electrodes were placed over bony prominences on the left upper extremity. Bioamplifiers (Coulbourn Instruments, Allentown, PA) were used to amplify (x 500-5,000) and band-pass filter (13-1000 Hz) surface and intramuscular EMG signals prior to sampling and storage at 2000 samples/s. Force signals were A/D converted at 200 samples/s (Power 1401, 16-bit resolution, Cambridge Electronic Design, Cambridge, UK) and stored on computer for subsequent analysis.
The average amplitude of the rectified EMG signal (aEMG) was calculated for a 0.5-s interval centered about the peak EMG of MVC trials for the first dorsal interosseus, second palmar interosseus, and abductor pollicis muscles. These values were used for normalization of EMG signals in order to compare the relative level of tonic activation for the intrinsic hand muscles during the force and position tasks. Tonic activation of the intrinsic hand muscles during the force and position tasks at 20% MVC force was determined by averaging the normalized aEMG across each trial for both tasks after excluding 1 s of data immediately following the onset of each stimulus and at the beginning and end of each trial. Similar analyses were performed for the biceps brachii and extensor digitorum muscles using the non-normalized EMG signal because no MVC trials were performed for these muscles. Tonic activation of the first dorsal interosseus was further examined by calculating the aEMG in the 50-ms period just prior to the onset of each stimulus. Consistent with the analysis of reflex responses to electrical and mechanical stimulation, tonic activation of the first dorsal interosseus just prior to stimulation was compared within the same session using the non-normalized EMG signal. This was done to avoid bias caused by small differences in the aEMG recorded during MVCs performed by the same subject prior to each task.
The aEMG records from 24 individual responses to each type of stimulus were averaged prior to subsequent analyses. Short-latency reflex responses were determined from the peak amplitude of the aEMG in the intervals from 29 ± 6 to 52 ± 4 ms for mechanical stimuli and from 28 ± 2 to 50 ± 4 ms for electrical stimuli. Long-latency reflex responses were determined similarly in the intervals from 52 ± 4 to 95 ± 2 ms for mechanical stimuli and from 50 ± 4 to 89 ± 6 ms for electrical stimuli. These reflex latencies differed between subjects, but remained constant across tasks within the same subject. Latencies were selected individually for each subject based on visual analysis of the time at which the averaged EMG signal on either side of the peak short- and long-latency responses for both tasks intersected the level of tonic aEMG as defined above. These latencies correspond well with values previously observed in the first dorsal interosseus muscle (Tarkka, 1986; Balestra et al., 1992; Duchateau and Hainaut, 1993). Peaks in the EMG response to stretch are less defined than those produced by electrical stimulation due to the asynchronous activation of Ia afferent fibers and the resulting temporal dispersion of afferent volleys to the spinal cord (Burke et al., 1983). Therefore, stretch reflexes were also assessed by calculating the area of the EMG signal that exceeded tonic levels of activity during the time intervals defined above. Analysis of variance for repeated measures was used to identify overall task differences in the magnitude of short- and long-latency reflex responses, followed by Student’s paired-sample t-tests for post hoc comparisons. Planned comparisons were restricted to within-subject differences between the force and position tasks and only two post-hoc tests were performed, therefore, no adjustment was made for multiple comparisons. Student’s paired-sample t-tests also were used for comparison of the maximum force and aEMG recorded during MVC of the first dorsal interosseus prior to each task. The level of significance was set at p < 0.05 for all analyses. Results are reported as mean (SD).
3. Results
Of the 16 subjects tested, 1 subject exhibited no identifiable reflex response to electrical or mechanical stimulation and was therefore excluded. Despite precautions to ensure a similar level of net muscle activation across conditions, two additional subjects had heightened (∼30%) agonist and antagonist EMG during the position task and were also excluded. Both of these subjects demonstrated a large increase in the short- and long-latency reflex responses to electrical (8-82%) and mechanical (60-110%) stimulation during the position task that was likely associated with heightened tonic activity of the agonist muscle. Finally, 3 subjects exhibited no heteronymous reflex in the first dorsal interosseus muscle following stimulation of the median nerve, and equipment malfunction prevented the measurement of reflex responses to mechanical stimulation in 1 subject. Statistical analyses were performed using reflex responses from the remaining 12 subjects for mechanical stimulation and 10 subjects for electrical stimulation (age: 27 ± 9 years, range 18-47 years; height: 1.8 ± 0.1 m, range 1.6-1.9 m; weight: 70.3 ± 12.8 kg, range 54.1-102.3 kg).
Maximal force (30.2 ± 6.9 versus 29.6 ± 1.8 N; P = 0.31) and aEMG (1.2 ± 0.4 versus 1.2 ± 0.5 mV; P = 0.96) recorded during MVC of the first dorsal interosseus prior to each task were similar for the force and position tasks, respectively. There were no task differences in the level of tonic activation of the first dorsal interosseus (23.8 ± 2.6 versus 25.8 ± 2.6 % EMG max; P = 0.27), second palmar interosseus (15.0 ± 5.7 versus 15.5 ± 4.9 % EMG max; P = 0.65), abductor pollicis brevis (5.4 ± 0.9 versus 6.0 ± 1.5 % EMG max; P = 0.75), biceps brachii (0.028 ± 0.01 versus 0.029 ± 0.01 mV; P = 0.93), or extensor digitorum (0.042 ± 0.01 versus 0.041 ± 0.01 mV; P = 0.84) during the force and position tasks, respectively. Similarly, there were no significant differences in baseline EMG for the first dorsal interosseus muscle just prior to the onset of mechanical (0.24 ± 0.12 versus 0.26 ± 0.15 mV; P = 0.10) or electrical (0.23 ± 0.10 versus 0.28 ± 0.15 mV; P = 0.10) stimuli for the force and position tasks. In addition, tonic muscle activation did not change significantly across the six trials for either task (main effect of trial for each muscle, P > 0.05). Finally, there were no significant effects of task order (first vs. second task, P > 0.05) or trial block (first vs. last 12 responses, P > 0.05) on the amplitude of short- or long-latency reflex responses to mechanical and electrical stimulation. These results indicate that tonic levels of agonist, antagonist, and accessory muscle activation were comparable for the two tasks, and did not change systematically during the experiment due to fatigue or other factors. Although a direct motor response (M-wave) cannot be used to test the constancy of stimulus conditions (Zehr, 2002) when assessing heteronymous reflexes, the similarity of the two tasks and the low forces that were required suggest that task variations in the stimulus conditions were likely minimal.
There were no significant differences in the peak amplitude of the short-latency (0.50 ± 0.25 versus 0.51 ± 0.29 mV) or long-latency (0.87 ± 0.47 versus 0.90 ± 0.46 mV) responses to mechanical stimulation during the force and position tasks, respectively (main effect of task, P = 0.69, n = 12). However, inter-subject variability was high as shown for three representative subjects in Figure 2. As can be seen in the top row of Figure 2, stretch kinematics were not always identical within the same subject across the two tasks. Although mean stretch displacement and velocity did not differ for the force and position tasks when considered as a group (see Methods), within-subject differences ranged from 0 to 3 degrees (mean 1 ± 1 degrees) and from 5 to 125 degrees/s (mean 37 ± 33 degrees/s). Therefore, analysis of covariance was used to assess whether short- and long-latency reflex responses differed for the two tasks after accounting for the variability caused by differences in stretch displacement and velocity. The effects of load type on the short- and long-latency reflex responses to mechanical stimulation remained insignificant (main effect of task, P = 0.90). Previous studies have reported similar variations in stretch kinematics (De Serres et al., 2002), (De Serres and Milner, 1991), which were considered necessary to accommodate the application of different load compliances.
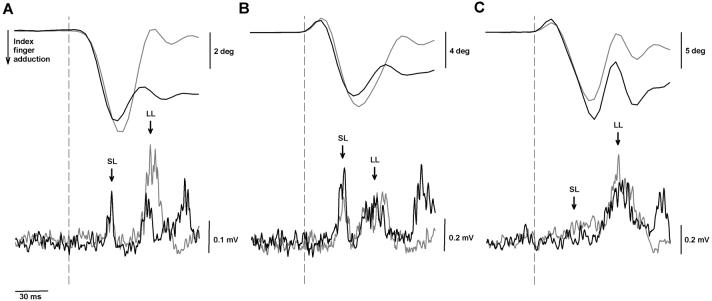
Fig 2.
Representative data illustrating task-dependent variations in the EMG response of the first dorsal interosseus muscle to mechanical perturbation of the index finger in three subjects (panels A-C). Traces represent an average of 24 trials for position (top row) and EMG (bottom row) signals recorded during the force (grey lines) and position (black lines) tasks. Dashed line indicates stimulus onset, which was verified using an accelerometer that detected the stimulus artifact (an initial upward deflection of the position signal). Short-latency (SL) and long-latency (LL) reflex responses are indicated by arrows. The third peak in the EMG record represents a voluntary response which was necessary to resume the target position after each stretch. The voluntary EMG response was greater for the position task in all subjects. The subject in panel A demonstrated no task differences in the magnitude of the SL response, whereas the LL response was greater for the force task. In contrast, the subject in panel B demonstrated a greater SL response during the position task with no corresponding difference in the magnitude of the LL response. The subject in panel C exhibited a minimal SL response during both tasks, but had a heightened LL response during the force task.
The amplitude of the stretch reflex is plotted for all subjects in Figure 4B; approximately half of the responses lie above and half lie below the line of identity. Consistent with the results for peak amplitude, the area of short-latency and long-latency responses to mechanical stimulation did not differ for the force and position tasks (P > 0.69).
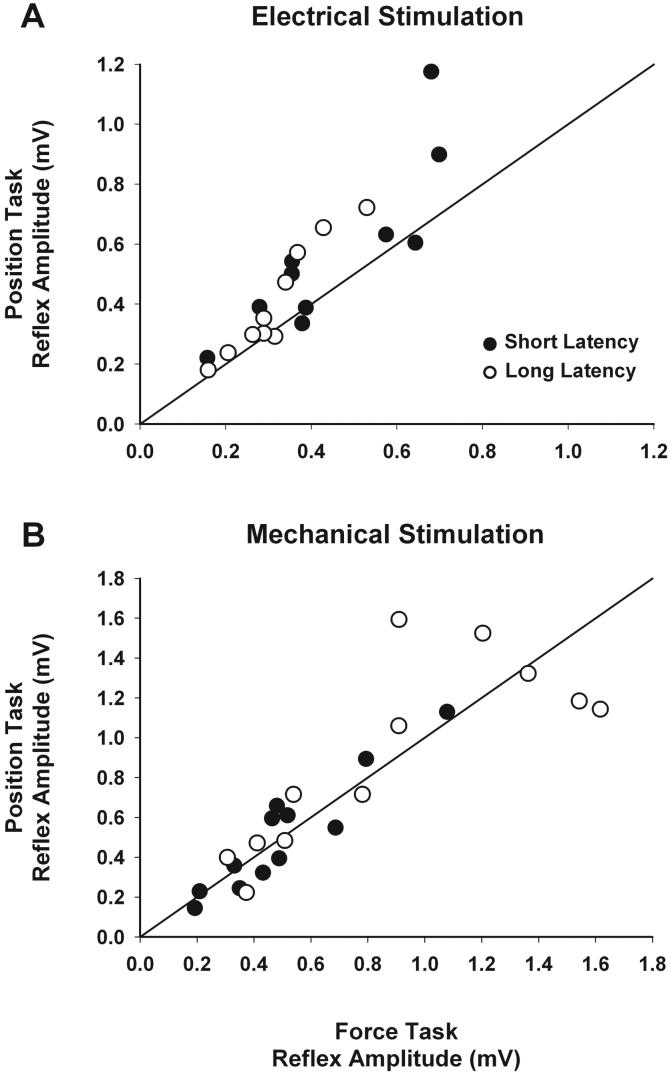
Fig 4.
Peak amplitude of the short- (closed symbols) and long- (open symbols) latency reflex responses to electrical stimulation of the median nerve (A) and mechanical perturbation of the index finger (B) during the force (x-axis) and position (y-axis) tasks for each subject. Most reflex responses to electrical stimulation lie above the line of identity, indicating a consistent increase in reflex sensitivity during the position task (P = 0.02). In contrast, reflex responses to mechanical perturbation were highly variable and were not significantly different across tasks (P = 0.69).
In contrast to the absence of task differences in the stretch reflex, Figures 3 and 4 demonstrate that both short-latency (0.45 ± 0.19 versus 0.57 ± 0.28 mV; P = 0.04) and long-latency (0.32 ± 0.11 versus 0.41 ± 0.19 mV; P = 0.01) reflex responses to electrical stimulation were significantly greater for the position task (main effect of task, P = 0.02, n = 10). No systematic differences were identified that could distinguish among individual subjects who did and did not exhibit heightened reflex responsiveness during the position task, as illustrated in Figure 4 by data points lying further from and closer to the line of identity, respectively.
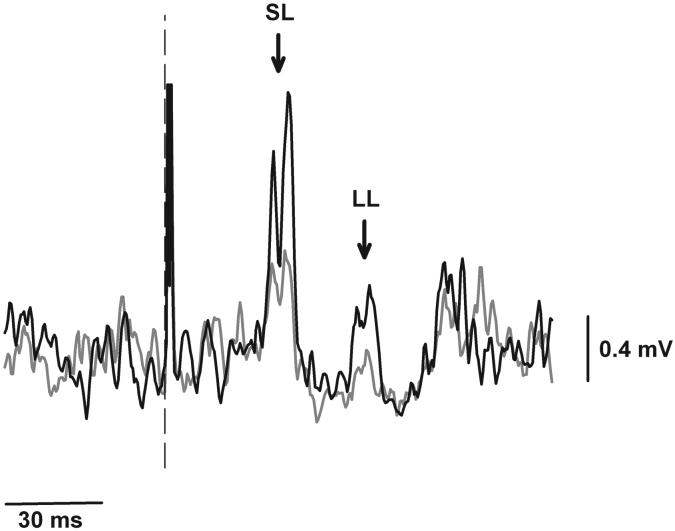
Fig 3.
EMG response of the first dorsal interosseus muscle to electrical stimulation of the median nerve is shown for a representative subject (same subject as illustrated in Fig 2, panel C). Traces represent an average of 24 EMG responses for the force (grey lines) and position (black lines) tasks. Dashed line indicates stimulus onset. Short-latency (SL) and long-latency (LL) reflex responses are indicated by arrows. SL and LL responses were greater for the position task compared with the force task.
4. Discussion
The lack of a consistent increase in the stretch reflex during the position task compared with the force task contrasts with previous investigations of reflex modulation during simulated load instability. For example, Akazawa and colleagues (1983) observed heightened reflex responsiveness of the flexor pollicis longus muscle when subjects attempted to maintain a steady thumb position against constant and variable loads generated by a torque motor. However, the subsequent observation that changes in the stretch reflex closely parallel changes in tonic muscle activation when destabilizing loads are applied to the wrist (De Serres and Milner, 1991) suggested that the findings of Akazawa et al. may have been due to elevated tonic activity of the agonist and antagonist muscles during the less stable loading conditions. Conversely, increases in the long-latency reflex response of the wrist flexor muscles can persist following paralysis of the wrist extensors (Doemges and Rack, 1992b), indicating that changes in reflex gain may occur without a corresponding change in tonic muscle activity. The present study sought to minimize coactivation by providing ample practice and closely monitoring the EMG signal during task performance. Although we did observe independent modulation of either the short- or long-latency component of the stretch reflex at similar levels of tonic activation in some subjects (Figure 2), no consistent pattern of task-dependent reflex modulation emerged for the two tasks examined in this study.
The discrepancy between changes in the stretch reflex observed by this and previous studies may be explained by differences in the stability demands of the experimental tasks. Consistent with this explanation, Doemges and Rack (1992a) also failed to observe modulation of stretch reflexes in the first dorsal interosseus muscle when a position-matching task was performed against a constant force applied by a torque motor. However, when the same task was performed against fluctuating forces to impose a greater challenge to stability, the long-latency component of the stretch reflex increased significantly. These observations underscore the importance of subtle variations in load stability on reflex modulation, and highlight the need to investigate the functional role of changes in reflex sensitivity that have previously been observed only during simulated loading conditions (Akazawa et al., 1983; Kanosue et al., 1983; De Serres and Milner, 1991; Doemges and Rack, 1992a; Doemges and Rack, 1992b; Dietz et al., 1994).
In contrast to inconsistent changes in the stretch reflex across tasks, short- and long-latency responses to electrical stimulation of the median nerve were significantly greater for the position task. These changes occurred despite a similar level of tonic activation present in agonist, antagonist, and accessory muscle groups. Duchateau and Hainaut (1993) previously described reflexes evoked in the first dorsal interosseus by stimulation of the median nerve that closely resemble those observed in the present study (cf. their Fig 5). The latencies of these reflexes are consistent with mono- and oligosynaptic pathways involving the Ia afferents of median-innervated muscles in the hand (Duchateau and Hainaut, 1993). However, contributions from other afferent fibers cannot be ruled out with mixed nerve stimulation. For example, selective simulation of digital nerves in the index finger are known to evoke cutaneomuscular reflexes in the first dorsal interosseus muscle (cf. Evans et al., 1989; Turner et al., 2002).
Given that similar levels of initial tonic excitation have been observed for the force and position tasks by this and previous studies (Maluf et al., 2005; Mottram et al., 2005), the available evidence does not support a change in fusimotor drive during the two tasks. Rather, the increase in reflex responsiveness to heteronymous nerve stimulation during the position task is likely mediated by reduced presynaptic inhibition of Ia afferent terminals. The absence of task differences in mechanically elicited responses might therefore be explained by diminished sensitivity of the stretch reflex to presynaptic inhibition. For example, human (Morita et al., 1998) and animal (Enriquez-Denton et al., 2002) experiments suggest that the nearly synchronous afferent volleys produced by electrical stimulation are less influenced by previous activation of Ia afferents compared with the temporally dispersed volleys produced by mechanical stretch. This difference results in greater sensitivity of the H-reflex to presynaptic effects. Electrical and mechanical stimuli may also have different effects on the input received from cutaneous and muscle afferents during the force and position tasks. The feedback transmitted by these afferents has been shown to modulate the level of presynaptic inhibition during a variety of motor tasks (reviewed in Aimonetti et al., 1999; Meunier, 1999; Rudomin, 2002).
Some evidence suggests that similar mechanisms regulate the sensitivity of the motor neuron pool to afferent input from heteronymous and homonymous sources (Nielsen and Kagamihara, 1993). However, it remains possible that differences in electrically and mechanically evoked reflexes in the present study were caused by differences in the modulation of afferent feedback from homonymous and heteronymous sources. This would suggest that the agonist motor neuron pool exhibits heightened sensitivity to afferent feedback from median-innervated accessory muscles when controlling the position of an inertial load, without a corresponding difference in sensitivity to stretch of the agonist. Selectively enhancing the gain of heteronymous reflexes may function to coordinate the responses of agonist and accessory muscles during postural contractions, and could increase joint stiffness without a potentially destabilizing effect on the reflex sensitivity of muscles directly responsible for supporting the inertial load.
In conclusion, this study provides the first direct evidence of heightened reflex responsiveness of the agonist motor neuron pool to heteronymous afferent feedback evoked by electrical stimulation when controlling the position of an inertial load. Surprisingly, task differences in the reflex response to electrical stimulation were not reflected in the response to mechanical perturbation. These preliminary observations are consistent with a reduced level of presynaptic inhibition during performance of the position task. However, future studies to examine the post-stimulus time histogram of single motor unit discharges in response to a conditioning stimulus will be necessary to confirm this interpretation. Given the non-linear properties of spinal motor neurons, it will also be necessary to determine the input-output relations for both motor tasks using a broad range of stimulus intensities. Finally, studies involving the selective activation or blockade of peripheral afferents are needed to clarify the effects of proprioceptive and cutaneous feedback on reflex modulation during the force and position tasks. Preliminary findings from this study suggest that modulation of spinal reflex pathways may contribute to differences in the control of force and position during isometric contractions of the first dorsal interosseus muscle. Future expansion of this line of research will further our understanding of agonist and accessory muscle coordination during postural contractions, and may help explain previously observed increases in muscle activation and fatigue during tasks that require accurate control of limb position while supporting an inertial load.
Acknowledgements
The National Institute of Neurological Disorders and Stroke supported this work with an award (R01 NS43275) to R.M. Enoka and the National Institute on Aging provided an award (T32 AG00279) to Robert Schwartz that supported K.S. Maluf. The authors gratefully acknowledge technical assistance from M.K. Anderson, S.S. Aidoor, and C.J. Barry. We also thank Professor Jacques Duchateau for helpful comments on an earlier version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aimonetti JM, Schmied A, Vedel JP, Pagni S. Ia presynaptic inhibition in human wrist extensor muscles: effects of motor task and cutaneous afferent activity. J Physiol Paris. 1999;93(4):395–401. doi: 10.1016/s0928-4257(00)80067-4. [DOI] [PubMed] [Google Scholar]
- Akazawa K, Milner TE, Stein RB. Modulation of reflex EMG and stiffness in response to stretch of human finger muscle. J Neurophysiol. 1983;49(1):16–27. doi: 10.1152/jn.1983.49.1.16. [DOI] [PubMed] [Google Scholar]
- Al-Falahe NA, Vallbo AB. Role of the human fusimotor system in a motor adaptation task. J Physiol. 1988;401:77–95. doi: 10.1113/jphysiol.1988.sp017152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestra C, Duchateau J, Hainaut K. Effects of fatigue on the stretch reflex in a human muscle. Electroencephalogr Clin Neurophysiol. 1992;85(1):46–52. doi: 10.1016/0168-5597(92)90101-g. [DOI] [PubMed] [Google Scholar]
- Basmajian JV, Blusmenstein R. In: Electrode placement in electromyographic biofeedback. Biofeedback. Principles and practice for clinicians. Basmajian JV, editor. Williams & Wilkins; Baltimore: 1983. pp. 363–378. [Google Scholar]
- Buchanan TS, Lloyd DG. Muscle activity is different for humans performing static tasks which require force control and position control. Neurosci Lett. 1995;194(12):61–4. doi: 10.1016/0304-3940(95)11727-e. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B. The afferent volleys responsible for spinal proprioceptive reflexes in man. J Neurophysiol. 1983;339:535–52. doi: 10.1113/jphysiol.1983.sp014732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Mazieres L, Morin C, Nielsen J, Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res. 1990;81(1):35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- De Serres SJ, Bennett DJ, Stein RB. Stretch reflex gain in cat triceps surae muscles with compliant loads. J Physiol. 2002;545(Pt 3):1027–40. doi: 10.1113/jphysiol.2002.027177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Serres SJ, Milner TE. Wrist muscle activation patterns and stiffness associated with stable and unstable mechanical loads. Exp Brain Res. 1991;86(2):451–8. doi: 10.1007/BF00228972. [DOI] [PubMed] [Google Scholar]
- Dietz V, Discher M, Trippel M. Task-dependent modulation of short- and long-latency electromyographic responses in upper limb muscles. Electroencephalogr Clin Neurophysiol. 1994;93(1):49–56. doi: 10.1016/0168-5597(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Doemges F, Rack PM. Changes in the stretch reflex of the human first dorsal interosseous muscle during different tasks. J Physiol. 1992a;447:563–73. doi: 10.1113/jphysiol.1992.sp019018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doemges F, Rack PM. Task-dependent changes in the response of human wrist joints to mechanical disturbance. J Physiol. 1992b;447:575–85. doi: 10.1113/jphysiol.1992.sp019019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Behaviour of short and long latency reflexes in fatigued human muscles. J Physiol. 1993;471:787–99. doi: 10.1113/jphysiol.1993.sp019928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez-Denton M, Morita H, Christensen LO, Petersen N, Sinkjaer T, Nielsen JB. Interaction between peripheral afferent activity and presynaptic inhibition of ia afferents in the cat. J Neurophysiol. 2002;88(4):1664–74. doi: 10.1152/jn.2002.88.4.1664. [DOI] [PubMed] [Google Scholar]
- Evans AL, Harrison LM, Stephens JA. Task-dependent changes in cutaneous reflexes recorded from various muscles controlling finger movement in man. J Physiol. 1989;418:1–12. doi: 10.1113/jphysiol.1989.sp017825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MA. AAEM Minimonograph #13: H reflexes and F waves: physiology and clinical indications. Muscle Nerve. 1992;15(11):1223–33. doi: 10.1002/mus.880151102. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Agarwal GC. Response to sudden torques about ankle in man: myotatic reflex. J Neurophysiol. 1979;42(1 Pt 1):91–106. doi: 10.1152/jn.1979.42.1.91. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Merletti R, et al. European recommendations for surface electromyography. Roessingh Research and Development; Enschede, Netherlands: 1999. [Google Scholar]
- Hunter SK, Ryan DL, Ortega JD, Enoka RM. Task differences with the same load torque alter the endurance time of submaximal fatiguing contractions in humans. J Neurophysiol. 2002;88(6):3087–96. doi: 10.1152/jn.00232.2002. [DOI] [PubMed] [Google Scholar]
- Kanosue K, Akazawa K, Fujii K. Modulation of reflex activity of motor units in response to stretch of a human finger muscle. Jpn J Physiol. 1983;33(6):995–1009. doi: 10.2170/jjphysiol.33.995. [DOI] [PubMed] [Google Scholar]
- Maluf KS, Enoka RM. Task failure during fatiguing contractions performed by humans. J Appl Physiol. 2005;99(2):389–96. doi: 10.1152/japplphysiol.00207.2005. [DOI] [PubMed] [Google Scholar]
- Maluf KS, Shinohara M, Stephenson JL, Enoka RM. Muscle activation and time to task failure differ with load type and contraction intensity for a human hand muscle. Exp Brain Res. 2005;167:165–77. doi: 10.1007/s00221-005-0017-y. [DOI] [PubMed] [Google Scholar]
- Meunier S. Modulation by corticospinal volleys of presynaptic inhibition to Ia afferents in man. J Physiol Paris. 1999;93(4):387–94. doi: 10.1016/s0928-4257(00)80066-2. [DOI] [PubMed] [Google Scholar]
- Morita H, Petersen N, Christensen LO, Sinkjaer T, Nielsen J. Sensitivity of H-reflexes and stretch reflexes to presynaptic inhibition in humans. J Neurophysiol. 1998;80(2):610–20. doi: 10.1152/jn.1998.80.2.610. [DOI] [PubMed] [Google Scholar]
- Mottram CJ, Jakobi JM, Semmler JG, Enoka RM. Motor-unit activity differs with load type during a fatiguing contraction. J Neurophysiol. 2005;93(3):1381–92. doi: 10.1152/jn.00837.2004. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. The regulation of presynaptic inhibition during co-contraction of antagonistic muscles in man. J Physiol. 1993;464:575–93. doi: 10.1113/jphysiol.1993.sp019652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osu R, Franklin DW, Kato H, Gomi H, Domen K, Yoshioka T, Kawato M. Short- and long-term changes in joint co-contraction associated with motor learning as revealed from surface EMG. J Neurophysiol. 2002;88(2):991–1004. doi: 10.1152/jn.2002.88.2.991. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The circuitry of the human spinal cord: Its role in motor control and movement disorders. Cambridge University Press; Cambridge: 2005. [Google Scholar]
- Rudomin P. Selectivity of the central control of sensory information in the mammalian spinal cord. Adv Exp Med Biol. 2002;508:157–70. doi: 10.1007/978-1-4615-0713-0_19. [DOI] [PubMed] [Google Scholar]
- Rudroff T, Barry BK, Stone AL, Barry CJ, Enoka RM. Accessory Muscle Activity Contributes to the Variation in Time to Task Failure for Different Arm Postures and Loads. J Appl Physiol. 2007;102(3):1000–6. doi: 10.1152/japplphysiol.00564.2006. [DOI] [PubMed] [Google Scholar]
- Rudroff T, Poston B, Shin IS, Bojsen-Moller J, Enoka RM. Net excitation of the motor unit pool varies with load type during fatiguing contractions. Muscle Nerve. 2005;31(1):78–87. doi: 10.1002/mus.20241. [DOI] [PubMed] [Google Scholar]
- Schieppati M. The Hoffmann reflex: A means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol. 1987;28:345–76. doi: 10.1016/0301-0082(87)90007-4. [DOI] [PubMed] [Google Scholar]
- Sjogaard G, Jorgensen LV, Ekner D, Sogaard K. Muscle involvement during intermittent contraction patterns with different target force feedback modes. Clin Biomech. 2000;15(Suppl 1):S25–9. doi: 10.1016/s0268-0033(00)00056-5. [DOI] [PubMed] [Google Scholar]
- Tarkka IM. Short and long latency reflexes in human muscles following electrical and mechanical stimulation. Acta Physiol Scand Suppl. 1986;557:1–32. [PubMed] [Google Scholar]
- Tax AA, Denier van der Gon JJ, Erkelens CJ. Differences in coordination of elbow flexor muscles in force tasks and in movement tasks. Exp Brain Res. 1990;81(3):567–72. doi: 10.1007/BF02423505. [DOI] [PubMed] [Google Scholar]
- Tax AA, Denier van der Gon JJ, Gielen CC, van den Tempel CM. Differences in the activation of m. biceps brachii in the control of slow isotonic movements and isometric contractions. Exp Brain Res. 1989;76(1):55–63. doi: 10.1007/BF00253623. [DOI] [PubMed] [Google Scholar]
- Turner LC, Harrison LM, Stephens JA. Finger movement is associated with attenuated cutaneous reflexes recorded from human first dorsal interosseous muscle. J Physiol. 2002;542(Pt 2):559–66. doi: 10.1113/jphysiol.2002.023846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr PE. Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol. 2002;86(6):455–68. doi: 10.1007/s00421-002-0577-5. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Kernell D, Kukulka CG. Spatial differences in fatigue-associated electromyographic behavior of the human first dorsal interosseus muscle. J Physiol. 1995;483:499–509. doi: 10.1113/jphysiol.1995.sp020601. [DOI] [PMC free article] [PubMed] [Google Scholar]