Abstract
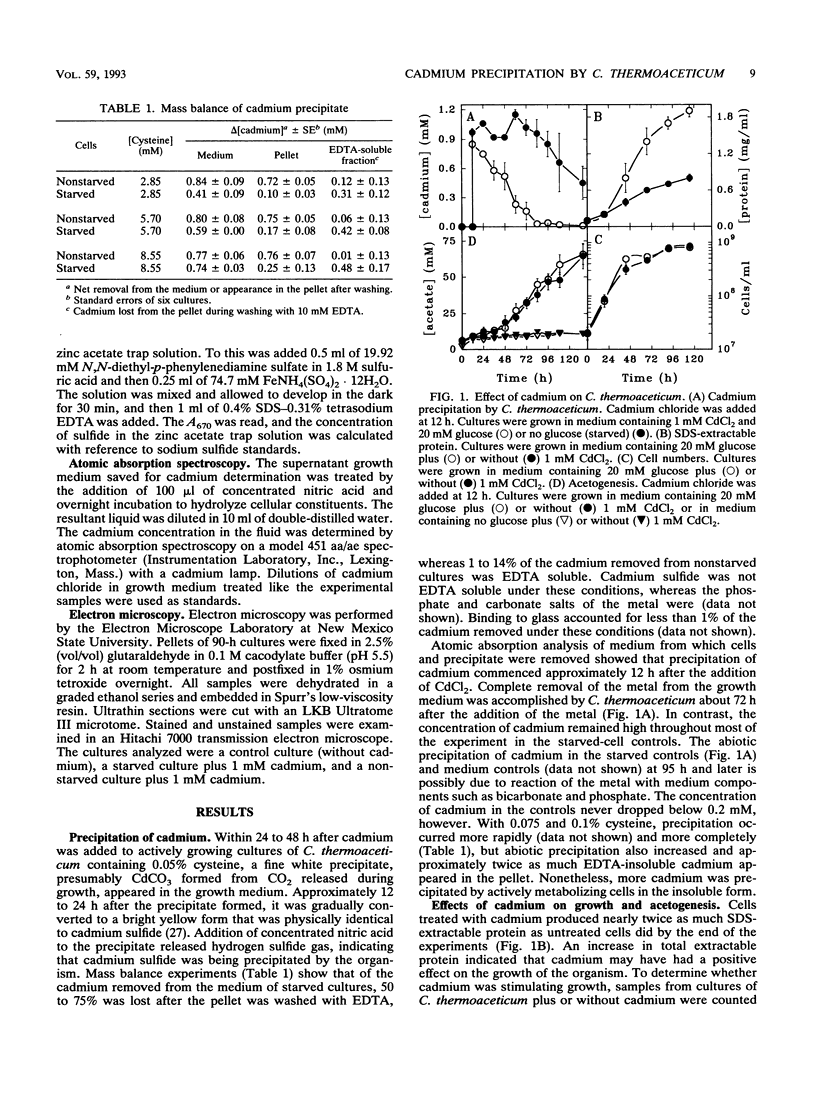
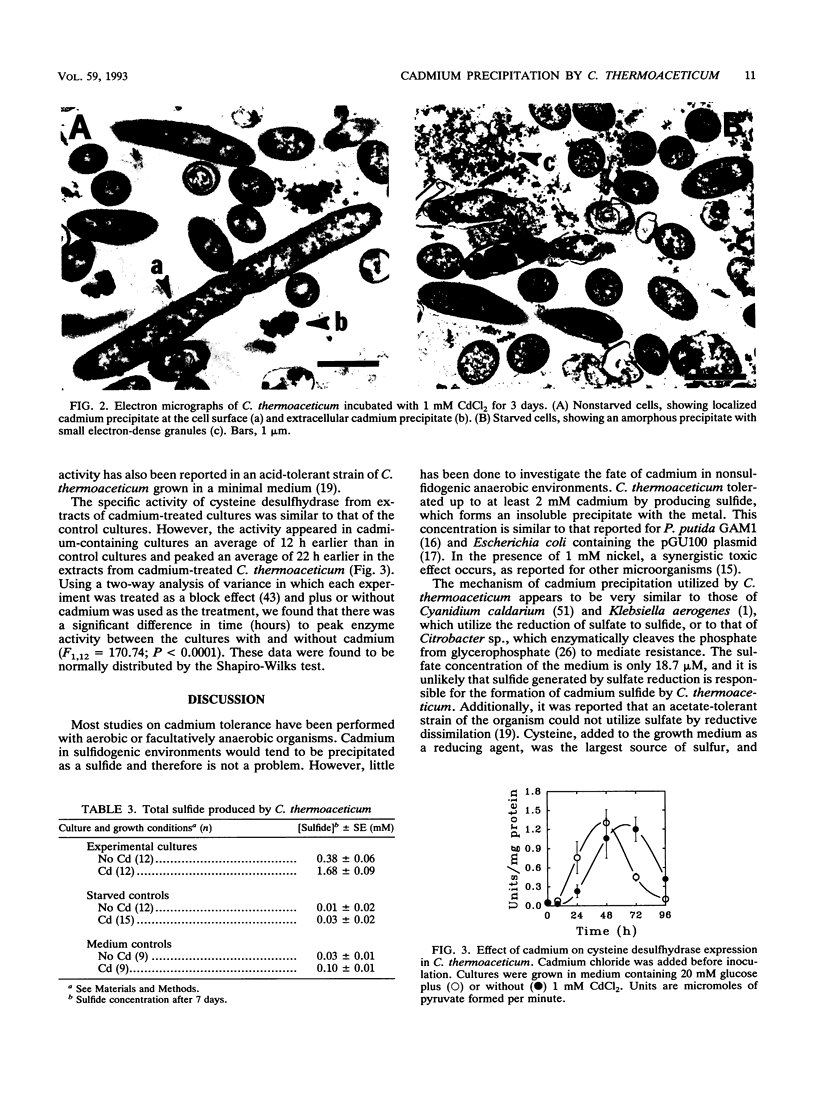
Cadmium at an initial concentration of 1 mM was completely precipitated by cultures of Clostridium thermoaceticum in complex medium. The precipitation was energy dependent and required cysteine, although cysteine alone did not act as a growth substrate. Electron microscopic analysis revealed localized areas of precipitation at the surfaces of nonstarved cells as well as precipitate in the surrounding medium. The addition of cadmium had no apparent effect on growth or acetogenesis. However, nickel and cadmium were synergistically toxic at a concentration (1 mM) at which neither alone was toxic. The amount of protein extracted from cadmium-treated cultures was twofold higher than that in control extracts, and the amount of total sulfide was fourfold higher in cultures containing cadmium than in control cultures. Comparable levels of cysteine desulfhydrase activity were observed in extracts of both cadmium-treated and control cultures, but the enzyme activity was expressed maximally about 24 h earlier in the cadmium-treated cultures than in the untreated controls.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiking H., Kok K., van Heerikhuizen H., van 't Riet J. Adaptation to Cadmium by Klebsiella aerogenes Growing in Continuous Culture Proceeds Mainly via Formation of Cadmium Sulfide. Appl Environ Microbiol. 1982 Oct;44(4):938–944. doi: 10.1128/aem.44.4.938-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiking H., Stijnman A., van Garderen C., van Heerikhuizen H., van 't Riet J. Inorganic phosphate accumulation and cadmium detoxification in Klebsiella aerogenes NCTC 418 growing in continuous culture. Appl Environ Microbiol. 1984 Feb;47(2):374–377. doi: 10.1128/aem.47.2.374-377.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaduri S., Demchick P. H. Simple and rapid method for disruption of bacteria for protein studies. Appl Environ Microbiol. 1983 Oct;46(4):941–943. doi: 10.1128/aem.46.4.941-943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DELAVIER-KLUTCHKO C., FLAVIN M. ENZYMATIC SYNTHESIS AND CLEAVAGE OF CYSTATHIONINE IN FUNGI AND BACTERIA. J Biol Chem. 1965 Jun;240:2537–2549. [PubMed] [Google Scholar]
- Fontaine F. E., Peterson W. H., McCoy E., Johnson M. J., Ritter G. J. A New Type of Glucose Fermentation by Clostridium thermoaceticum. J Bacteriol. 1942 Jun;43(6):701–715. doi: 10.1128/jb.43.6.701-715.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneros G., Ortega M. V. Cysteine desulfhydrase activities of Salmonella typhimurium and Escherichia coli. Biochim Biophys Acta. 1970 Jan 14;198(1):132–142. doi: 10.1016/0005-2744(70)90041-0. [DOI] [PubMed] [Google Scholar]
- Harwood-Sears V., Gordon A. S. Copper-induced production of copper-binding supernatant proteins by the marine bacterium Vibrio alginolyticus. Appl Environ Microbiol. 1990 May;56(5):1327–1332. doi: 10.1128/aem.56.5.1327-1332.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham D. P., Sadler P. J., Scawen M. D. Cadmium-Resistant Pseudomonas putida Synthesizes Novel Cadmium Proteins. Science. 1984 Sep 7;225(4666):1043–1046. doi: 10.1126/science.225.4666.1043. [DOI] [PubMed] [Google Scholar]
- Hsu T. D., Lux M. F., Drake H. L. Expression of an aromatic-dependent decarboxylase which provides growth-essential CO2 equivalents for the acetogenic (Wood) pathway of Clostridium thermoaceticum. J Bacteriol. 1990 Oct;172(10):5901–5907. doi: 10.1128/jb.172.10.5901-5907.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M., Foote L. J., Keenan B. S. The stoichiometry and kinetics of the inducible cysteine desulfhydrase from Salmonella typhimurium. J Biol Chem. 1973 Sep 10;248(17):6187–6196. [PubMed] [Google Scholar]
- Laddaga R. A., Bessen R., Silver S. Cadmium-resistant mutant of Bacillus subtilis 168 with reduced cadmium transport. J Bacteriol. 1985 Jun;162(3):1106–1110. doi: 10.1128/jb.162.3.1106-1110.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundie L. L., Jr, Drake H. L. Development of a minimally defined medium for the acetogen Clostridium thermoaceticum. J Bacteriol. 1984 Aug;159(2):700–703. doi: 10.1128/jb.159.2.700-703.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundie L. L., Jr, Yang H. C., Heinonen J. K., Dean S. I., Drake H. L. Energy-dependent, high-affinity transport of nickel by the acetogen Clostridium thermoaceticum. J Bacteriol. 1988 Dec;170(12):5705–5708. doi: 10.1128/jb.170.12.5705-5708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaskie L. E., Dean A. C. Cadmium accumulation by a Citrobacter sp. J Gen Microbiol. 1984 Jan;130(1):53–62. doi: 10.1099/00221287-130-1-53. [DOI] [PubMed] [Google Scholar]
- Mullen M. D., Wolf D. C., Ferris F. G., Beveridge T. J., Flemming C. A., Bailey G. W. Bacterial sorption of heavy metals. Appl Environ Microbiol. 1989 Dec;55(12):3143–3149. doi: 10.1128/aem.55.12.3143-3149.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T., Kanzaki H., Yamada H. Cystathionine gamma-lyase of Streptomyces phaeochromogenes. The occurrence of cystathionine gamma-lyase in filamentous bacteria and its purification and characterization. J Biol Chem. 1984 Aug 25;259(16):10393–10403. [PubMed] [Google Scholar]
- Nies D. H. Resistance to cadmium, cobalt, zinc, and nickel in microbes. Plasmid. 1992 Jan;27(1):17–28. doi: 10.1016/0147-619x(92)90003-s. [DOI] [PubMed] [Google Scholar]
- Nies D. H., Silver S. Metal ion uptake by a plasmid-free metal-sensitive Alcaligenes eutrophus strain. J Bacteriol. 1989 Jul;171(7):4073–4075. doi: 10.1128/jb.171.7.4073-4075.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafson R. W., Abel K., Sim R. G. Prokaryotic metallothionein: preliminary characterization of a blue-green alga heavy metal-binding protein. Biochem Biophys Res Commun. 1979 Jul 12;89(1):36–43. doi: 10.1016/0006-291x(79)90939-2. [DOI] [PubMed] [Google Scholar]
- Perry R. D., Silver S. Cadmium and manganese transport in Staphylococcus aureus membrane vesicles. J Bacteriol. 1982 May;150(2):973–976. doi: 10.1128/jb.150.2.973-976.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezacka E., Wood H. G. The autotrophic pathway of acetogenic bacteria. Role of CO dehydrogenase disulfide reductase. J Biol Chem. 1986 Feb 5;261(4):1609–1615. [PubMed] [Google Scholar]
- ROSE I. A., GRUNBERG-MANAGO M., KOREY S. R., OCHOA S. Enzymatic phosphorylation of acetate. J Biol Chem. 1954 Dec;211(2):737–756. [PubMed] [Google Scholar]
- Ragsdale S. W. Enzymology of the acetyl-CoA pathway of CO2 fixation. Crit Rev Biochem Mol Biol. 1991;26(3-4):261–300. doi: 10.3109/10409239109114070. [DOI] [PubMed] [Google Scholar]
- Remacle J., Vercheval C. A zinc-binding protein in a metal-resistant strain, Alcaligenes eutrophus CH34. Can J Microbiol. 1991 Nov;37(11):875–877. doi: 10.1139/m91-150. [DOI] [PubMed] [Google Scholar]
- Silver S. Plasmid-determined metal resistance mechanisms: range and overview. Plasmid. 1992 Jan;27(1):1–3. doi: 10.1016/0147-619x(92)90001-q. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stipanuk M. H., Beck P. W. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982 Aug 15;206(2):267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Gos Z., Zajac J. Reduced cadmium transport determined by a resistance plasmid in Staphylococcus aureus. J Bacteriol. 1981 Aug;147(2):305–312. doi: 10.1128/jb.147.2.305-312.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Malm A., Skwarek T. Effect of Cd2+ on growth of the cadmium-resistant and -sensitive Staphylococcus aureus. Acta Microbiol Pol. 1989;38(2):117–129. [PubMed] [Google Scholar]
- Tynecka Z., Malm A., Skwarek T., Szcześniak Z. Plasmid-linked protection of [14C]-glutamate transport and its oxidation against Cd2+ in Staphylococcus aureus. Acta Microbiol Pol. 1989;38(2):131–141. [PubMed] [Google Scholar]
- Tynecka Z., Szcześniak Z. Effect of Cd2+ on phosphate uptake by cadmium-resistant and cadmium-sensitive Staphylococcus aureus. Microbios. 1991;67(274):53–63. [PubMed] [Google Scholar]




