Abstract
The objective of the present work was to develop a simple and sensitive radioenzymatic assay to quantify lysophosphatidic acid (LPA). For that, a recombinant rat lysophosphatidic acid acyl transferase (LPAAT) produced in Escherichia coli was used. In the presence of [14C]oleoyl CoA, LPAAT selectively catalyzes the transformation of LPA and alkyl-LPA into [14C]phosphatidic acid. Acylation of LPA was complete and linear from 0 to 200 pmoles with a minimal detection of 0.2 pmoles. This method was used to quantify LPA in butanol- extracted lipids from bovine sera, as well as from human and mouse plasma. This radioenzymatic assay represents a new, simple, and highly sensitive method to quantify LPA in various biological fluids.
Keywords: Acyltransferases; chemistry; Animals; Chromatography, Thin Layer; Lysophospholipids; analysis; Methods; Rats; Recombinant Proteins; chemistry; Sensitivity and Specificity
Introduction
Lysophosphatidic acid (LPA: 1-acyl-2-hydroxy-sn-glycero-3-phosphate), is a key intermediate in glycerolipid synthesis, and a bioactive phospholipid (1, 2). It controls a wide variety of cellular responses (mitogenesis, cytoskeletal rearrangements, cell adhesion, ion transport, apoptosis) through the activation of specific G-protein coupled receptors (3, 4).
LPA has been detected in various biological fluids including blood (5–9), aqueous humors (10), or adipose tissue microdialysates (11). Abnormally high concentrations of LPA have been found in ascites and plasma from patients bearing ovarian cancer (12, 13). Several cell types have been shown to produce LPA in vitro: platelets (14, 15), adipocytes (11), ovarian cancer cells 16.. LPA has also been shown to be produced from oxidized LDL (17). Several possible metabolic pathways could be involved in cellular production of LPA: phospholipase A2-dependent deacylation of phosphatidic acid (18), lysophospholipase D-dependent hydrolysis of lysophosphatidylcholine (19, 20), acyl transferase-dependent acylation of monoacyl glycerol-phosphate (21), or acyl dihydroxyacetone-phosphate reductase-dependent reduction of acyl dihydroxyacetone-phosphate(22).
In order to understand the precise role of LPA in the development of particular pathologies such as cancer or obesity, and to clarify the metabolic origin of LPA produced by the cells, a precise and sensitive detection of LPA is necessary. In the present work, a recombinant lysophosphatidic acid acyltransferase (LPAAT) was used to develop a simple and highly sensitive radioenzymatic assay to quantify LPA in various biological fluids.
Material and Methods
Preparation of recombinant LPAAT
The rat 1-acyl-sn-glycerol-3-phosphate acyltransferase cDNA encoding for an enzyme behaving as a LPAAT was previously cloned in a bacterial expression vector pTrcHis (23). Fifty μl of DH5α-competent-cell suspension (Life Technologies) were transformed with 0.5 μg of pTrcHis-AGPAT plasmide (generous gift from Dr. Kazuhiko Kume) and grown in LB media containing 50 μg/ml ampicilin. When A600 reached approximately 0.6, bacteria were cultured for 3 hours in the presence of 1 mM IPTG (isopropyl-β-D-thiogalactopyranoside). Bacteria were collected by centrifugation at 3000 g for 10 min, and the pellet was disrupted by sonication in 0.2 M Tris-HCl (pH 7.4). After centrifugation at 10,000 x g for 20 min, the supernatant was recovered and further centrifuged at 100,000 x g (microsomes) for 90 min. The yield of a typical preparation is 6 to 7 mg microsomal protein per liter of culture. The resulting pellet was resuspended in the same buffer to a final protein concentration of 1 μg/μl and was used for radioenzymatic detection of LPA. Kinetic analysis allowed to determined that the activity of transformation of LPA into PA of a typical microsome preparation varied from 0.8 to 1 nmol/min/mg protein.
Preparation of acyl- and alkyl-LPA stock solutions
Na+ salt 1-oleoyl-sn-glycero-3-phosphate (LPA) (from SIGMA) was solubilized in PBS buffer containing 1% lipid-free albumin (from ICN). 1-O-hexadecyl-sn-glycero-3-phosphate (alkyl-LPA) was obtained from 1-O-hexadecyl-2-acetyl-sn-glycerol-3-phosphocholine (PAF) (from SIGMA). PAF was dispersed by sonication in a 50 mM Tris buffer (pH 7.4) containing 5 mM Ca++, and incubated first with pig pancreas PLA2 (from Sigma) for 2h followed by 30 min treatment with Streptomyces chromofuscus PLD (from SIGMA). Lipids were extracted using Bligh and Dyer procedure (24) in acid conditions. After evaporation alkyl-LPA was solubilized in ethanol. The real concentration of the stock solutions of LPA and alkyl-LPA was determined by phosphorus measurement (25).
Lipid extraction from serum and plasma
1-oleoyl-LPA or lipids contained in serum or plasma were extracted with 1 volume of 1-butanol. After vigorous shaking and centrifugation (5 min at 3000 g), the upper butanol phase was collected and evaporated under nitrogen at 50°C. This procedure allowed 85 to 90% extraction of LPA.
In vitro acylation of LPA
Dry lipid extract was resuspended in 200 μl of reaction medium (1 μl [14C]oleoyl CoA (RAS 55 mCi/mmole, NEN), 20 μl Tris (pH 7.5) 200 mM, 10 μl of recombinant LPAAT, 8 μl of sodium orthovanadate 500 μM, and 161 μl H2O containing 1mg/ml Tween 20), and incubated for 120 min at 20°C. The mixture was vortexed every 15 min. The reaction was stopped by addition of 400 μl of CHCl3/MeOH/HCl 12M (40/40/0.26) followed by a vigorous shaking and a 10 min centrifugation at 3000 g. The lower CHCl3 phase was evaporated under nitrogen, resuspended in 20 μl of CHCl3/MeOH (1/1), and spotted on a silica gel 60 TLC glass plate (Merck), then separated using CHCl3/MeOH/NH4OH/H2O (65/25/0.9/3) as a solvent. Alternatively, a two dimensional TLC using CHCl3/MeOH/NH4OH/H2O (65/25/0.9/3) as the solvent of the first migration, and CHCl3/MeOH/acetic acid/H2O (40/20/5/0.5) as the solvent of the second migration, was performed. The plate was autoradiographed overnight to localize [14C]-labeled lipids. Identification of radioactive spots was performed by co-migration with cold lipids visualized under iodine vapors. [14C]phosphatidic acid spots were then scraped and counted with 3 ml of scintillation cocktail. The following formula was used to convert radioactivity in pmole: dpm = 1/(2.22xRAS) pmoles, RAS being the specific radioactivity (of [14C]oleoyl CoA.
Origin of bovine sera
Fetal calf sera were from BioWhittaker Europe (Lot #6SB0011), Gibco Life Technologies (Lot #4001585K)) and Boehringer Mannheim France (Lot #210463). Donor calf sera were from BioWhittaker Europe (Lot #6M1955) and Gibco Life Technologies (Lot #3016082D).
Blood collection
Human plasma was collected on EDTA with the consent of the subjects and was approved by the Ethical Committee of the Hospital. Mouse plasma was collected from FVB mice following carotid section. Collection was performed in tubes containing 50 μl of a 2% solution of EDTA, disodium salt. The tubes were centrifuged for 5 min at 1500 g and LPA was measured in the platelet poor plasma. Animals were handled in accordance with the principles and guidelines established by the National Institutes of Medical Research (INSERM).
Results
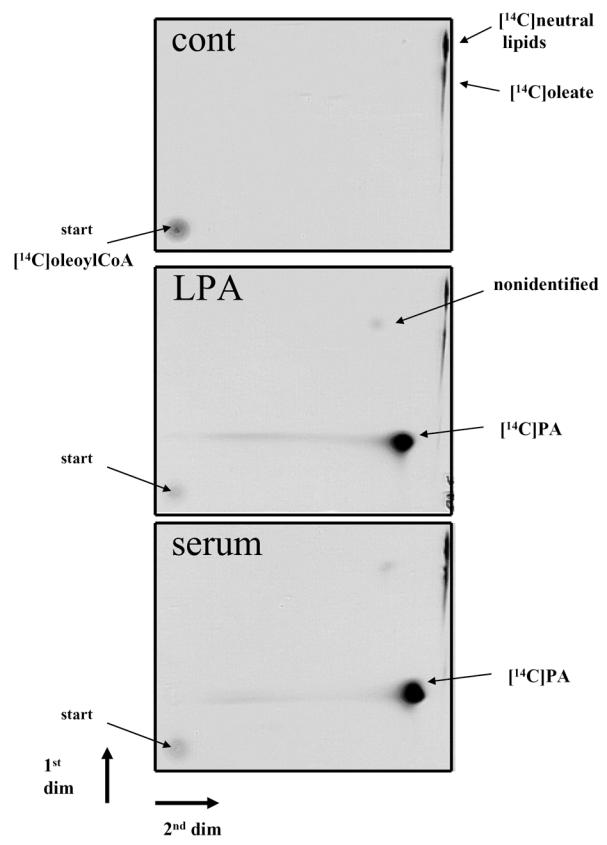
The ability of the LPAAT to acylate LPA into PA was tested after semi-purification of the enzyme from rat LPAAT cDNA-transformed E. coli. For that, 1-oleoyl-LPA or butanol-extracted lipids from serum (known to contain significant amount of LPA (6–9)) were incubated in the presence of LPAAT-transformed E. coli microsomes and [14C]oleoylCoA. In order to identify the products of the reaction, they were separated by two dimensional thin layer chromatography (Figure 1).
Figure 1. LPAAT-dependent acylation of LPA into [14C]PA.
Lipids were butanol-extracted from 2ml PBS buffer containing either 200 pmoles of 1-oleoyl-LPA (LPA), or 300 μl of fetal calf serum (serum), or nothing (cont). After evaporation, lipids were incubated in the presence of semi-purified LPAAT and [14C]oleoylCoA as described in Material and Methods. The products of the reaction were separated by two dimensional TLC and autoradiographed as described in Material and Methods.
In the absence of LPA (cont), no [14C]PA was detected. A radioactive spot was found at the depot (start). It corresponded to traces of [14C]oleoylCoA extracted with 1-butanol. Two additional spots (up right corner) were also observed. The lower spot comigrated with cold oleic acid (not shown) and was therefore identified as [14C]oleate. Since [14C]oleate was also observed in the absence of microsomes (not shown), and resulted from a partial degradation of [14C]oleoylCoA. The upper spot comigrated with diacylglycerol and triacylglycerol and was therefore identified as [14C]neutral lipids likely resulting from the presence of monoacyl- and/or diacyl-glycerol acyltransferases endogenously expressed in E. coli and co-purified with LPAAT in microsomes.
In the presence of LPA (standard or from serum) a major spot comigrating with cold PA was observed. [14C]PA was interpreted as the result of the transfert of [14C]oleate onto LPA. This confirming the presence of LPAAT in E. coli microsomes. A minor but nonidentified spot was also observed. Because it represented less than 2% of [14C]PA this minor spot was further neglected.
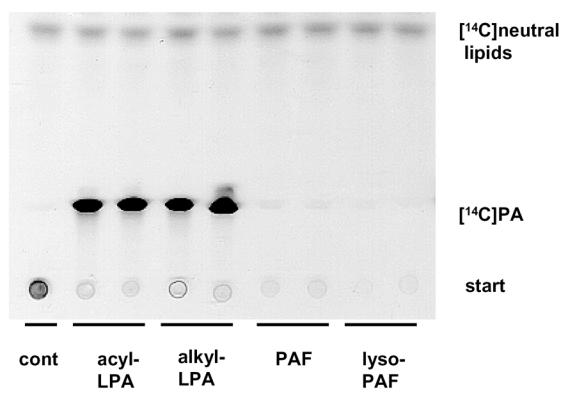
Whereas the vast majority (about 90 %) of LPA species found in biological fluids and tissues are acyl-LPA, a small proportion (about 10%) are alkyl-LPA (26). LPAAT-dependent acylation of alkyl-LPA was tested. Alkyl-LPA was synthesized from PAF (see Methods) and exposed to semi-purified LPAAT and [14C]oleoylCoA. As shown in figure 2, both acyl-LPA or alkyl-LPA led to the formation of [14C]PA. No [14C]PA was formed from PAF or lyso-PAF.
Figure 2. LPAAT-dependent acylation of alkyl-LPA in [14C]PA.
Alkyl-LPA was synthetized from PAF as described in Material and Methods. 200 pmoles of LPA, PAF, lyso-PAF and alkyl-LPA were incubated in the presence of semi-purified LPAAT and [14C]oleoylCoA as described in Material and Methods. The products of the reaction were separated by one dimensional TLC and autoradiographed as described in Material and Methods.
The sensitivity of radioenzymatic assay was then tested. For that, increased quantities of 1-oleoyl-LPA were incubated in the presence of semi-purified LPAAT and [14C]oleoylCoA. This led to the formation of increased amount of [14C]PA (Figure 3A). Conversion of the radioactivity into pmoles revealed that LPA acylation into PA was complete and linear (slope close to 1) from zero to 200 pmoles, with a detection set point of 0.2 pmole (Figure 3-B).
Figure 3. Sensitivity and linearity of LPAAT-dependent acylation of LPA into [14C]PA.
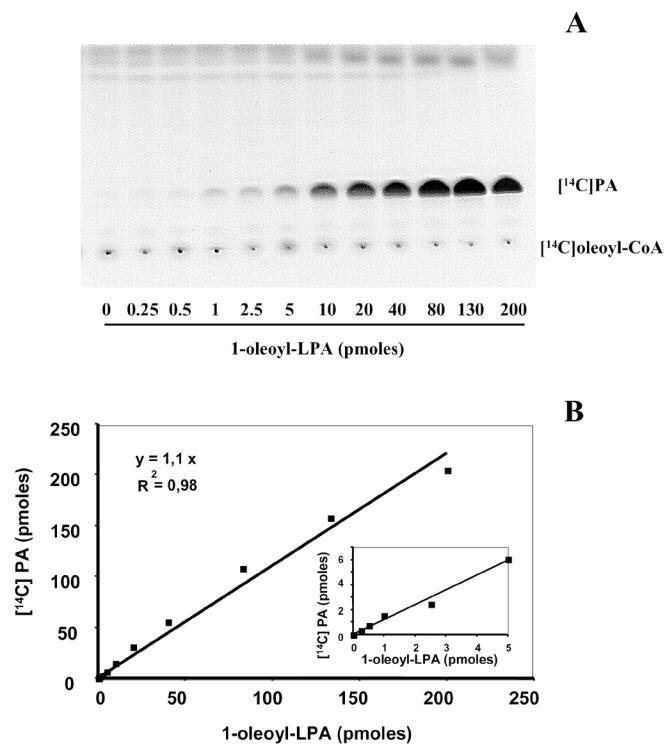
Butanol-extracted 1-oleoyl-LPA (0 to 200 pmoles) was evaporated and incubated in the presence of semi-purified LPAAT and [14C]oleoylCoA as described in Material and Methods. The products of the reaction were separated by one dimensional TLC and autoradiographed (A) as described in Material and Methods. Each spot of [14C]PA was scraped, counted, and the radioactivity was converted into pmoles (B). The insert in (B) represents the lowest amounts of LPA.
The radioenzymatic assay was finally used to quantify LPA in bovine sera from different origin. As shown in Table 1, the concentration of LPA measured in 20 μl of serum varied from 0.28 to 2.56 μM. Concentration of LPA in donor calf sera was significantly higher (2.4 to 4.7 fold) than the mean concentration (0.54 μM) of LPA found in fetal calf sera. Although present at much lower concentrations than in serum, significant concentrations of LPA (from 51 to 283 nM) were also found in human and mouse platelet -poor plasma (Table 2).
Table 1.
Quantification of LPA in different bovine sera.
| Calf Sera | Company | Concentration of LPA (μM) |
|---|---|---|
| Fetal | BioWhittaker | 0.65 ± 0.21 |
| GIBCO | 0.28 ± 0.04 | |
| Boehringer | 0.71 ± 0.07 | |
| Donor | BioWhittaker | 2.56 ± 0,43 |
| GIBCO | 1.31 ± 0.11 |
Lipids present in 25 μl of fetal or donor calf serum were extracted with butanol, evaporated, and incubated in the presence of semi-purified LPAAT and [14C]oleoylCoA as described in Material and Methods. The products of the reaction were separated by one dimensional TLC and autoradiographed as described in Material and Methods. Each spot of [14C]PA was scraped and counted. The radioactivity was converted into pmoles and the concentration of LPA was calculated. Values corresponded to mean ± SEM from 3 separate determinations.
Table 2.
Quantification of LPA in human and mice plasma
| Concentration of LPA (nM) | n | |
|---|---|---|
| Human | 83 ± 8 (51–136) | 14 |
| Mouse | 170 ± 50 (80–283) | 12 |
Lipids present in 200 μl of human or mouse platelet-poor plasma were extracted with butanol, evaporated, and incubated in the presence of semi-purified LPAAT and [14C]oleoylCoA as described in Material and Methods. The products of the reaction were separated by one-dimensional TLC and autoradiographed as described in Material and Methods. Each spot of [14C]PA was scraped, counted. The radioactivity was converted into pmoles and the concentration of LPA was calculated. Values correspond to mean ± SEM from n separate determinations. Extreme values are reported in parenthesis.
Discussion
Several procedures have previously been used to detect LPA. Gas chromatography (GC) allows quantification of methylated fatty acids derived from TLC-purified LPA (15). This is an indirect assay allowing determination of the relative fatty acid composition of LPA. However, this method is not very sensitive and requires relatively high amounts of purified LPA. In addition, classical GC does not allow to determine the presence alkyl-LPA. For that it is necessary to employ both mild alkaline hydrolysis and gas chromatography coupled to mass spectrometry (26).
The presence of LPA in a biological fluid can also be demonstrated by using bioassays. This type of assay consists in measuring the influence of a LPA-containing fluid on a cellular response known to be controlled by LPA, such as platelet aggregation (17), calcium mobilization in xenope oocytes (10), or cell spreading (11). Since bioactive phospholipids other than LPA are also able to generate those biological responses, bioassays cannot be considered specific enough for LPA quantification. LPA can also be detected following labeling cellular phospholipid with [32P]ortho-phosphate, and separation of [32P]LPA by TLC (7, 11, 18). Although very sensitive, this method is only applicable on cultured cells, and is not quantitative.
The LPAAT, also known as 1-acyl-sn-glycerol-3-phosphate acyltransferase (EC 2.3.1.51) catalyses LPA conversion into phosphatidic acid (PA), a key step in glycerolipid synthesis. LPAAT has been demonstrated to be highly specific for LPA and not influenced by LPA fatty acid composition (27). Therefore the high specificity of LPAAT made this enzyme an interesting partner to develop a new assay for LPA quantification.
By using a rat recombinant LPAAT produced in E. coli (23) we have developed a simple, rapid, and highly sensitive radioenzymatic assay to quantify LPA in various biological fluids. By using this assay it is possible to quantify LPA in crude butanol-lipid extracts without prior time consuming and poorly efficient TLC-purification of LPA. It is also important to notice that, this assay allows to quantify both acyl- and alkyl-LPA. Although present to a much lower concentration than acyl-LPA, alkyl-LPA has been reported to be much more active as acyl-LPA on platelet aggregation( 28). Although our assay does not discriminate between acyl-LPA and alkyl-LPA, the amount of alkyl-LPA present in a biological fluid can nevertheless be determined. For that the biological fluid can be treated with a lysophospholipase, such as the phospholipase B, known to specifically hydrolyze acyl-LPA without altering alkyl-LPA. Phospholipase B-resistant LPA will correspond to alkyl-LPA.
Our radioenzymatic assay is also highly sensitive. It is possible to detect less than 0.5 pmole LPA. This high sensitivity allows determination of LPA concentration from low volumes of biological samples. It was for example possible to quantify LPA in 200 μl of plasma or 25 μl of serum. Those volumes are entirely compatible with current blood sampling in both human and animals. This high sensitivity allowed us to easily quantify as low as 51 nM LPA in human and mouse plasma. It is important to notice that, by using gas chromatography, Xu et al. considered that the lower detectable concentration of LPA in human plasma was 100 nM (5).
Finally, the method is reliable since the concentration values determined in bovine sera are in agreement with those previously published (6–9). However, it is noticeable that donor calf sera contain a higher concentration of LPA than fetal calf sera. Although the precise reasons of this difference remain to be determined, this information needs to be taken into consideration to choose cell culture serum.
In conclusion, this radioenzymatic assay represents a new, and highly sensitive method for LPA quantification. Because of its simplicity and rapidity of execution, this method could easily be introduced in any research or hospital laboratory interested in fundamental or pathological issues concerning LPA.
Acknowledgments
We thank Dr. Kazuhiko Kume (University of Tokyo) for having generously provided us with pTrcHis-AGPAT vector. We also thank David Sibrac for his skillful technical assistance and Stéphane Gesta for fruitful discussion. This work was supported by grants from the “Institut National de la Santé et de la Recherche Médicale” (APEX #4X405D), the “Association pour la Recherche sur le Cancer” (#5381), the “Institut de Recherche Servier”, and the “Laboratoires Clarins”.
Abbreviations
- LPA
lysophosphatidic acid
- LPAAT
lysophosphatidic acid acyl-transferase
- PA
phosphatidic acid
- AGPAT
acyl glycerol phosphate acyl transferase
- LB
Lubroth
- GC
gas chromatography
- PAF
platelet activating factor
- PLD
phospholipase D
- PLA2
phospholipase A2
- LDL
low density lipoprotein
References
- 1.Gaits F, Fourcade OFLB, Gueguen G, Gaigé B, Gassama-Diagne A, Fauvel J, Salles J-P, Mauco G, Simon M-F, Chap H. Lysophosphatidic acid as a phospholipid mediator: pathways of synthesis. FEBS Lett. 1997;410:54–58. doi: 10.1016/s0014-5793(97)00411-0. [DOI] [PubMed] [Google Scholar]
- 2.Jalink K, Hordijk PL, Moolenaar WH. Growth factor-like effects of lysophosphatidic acid, a novel lipid mediator. Biochim Biophys Acta. 1994;1198:185–196. doi: 10.1016/0304-419x(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 3.Goetzl E, An S. Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. FASEB J. 1998;12:1589–1598. [PubMed] [Google Scholar]
- 4.Chun J, Contos JJ, Munroe D. A growing family of receptor genes for lysophosphatidic acid (LPA) and other lysophospholipids (LPs) Cell Biochemistry and Biophysics. 1999;30:213–242. doi: 10.1007/BF02738068. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, Shen Z, Wiper DW, Wu M, Morton R, Elson P, Kennedy A, Belinson J, Markman M, Casey G. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998;280:719–723. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
- 6.Tigyi G, Miledi R. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochomocytoma cells. J Biol Chem. 1992;267:21360–21367. [PubMed] [Google Scholar]
- 7.Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993;291:677–680. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tokumura A, Iimori M, Nishioka Y, Kitahara M, Sakashita M, Tanaka S. Lysophosphatidic acids induce proliferation of cultured vascular smooth muscle cells from rat aorta. Am J Physiol. 1994;267:C204–C210. doi: 10.1152/ajpcell.1994.267.1.C204. [DOI] [PubMed] [Google Scholar]
- 9.Sasagawa T, Suzuki K, Shiota T, Kondo T, Okita M. The significance of plasma lysophospholipids in patients with renal failure on hemodialysis. J Nutr Sci Vitaminol. 1998;44:809–18. doi: 10.3177/jnsv.44.809. [DOI] [PubMed] [Google Scholar]
- 10.Liliom K, Guan Z, Tseng J-L, Desiderio DM, Tigyi G, Watsky MA. Growth factor-like phospholipids generated after corneal injury. Am J Physiol. 1998;274:C1065–C1074. doi: 10.1152/ajpcell.1998.274.4.C1065. [DOI] [PubMed] [Google Scholar]
- 11.Valet P, Pagès C, Jeanneton O, Daviaud D, Barbe P, Record M, Saulnier-Blache J, Lafontan M. Alpha2-adrenergic receptor-mediated release of lysophosphatidic acid by adipocytes. J Clin Invest. 1998;101:1431–1438. doi: 10.1172/JCI806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y, Gaudette D, Boynton J, Frankel A, Fang X, Sharma A, Hurteau J, Casey G, Goodbody A, Mellors A, Holub B, Mills G. Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clin Cancer Res. 1995;1:1223–1232. [PubMed] [Google Scholar]
- 13.Westermann AM, Havik E, Postma FR, Beijnen JH, Dalesio O, Moolenar MW, Rodenhuis S. Malignant effusions contain lysophosphatidic acid (LPA)-like activity. Ann Oncol. 1998;9:437–442. doi: 10.1023/a:1008217129273. [DOI] [PubMed] [Google Scholar]
- 14.Mauco G, Chap H, Simon MF, Douste-Blazy L. Phosphatidic and lysophosphatidic acid production in phospholipase C-and thrombin-treated platelets. Possible involvement of a platelet lipase. Biochimie. 1978;60:653–661. doi: 10.1016/s0300-9084(78)80784-6. [DOI] [PubMed] [Google Scholar]
- 15.Gerrard JM, Robinson P. Identification of the molecular species of lysophosphatidic acid produced when platelets are stimulated by thrombin. Biochim Biophys Acta. 1989;1001:282–285. doi: 10.1016/0005-2760(89)90112-4. [DOI] [PubMed] [Google Scholar]
- 16.Shen Z, Belinson J, Morton R, Yan X. Phorbol 12-myristate 13-acetate stimulates lysophosphatidic acid secretion from ovarian and cervical cancer cells but not from breast or leukemia cells. Gynecol Oncol. 1998;71:364–368. doi: 10.1006/gyno.1998.5193. [DOI] [PubMed] [Google Scholar]
- 17.Siess W, Zangl K, Essler M, Bauer M, Brandl R, Corrinth C, Bittman R, Tigyi G, Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci U S A. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fourcade O, Simon MF, Viodé C, Rugani N, Leballe F, Ragab A, Fournié B, Sarda L, Chap H. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. 1995;80:919–927. doi: 10.1016/0092-8674(95)90295-3. [DOI] [PubMed] [Google Scholar]
- 19.Tokumura A, Harada K, Fukuzawa K, Tsukatani H. Involvement of lysophospholipase D in the production of lysophosphatidic acid in rat plasma. Biochim Biophys Acta. 1986;875:31–38. [PubMed] [Google Scholar]
- 20.Tokomura A, Miyake M, Nishioka Y, Yamano S, Aono T, Fukuzawa K. Production of lysophosphatidic acids by lysophospholipase D in human follicular fluids of in vitro fertilization patients. Biol Reprod. 1999;61:195–199. doi: 10.1095/biolreprod61.1.195. [DOI] [PubMed] [Google Scholar]
- 21.Haldar D, Vancura A. Glycerophosphate acyltransferase from liver. Methods Enzymol. 1992;209:64–72. doi: 10.1016/0076-6879(92)09008-q. [DOI] [PubMed] [Google Scholar]
- 22.Dunlop ME, Larkins RG. Pancreatic islets synthesize phospholipids de novo from glucose via acyl-dihydroxyacetone phosphate. Biochem Biophys Res Commun. 1985;132:467–473. doi: 10.1016/0006-291x(85)91157-x. [DOI] [PubMed] [Google Scholar]
- 23.Kume K, Shimizu T. cDNA cloning and expression of murine 1-acyl-sn-glycerol-3-phosphate acyltransferase. Biochem Biophys Res Com. 1997;237:663–666. doi: 10.1006/bbrc.1997.7214. [DOI] [PubMed] [Google Scholar]
- 24.Bligh E, Dyer W. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 25.Böttcher C, Gent CV, Priest C. A rapid and sensitive sub microphosphorus determination. Anal Chim Acta. 1961;24:203–204. [Google Scholar]
- 26.Sugiura T, Nakane S, Kishimoto S, Waku K, Yoshioka Y, Tokomura A, Hanahan D. Occurence of lysophosphatidic acid and its alkyl ether-linked analog in rat brain and comparison of their biological activities toward culture neural cells. Biochim Biophys Acta. 1999;1440:194–204. doi: 10.1016/s1388-1981(99)00127-4. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita S, Hosaka K, Miki Y, Numa S. Glycerolipid acyltransferases from rat liver: 1-acylglycerophosphate acyltransferase, 1-acylglycerophosphorylcholine acyltransferase and diacylglycerol acyltranserase. Methods Enzymol. 1981;71:528–536. doi: 10.1016/0076-6879(81)71063-2. [DOI] [PubMed] [Google Scholar]
- 28.Simon MF, Chap H, Douste-Blazy L. Human platelet aggregation induced by 1-alkyl-lysophosphatidic acid and its analogs: a new group of phospholipid mediators? Biochem Biophys Res Commun. 1982;108:1743–50. doi: 10.1016/s0006-291x(82)80113-7. [DOI] [PubMed] [Google Scholar]


