Abstract
Withdrawal from high levels of progesterone in rodents has been proposed as a model for premenstrual syndrome or postpartum depression. Forced swim test (FST) immobility, used to model depression, was assessed in intact female DBA/2J mice following progesterone withdrawal (PWD) or treatment with the 5α-reductase inhibitor finasteride. Following 5 daily progesterone injections (5 mg/kg IP) FST immobility increased only in mice withdrawn for 3 days (p < .05). In another experiment, 3 days of PWD significantly decreased levels of progesterone compared to 0 days of withdrawal, but progesterone levels at 3 days of PWD did not differ from vehicle-treated controls. In a final study, mice received daily injections of progesterone (5 mg/kg IP) for 8 days, with 0 mg/kg, 50 mg/kg, or 100 mg/kg finasteride co-administered for the last three days. Mice that received 100 mg/kg finasteride, but not 50 mg/kg finasteride, displayed increased FST immobility. PWD and finasteride treatment, both of which reduce allopregnanolone levels, were associated with increased FST immobility in female DBA/2J mice. These findings suggest that decreased levels of the GABAergic neurosteroid allopregnanolone contributes to symptoms of PWD. Future studies of PWD may provide information about human conditions that are associated with hormone changes such as premenstrual syndrome or postpartum depression.
Keywords: Depression, premenstrual syndrome, postpartum depression, progesterone withdrawal, behavioral despair, GABA-A receptor, allopregnanolone, finasteride
Beginning in adolescence, females experience affective disorders at higher rates than males, partially due to sex-specific disorders such as premenstrual syndrome (PMS) and postpartum depression (McGrath et al., 1990, Steiner et al., 2003). Progesterone secretion is dramatically reduced prior to menstruation or following childbirth. These periods of progesterone clearance have been called “progesterone withdrawal” (PWD), and are associated with dynamic (but species-dependent) biological events in females (MacDonald et al., 1991). Given the temporal association between PWD and PMS or postpartum depression, studies that elucidate the effects of PWD may lead to increased understanding of these disorders.
Emerging evidence about the cellular actions of progesterone and its metabolites in the brain has stimulated research regarding neuroendocrine correlates of behaviors. In addition to the actions of progesterone on intracellular and membrane-bound receptors, progesterone can be metabolized to a number of neuroactive steroids. Reports have demonstrated roles for progesterone or its metabolites in a variety of scenarios including pain perception (Choi et al., 2006; Frye et al., 2004), anxiety (Löfgren et al., 2006; Smith et al., 2004, 2006), learning and memory (Djebaili et al., 2004), seizure susceptibility (Belelli et al., 1989; Finn & Gee, 1994; Frye et al., 2002; Hsu & Smith, 2003; Kokate et al., 1994, 1996), depression (Rasmusson et al., 2006), and alcohol dependence (Cagetti et al., 2004; Devaud et al., 1996; Finn et al., 2004a, 2004b, 2006b).
Among these metabolites, allopregnanolone (ALLO, 3α-hydroxy-5α-pregnan-20-one) has been of particular interest. ALLO is an A-ring reduced metabolite of progesterone that is a positive allosteric modulator of the type-A γ-aminobutyric acid receptor (GABAAR), an inhibitory neurotransmitter receptor found on most neurons in the central nervous system (Majewska, 1992). Low nanomolar concentrations of ALLO can increase Cl− influx at GABAARs (e.g., Belelli et al., 1990; Majewska, 1992; Morrow et al., 1987), thereby enhancing GABAergic neurotransmission. Since plasma concentrations of progesterone are correlated with neural ALLO levels (Corpéchot et al., 1993), changes in progesterone secretion across the menstrual cycle should alter ALLO concentrations in the brain, with a concomitant change in GABAAR modulation.
There is conflicting evidence on the role of ALLO in psychopathology. In one of the early investigations in this line of research, Schmidt and coworkers (1994) found no difference in plasma ALLO levels at a single time point during the luteal phase in women with and without PMS. A subsequent study found that women with PMS had lower plasma ALLO concentrations compared to control participants at 12 days, but not 5 days after luteinizing hormone (LH) surge (Rapkin et al., 1997). Importantly, Rapkin and coworkers (1997) used time points that surrounded a period of progesterone withdrawal, as confirmed by a main-effect of time (5 day vs. 12 day post-LH surge) on plasma progesterone levels. Thus, while the evidence is mixed, reports such as those of Rapkin and colleagues (1997) have supported the idea that women with PMS have lower ALLO during the luteal phase of the menstrual cycle than women without PMS (Bernardi et al., 2004), consistent with the idea that it is the decline in ALLO that precipitates the symptoms of PMS.
There is also evidence that women have decreased ALLO following childbirth (Gilbert Evans et al., 2005), the period when they are susceptible to postpartum depression. Interestingly, clinical studies in small cohorts of patients with unipolar depression have documented an inverse relationship between endogenous ALLO levels and depression severity (discussed in Uzunova et al., 2006). Furthermore, in vitro methods have demonstrated that several antidepressant medications favor the production or synaptic accumulation of ALLO (Griffin & Mellon, 1999; Pinna et al., 2006; Schüle et al., 2006). The hypothesis that GABAARs are involved in depression has been strengthened by the finding that the selective serotonin reuptake inhibitor fluoxetine increased ALLO levels in depressed patients during the same time frame as the onset of its antidepressant efficacy (Romeo et al., 1998; but see Uzunova et al., 2006). Notably, ALLO levels were not increased in patients that did not respond to the antidepressant therapy (i.e., no improvement in symptoms of depression).
Progesterone, ALLO, and other steroid manipulations have been used to model symptoms related to steroid withdrawal in behavioral studies with laboratory animals. Animal models of anxiety-related behaviors have consistently shown an increase in anxiety during steroid withdrawal (e.g., Bitran & Smith, 2005; Smith et al., 1998, 2004, 2006; see also discussion), while animal models of depression-like behaviors have received less attention. In procedures designed to model menopause-associated changes or postpartum depression, hormone withdrawal has been shown to increase depression-like behavior, measured in the forced swim test (FST). An increase in FST immobility is interpreted as an increase in depression-like behavior whereas a decrease in FST immobility is interpreted as an antidepressant effect (for review, see Cryan et al., 2002). Bekku and coworkers (2007) found that ovariectomy in mice resulted in increased FST immobility from approximately 10–18 days post surgery, compared to sham-operated females (see also the results of Galea et al., 2001, and Stoffel & Craft, 2004). However, while these studies clearly demonstrate that steroid withdrawal resulted in altered FST behavior, estrogens and progestins were both manipulated in these procedures making it difficult to attribute the behavioral changes to one hormone family.
Taken together, two recent reports offer support for the hypothesis that ALLO withdrawal, as a result of PWD, results in a depression-like state in rats. In the first report, anxiety-like and depression-like behaviors in rats were increased by rapid PWD, compared to slower PWD. Saavedra and coworkers (2006) injected ovariectomized rats with progesterone such that one group received equal daily amounts of progesterone for five days, while the other group received a higher dose of progesterone on the first day, which was gradually tapered over the following four days. These two groups received the same cumulative dose of progesterone, but when tested on the sixth day the group that had received tapered progesterone injections spent less time immobile in the FST versus the group that had progesterone abruptly discontinued. In the second study, rats injected with ALLO showed decreased immobility in the FST, but coadministration of the GABAAR antagonist picrotoxin blocked ALLO’s effect on FST immobility (Rodríguez-Landa et al., 2007). Collectively, PWD increased FST immobility (depression-like behavior), ALLO decreased FST immobility (an antidepressant-like effect), and application of a GABAAR antagonist blocked the antidepressant effect of ALLO’s. These studies support the current hypothesis that ALLO withdrawal may underlie depression-like behavior following PWD.
The current studies build on the work described above by examining the behavioral effects of PWD without simultaneously manipulating estrogens. This was accomplished by adapting procedures previously used in electrophysiological and neurochemical studies. Costa and coworkers (1995) found that treating intact female rats with daily 5 mg progesterone injections (5 days/wk for 1–3 wks) significantly increased brain ALLO levels at 0 days of PWD, but ALLO levels were diminished after one, three, or five days of withdrawal. Dazzi and coworkers (2002) found that treating male rats with 5 mg/kg progesterone for 5 days resulted in increased brain levels of ALLO that remained elevated 25–30 hr after the fifth injection. Taking both of these studies into consideration, we predicted that the 5 mg/kg treatment regimen in intact female mice would result in a withdrawal syndrome after 3 days, if not after 1 day.
The present experiments were conducted to test the hypothesis that PWD and ALLO withdrawal result in depression-like behavior in intact female mice, measured by FST immobility. In our first experiment we sought to identify the time point(s) when FST immobility increased following discontinuation of progesterone administration. We next confirmed that the progesterone injection regimen decreased plasma progesterone levels during PWD. Finally, we tested whether blocking the metabolism of progesterone to neuroactive steroids (such as ALLO) was sufficient to induce FST immobility. For this experiment we used the 5α-reductase inhibitor finasteride, which blocks the conversion of progesterone to 5α-dihydroprogesterone, the metabolic precursor of ALLO, and causes a downstream reduction in ALLO levels (e.g., Frye & Walf, 2002; Reddy et al., 2001; Rhodes & Frye, 2001; VanDoren et al., 2000).
Methods
Animals
Female DBA/2J mice were obtained from The Jackson Laboratory (Davis, CA) and housed in the Veterinary Medical Unit at the Veterans Affairs Medical Center (VAMC) in Portland, OR. Mice were housed four to a cage except two cages (3 mice/cage) in Experiment 1a. All mice were housed in Maxi-Miser #1 cages (Thoren Caging Systems, Hazelton, PA) with 0.25-in Bed-o’cobs bedding (Andersons Inc., Maumee OH). All mice were housed in a temperature controlled room (21 ± 1 °C). Throughout each experiment, mice were maintained on a 12 hr/12 hr light cycle (lights on at 0600 hours) with ad libitum access to mouse chow (LabDiet 5001 Rodent Diet, PMI International) and tap water. All mice were allowed to acclimate to their caging location and conditions for at least a week prior to commencement of injections, and received ear punches for identification prior to receiving their first injection.
General Experimental Design
The following experiments were designed to test associations between PWD and depression-like behavior. Based on the procedures used by Costa and coworkers (1995) and Dazzi and coworkers (2002), our general experimental design was to give all experimental groups of mice a five- or six-day treatment of progesterone, followed by a three-day treatment specific to the experimental group. Table 1 presents the schedule of injections and behavioral testing in the FST (Experiments 1a and 2). Table 2 shows the schedule of injections and blood collection for measurement of progesterone levels (Experiment 1b). All procedures described herein were approved by the Portland VAMC Institutional Animal Care and Use Committee and were conducted in accordance with the guidelines discussed in the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council, 2003).
Table 1.
Schedule of Injections and Testing in the FST
| Days
|
|||||
|---|---|---|---|---|---|
| Group | 1–4 | 5 | 6 | 7 | 8 |
| Experiment 1a | |||||
| VEH | VEH | VEH, FST | |||
| 0 d PWD | PRO | PRO, FST | |||
| 1 d PWD | PRO | PRO | FST | ||
| 2 d PWD | PRO | PRO | FST | ||
| 3 d PWD | PRO | PRO | FST | ||
| Experiment 2 | |||||
| 0 mg | PRO | PRO | PRO, VEH | PRO, VEH | PRO, VEH, FST |
| 50 mg | PRO | PRO | PRO, FIN | PRO, FIN | PRO, FIN, FST |
| 100 mg | PRO | PRO | PRO, FIN | PRO, FIN | PRO, FIN, FST |
Note. Drug-injection abbreviations used: PRO, progesterone; VEH, vehicle; FIN, finasteride. “FST” indicates the day of FST testing.
Table 2.
Schedule of Injections and Blood Collection for Progesterone Measurement
| Days
|
|||||||
|---|---|---|---|---|---|---|---|
| Group | 1 | 2–4 | 5 | 6 | 7 | 8 | 9 |
| Experiment 1b | |||||||
| 0.5 hr | PRO (0.5 hr) | ||||||
| 2 hr | PRO (2 hr) | ||||||
| 8 hr | PRO (8 hr) | ||||||
| 0 d PWD | PRO | PRO | PRO (2 hr) | ||||
| VEH | VEH | VEH | VEH | VEH (2 hr) | |||
| 1 d PWD | PRO | PRO | PRO | PRO | VEH (2 hr) | ||
| 3 d PWD | PRO | PRO | PRO | PRO | VEH | VEH | VEH (2 hr) |
Note. Drug-injection abbreviations used: PRO, progesterone; VEH, vehicle. Time intervals in parentheses indicate the interval between the final injection and blood collection.
Mice were not ovariectomized for these experiments, because we wanted the control group to experience normal fluctuations in steroid hormones (which would not be the case with ovariectomy, even with hormone replacement). Additionally, withdrawal from daily progesterone injections in intact female mice (3 injections of 5 mg/kg over 48 hrs; Gulinello & Smith, 2003) and rats (Costa et al., 1995) significantly increased anxiety-related behavior and decreased GABAAR sensitivity, respectively.
Drugs
All drugs were injected intraperitoneally (IP). The vehicle for all injections was 20% w/v 2-hydroxypropyl-β-cyclodextrin (Cargill, Cedar Rapids, IA) in 0.9% saline (Baxter Healthcare, Deerfield, IL), and all injections were given in volumes of 10 mL/kg. Progesterone (Experiment 1a, Sigma, St. Louis, MO; Experiments 1b and 2, Steraloids, Newport, RI) was injected at a dose of 5.0 mg/kg. In Experiment 2, finasteride (Steraloids) was administered as an additional injection of 0 mg/kg (control), 50 or 100 mg/kg. Drug or vehicle injections were performed at 1000–1230 hours.
Porsolt Forced Swim Test (FST)
As a measure of depression-like behavior, the FST was performed using slight modifications of the methods described by Lucki and coworkers (2001). Prior to testing each mouse, the clear, colorless, cylindrical testing tank measuring 21.5 cm × 24.5 cm (inner diameter × height) was filled with 25 ± 2 °C water to a height of 15 ± 1 cm. To test a subject, the mouse was dropped from a height of approximately 20 cm above the upper rim of the testing tank while a video camera recorded the mouse’s behaviors during the testing session. Six min after the mouse was dropped into the testing tank, she was removed and transferred to a holding cage. All testing occurred from 1300–1800 hours. Mice were euthanized following testing by CO2 asphyxiation. A single, experienced observer, blinded to the experimental conditions of individual mice, scored each mouse from videotape. In keeping with the methods of Lucki and coworkers, only the last 4 min of each 6 min test session was used for scoring. Since mice swim in bouts interspersed with periods of floating, a stopwatch was used to record cumulative immobility of each subject. A mouse was considered to be immobile when the observer judged that she was exhibiting no overt behaviors other than small postural movements.
Experiment 1a: Time Course of PWD, Measured by FST Behavior
DBA/2J mice aged approximately 11–12 weeks on the test days were used. Mice were injected with progesterone once daily for five days and tested in the FST on the same day as the last injection of progesterone (n = 12, “0 d PWD” group), or one day, two days, or three days (“1 d PWD,” “2 d PWD,” and “3 d PWD” groups, respectively; n = 14/group) after the last progesterone injection. A fifth group (n = 12, “VEH” group) received vehicle injections for five days and was then tested in the FST. Table 1 shows the daily schedule of progesterone or vehicle injections, and indicates the days of forced swim testing for each group.
Experiment 1b: Time Course of Progesterone Levels During PWD
General methods
DBA/2J mice aged approximately 11–12 weeks on test days (n = 5–7/group) were used to determine plasma progesterone levels at three time points following a single 5 mg/kg IP injection (“0.5 hr,” “2 hr,” and “8 hr” groups), or at three time points following repeated daily injections of 5 mg/kg progesterone IP for 5–6 days (“0 d PWD,” “1 d PWD,” and “3 d PWD” groups). Blood was collected from 0 d PWD group two hours after the last progesterone injection. Mice in the 1 d PWD and 3 d PWD groups received daily vehicle injections for one or three days (respectively) following the last progesterone injection, and blood was collected from these groups two hours after the last vehicle injection. A seventh group (“VEH”) was used to determine average plasma progesterone levels in mice receiving daily IP injections of vehicle for six days. Blood was collected from the VEH group two hours after the last vehicle injection. Table 2 shows the daily schedule of progesterone or vehicle injections, and interval after which blood was collected following the last injection.
An original goal was to measure ALLO levels, in addition to progesterone levels. However, decreased sensitivity of the antibody following long-term storage prohibited these determinations. Nonetheless, previous work has confirmed that the progesterone injection regimen significantly increased brain ALLO levels and produced a decrease during PWD (e.g., Costa et al., 1995; Dazzi et al., 2002; Gulinello & Smith, 2003).
Plasma progesterone determination
Mice were decapitated at the times indicated above. Trunk blood was collected into 4 mL tubes containing 7.2 mg EDTA on ice, which were then centrifuged at 850 g and 4 °C for 20 min. Following centrifugation, the plasma fraction was aspirated and stored in separate tubes at −80 °C until assayed. Plasma progesterone concentrations were determined by radioimmunoassay using a commercially available kit (Coat-A-Count progesterone kit, Diagnostic Products Corporation, Los Angeles, CA) according to the manufacturer’s instructions. Briefly, 100 μL of each standard (in duplicate), sample, or diluted sample was vortexed with 1.0 mL [125I] progesterone and incubated in the provided tubes coated with rabbit antibodies for progesterone at room temperature for 3 hr. Following incubation, tubes were decanted and bound radioactivity was quantified with a standard gamma counter. Because progesterone concentrations were expected to vary widely among treatment groups, all samples were assayed at their full concentration but some samples were additionally assayed after being diluted. To improve precision, reported plasma progesterone concentrations are based on the dilution at which counts were best contained within the linear portion of the standard curve.
Counts per minute were normalized and fit to a least-squares regression equation produced by log-logit transformation of the standards (0.1–40 ng/mL) using Prism 4 (GraphPad Software, Inc., San Diego, CA). Mass of samples was calculated by interpolation of the standards. The minimal detectable limit of the assays ranged from 0.14–0.22 ng/mL. Intra-assay coefficients of variation were less than or equal to 4.1%, and the inter-assay coefficient of variation was less than 8.6%.
Experiment 2: Effect of Finasteride on PWD-Induced Behavior in the FST
DBA/2J mice aged approximately 12–13 weeks on test day were used. On days 1–5 of the experiment, mice (n = 12/group) received daily injections of progesterone. Then on days 6–8 of the experiment, separate groups of mice received daily injections of progesterone with additional injections of vehicle (“0 mg/kg” group), 50 mg/kg finasteride (“50 mg/kg” group), or 100 mg/kg finasteride (“100 mg/kg” group). All mice were tested in the FST on day 8 of the experiment, approximately 2–4 hours after the last injection. Table 1 shows the daily schedule of progesterone, vehicle, or finasteride injections, and indicates the day of testing for all groups. The doses of finasteride that were chosen have previously been shown to significantly decrease ALLO levels following a systemic progesterone injection (e.g., Rhodes & Frye, 2005) or in pseudopregnant animals (e.g., Reddy et al., 2001).
Statistical Analysis
One-way analysis of variance (ANOVA) was used to test for omnibus differences in FST immobility or plasma progesterone concentrations for all experiments. To minimize type I error inflation, significant omnibus tests were followed by Tukey HSD tests in all experiments. One mouse was removed from the analysis of Experiment 1a (from the 1 d PWD group) after being identified as a statistical outlier using the Studentized Residual test. The .05 α-level (two-tailed) was adopted as the cutoff for statistical significance in all analyses. Statistical procedures were performed using SYSTAT 11 (Systat Software, San Jose, CA).
Results
Experiments 1a: Time Course of PWD, Measured by FST Behavior
ANOVA was used to compare group means of FST immobility (figure 1). The omnibus test revealed that depression-like behavior differed among treatment groups, F4,60 = 3.97, p < .05. Tukey HSD comparisons revealed that the 3 d PWD group exhibited significantly more immobility in the FST than each of the other groups. No other difference was determined among these groups.
Figure 1.

Mean (± SEM) seconds of immobility in the FST in female DBA/2J mice following different intervals of withdrawal (group labels) from repeated 5 mg/kg IP injections of progesterone, or following repeated vehicle injections. * p < .05 compared to 3 d PWD group by Tukey HSD.
Experiment 1b: Time Course of Progesterone Levels During PWD
ANOVA was performed on plasma progesterone concentrations of blood collected from mice that received one 5 mg/kg progesterone injection, and from mice that received repeated progesterone or vehicle injections. An overall difference was found among plasma progesterone concentrations taken at different time points, F6,34 = 60.6, p < .05. Post hoc tests confirmed that plasma progesterone was significantly increased at 0.5 hr after injection, compared to all other groups. However, progesterone levels did not differ significantly between 2 hr or 8 hr after injection (figure 2a). Progesterone levels were significantly higher in the 0 d PWD group compared to the 3 d PWD group, but progesterone levels in the 1 d PWD were not significantly different from either the 0 d PWD or 3 d PWD groups. Progesterone levels in the VEH group were significantly lower than the 0 d PWD group and the 2 hr group, but were not different from the 3 d PWD group. Plasma progesterone levels in the 8 hr group were significantly lower than levels in the 0.5 hr group, but were not significantly different from any other group.
Figure 2.
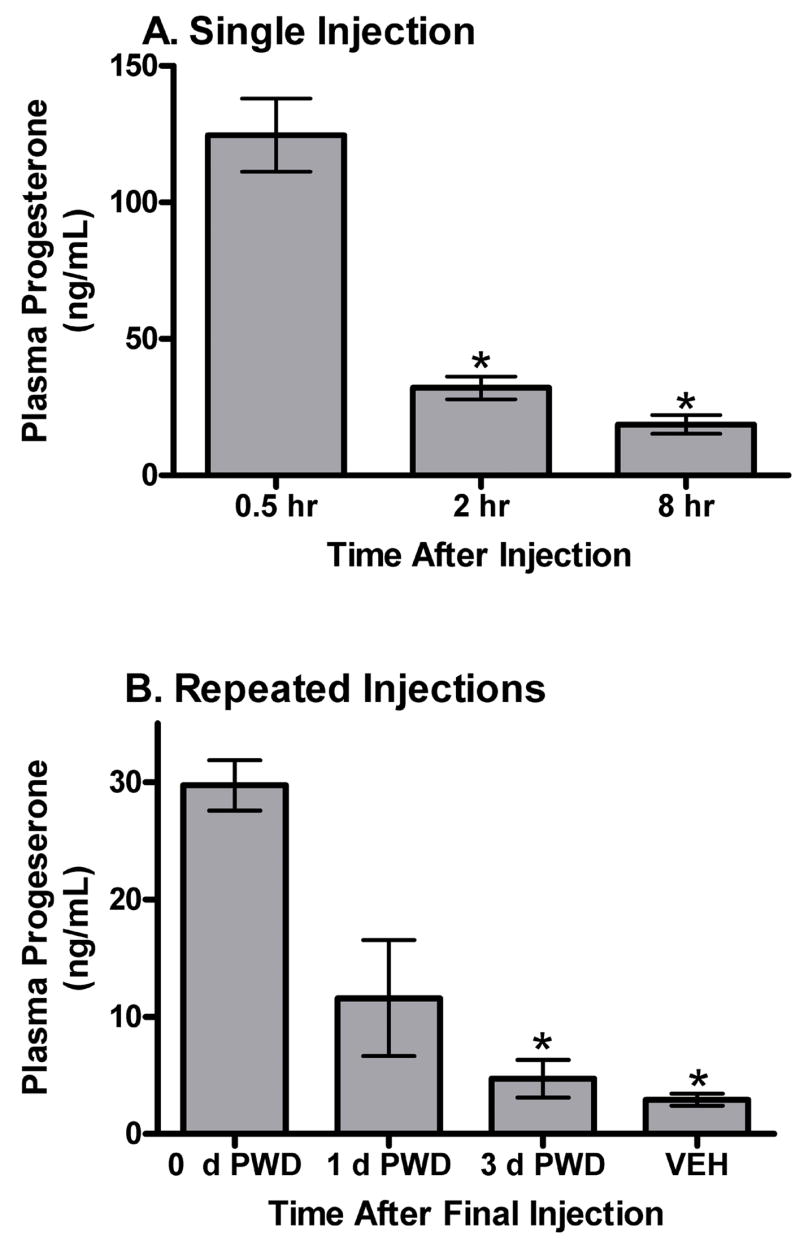
Mean (± SEM) ng/mL of progesterone in plasma from female DBA/2J mice. (A) Blood was collected at various times (group labels) following a single 5 mg/kg IP injection of progesterone. *p < .05 compared to 0.5 hr group by Tukey HSD. (B) Blood was collected at various times of withdrawal (group labels) following repeated 5 mg/kg IP injections of progesterone, or after repeated vehicle injections. *p < .05 compared to 0 d PWD group by Tukey HSD.
Experiment 2: Effect of Finasteride on PWD-Induced Behavior in the FST
ANOVA was used to determine the effects of finasteride treatment on FST immobility. An overall difference was found among the finasteride dose groups, F2,33 = 5.18, p < .05. Post hoc Tukey HSD tests revealed that 100 mg/kg finasteride significantly increased immobility in the FST, when compared to the 50 mg/kg or 0 mg/kg doses (figure 3). There was no difference in immobility detected between the 0 mg/kg and 50 mg/kg doses.
Figure 3.

Mean (± SEM) seconds of immobility in the FST in female DBA/2J mice following repeated 5 mg/kg IP injections of progesterone, co-administered with different doses of finasteride (group labels). *p < .05 compared to 100 mg/kg finasteride dose by Tukey HSD.
Discussion
These experiments were designed to adapt a previously described PWD procedure for behavioral analysis, and to determine whether the behavioral effect of PWD could be replicated by withdrawing 5α-reduced neurosteroids (such as ALLO). Experiment 1a determined that three days of PWD was required to detect a significant increase in FST immobility. This finding is consistent with recent reports of delayed increases in FST immobility following steroid withdrawal (Bekku et al., 2007; Stoffel & Craft, 2004), and of increased depression-like behavior in rats at three days postpartum, as assessed in the differential reinforcement of low response-rate model of depression (Molina-Hernández et al., 2000). In the case of Stoffel and Craft (2004), rats received progesterone and estradiol injections, separately or in combination, for a period of more than three weeks. Bekku and colleagues (2007) used multiple strains of mice (ICR, C57BL/6J, DBA/2N, and CD-1) and assessed the effect of ovariectomy on FST immobility. Although their results varied from the current data in some ways, such differences may be the result of differences in methodology. Importantly, despite differences in methods, each of these studies reported an effect of steroid withdrawal on FST that is consistent with the current work.
Experiment 1b was used to confirm the effects of this procedure on plasma progesterone levels. Plasma progesterone concentrations were significantly increased at 0.5 hr after injection and remained elevated at 2 hr after injection when compared to levels observed in mice treated with vehicle or withdrawn for three days. Progesterone levels two hours after a single progesterone injection (2 hr group) or two hours after repeated progesterone injections (0 d PWD group) did not differ, suggesting similar kinetics of progesterone during this procedure. This experiment also demonstrated a significant decrease in plasma progesterone levels at three days of withdrawal from repeated progesterone injections, but not at one day of withdrawal. However, plasma progesterone levels were not different between vehicle-treated mice and mice allowed to withdraw from progesterone for three days.
These findings indicate that there is not a simple relationship between progesterone levels and FST immobility. Comparing the results of Experiments 1a and 1b, these data show that low levels of progesterone were not associated with increased FST immobility among vehicle-treated mice, but that similarly low levels of progesterone were associated with significantly increased FST immobility following withdrawal from high levels of progesterone. Taken in conjunction with the finding that an abrupt drop in progesterone levels was associated with increased FST immobility (Saavedra et al., 2006, see Introduction for experimental methods), these data suggest that a withdrawal-induced change in progesterone levels reflects neuroadaptation to high levels of progesterone or its metabolites.
Despite the temporal continuity between hormonal fluctuations and PMS symptoms, several reports have suggested that hormones are normal throughout the cycle in women with PMS (Halbreich, 2003). Thus, it is of great interest to explore hormone changes outside the estrogen/progesterone dichotomy. One potential mechanism through which PWD might result in depression is through a corresponding decrease in its GABAergic metabolite ALLO. This hypothesis was tested by administering the 5α-reductase enzyme inhibitor finasteride, which produces a concomitant decrease in 5α-dihydroprogesterone and ALLO. In Experiment 2, mice that received 100 mg/kg finasteride and progesterone exhibited a significant increase in FST immobility versus mice that received continuous progesterone. Also, the degree to which FST immobility was increased by 100 mg/kg finasteride administration was consistent with the degree of FST immobility produced by three days of PWD. Since progesterone levels should not be decreased in the mice receiving progesterone and finasteride injections, these results demonstrate that withdrawal of 5α-reduced steroids can mimic the behavioral effects of PWD. Thus, these findings support the hypothesis that the effects of PWD on FST immobility are mediated through ALLO withdrawal.
Several lines of evidence indicate that the progesterone injection regimen utilized in the present study produces fluctuations in ALLO levels that are temporally related to progesterone levels, and that the use of finasteride can block the progesterone-induced increase in ALLO levels. First, in intact female mice and rats 3–5 daily progesterone injections produced high-physiological levels of ALLO (Costa et al., 1995; Gulinello & Smith, 2003) that are significantly decreased during PWD. Second, data from our laboratory has shown an 80% decrease in brain ALLO levels at 24 hr following injection of the 50 mg/kg dose of finasteride in male mice (for a review on finasteride see Finn et al., 2006a), which is consistent with data in rats (VanDoren et al., 2000). Since female mice have higher endogenous ALLO levels than male mice (Finn et al., 2004b) and since we administered exogenous progesterone, we presumed that the 100 mg/kg dose of finasteride would be more efficacious at decreasing endogenous ALLO levels. Third, the 50 mg/kg dose of finasteride significantly decreased ALLO levels following a single progesterone injection (Rhodes and Frye, 2005), whereas the 100 mg/kg finasteride dose significantly reduced ALLO levels by 85% in pseudopregnant female rats without altering plasma progesterone concentrations (Reddy et al., 2001). Collectively, these findings suggest that the use of the 100 mg/kg dose of finasteride in the present study should significantly reduce ALLO to levels that have been reported to occur during PWD.
The present work adds to a line of research indicating a role for ALLO in depression-like behavior in mice. For example, Frye and coworkers (2004) previously reported that progesterone decreased FST immobility in wildtype mice but not in mice with a null mutation for the Srd5a1 gene (encodes the type 1 5α-reductase enzyme). Also, systemic or intra-hippocampal administration of finasteride (Frye & Walf, 2002) as well as intra-amygdala injections of finasteride (Walf et al., 2006) increased FST immobility in female rats. These bidirectional manipulations of ALLO levels provide evidence for an inverse relationship between ALLO levels and FST immobility, albeit not in the context of PWD. In contrast, the efforts of other researchers (e.g., Bekku et al., 2007; Molina-Hernández et al., 2000) have reported depression-like effects of PWD, but without specifically assessing potential mechanisms underlying the effect. Thus, FST immobility was previously studied in the context of acute ALLO manipulation or progesterone withdrawal, and the current research combines these lines of research.
Anxiety-like behaviors have been better characterized within the context of PWD. Interestingly, while onset of FST immobility appears to be delayed by some number of days, rodent models of anxiety such as the elevated plus maze (EPM) show anxiogenic-like responses as early as one day following PWD. For example, Bitran and Smith (2005) induced PWD by ovariectomizing rats after 10 days of hormonally-induced pseudopregnancy and found that one day of PWD increased anxiety-like behavior in the EPM compared to ovariectomized control rats. Similar effects in the EPM were detected in rats after one day of PWD when progesterone was administered via implantation of a progesterone-filled, silicone capsule, and withdrawal was achieved by removing the capsule (Smith et al. 2004). Finally, three injections of progesterone (5 mg/kg) or ALLO (10 mg/kg) significantly increased anxiety-like behavior in the EPM at 3–4 hrs after the last injection in male and female mice (Gulinello & Smith, 2003). Differences between the effects of ALLO and progesterone on anxiety and depression may have relevance to syndromes such as PMS, which has been characterized as having highly variable symptom profiles (Chrisler & Levy, 1990; Gotts et al., 1995; Halbreich, 2003).
It should be noted that research examining animal models of PWD has utilized several methods to manipulate progesterone levels (e.g., injections, osmotic capsules, pseudopregnancy, ovariectomy). In addition, different species of rodents and different strains within a given species can complicate direct comparisons among these studies. However, despite variation in progesterone methods, choice of animals, and other methodological details, many groups have found consistent effects of steroid withdrawal on rodent behavior in models of anxiety or depression. Thus, the effects of PWD appear to be robust, despite methodological diversity.
These studies demonstrate that the present model of PWD, chosen to reflect the shorter period of exposure to progesterone during the menstrual cycle (as opposed to pregnancy), is effective at increasing depression-like behavior in female mice from an inbred strain. The procedures are quick and easy to perform, requiring only eight days of experimentation, and produce behavioral differences without resorting to surgeries or manipulation of non-progestin steroids such as estrogens. While not definitive, the results of Experiments 2 offer support for the hypothesis that ALLO withdrawal may contribute to depression-like behavior associated with PWD. This model has the potential to be incorporated into a variety of experimental procedures that may further elucidate electrophysiological changes in the nervous system, neuroanatomical correlates of behavioral changes, and pharmacological dissociations of the substrates underlying depression-like behavior following PWD.
Acknowledgments
This work was supported by National Institutes of Health grants AA10760 and AA12439, and a Merit Award from the Department of Veterans affairs (D. A. F.). E. H. B. was also supported by the NIH training grant T32-AA07468 (awarded to Dr. Christopher L. Cunningham), and by the Nancy and Dodd Fischer Scholarship, administered by the Portland, OR chapter of the ARCS Foundation. Drs. Suzanne H. Mitchell and John C. Crabbe and two anonymous faculty members provided valuable feedback on some of the work reported herein. Drs. James J. Crowley and Irwin Lucki provided expert information on the technical parameters for the forced swim test. Ms. Andrea Fretwell provided invaluable assistance with animal care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bekku N, Yoshimura H, Araki H. Factors producing a menopausal depressive-like state in mice following ovariectomy. Psychopharmacology (Berl) 2007;187:170–80. doi: 10.1007/s00213-006-0395-2. [DOI] [PubMed] [Google Scholar]
- Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5α-pregnan-3α-ol-20-one. Eur J Pharmacol. 1989;166:325–9. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lan NC, Gee KW. Anticonvulsant steroids and the GABA/benzodiazepine receptor-chloride ionophore complex. Neurosci Biobehav Rev. 1990;14:315–22. doi: 10.1016/s0149-7634(05)80041-7. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Pluchino N, Begliuomini S, Lenzi E, Palumbo M, Luisi M, et al. Disadaptive disorders in women: Allopregnanolone, a sensitive steroid. Gynecol Endocrinol. 2004;19:344–53. doi: 10.1080/09513590400018223. [DOI] [PubMed] [Google Scholar]
- Bitran D, Smith SS. Termination of pseudopregnancy in the rat produces an anxiogenic-like response that is associated with an increase in benzodiazepine receptor binding density and a decrease in GABA-stimulated chloride influx in the hippocampus. Brain Res Bull. 2005;64:511–8. doi: 10.1016/j.brainresbull.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Pinna G, Guidotti A, Baicy K, Olsen RW. Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompanied by altered behavioral responses to neurosteroids and memory function. Neuropharmacology. 2004;46:570–9. doi: 10.1016/j.neuropharm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Choi JC, Park SK, Kim YH, Shin YW, Kwon JS, Kim JS, et al. Different brain activation patterns to pain and pain-related unpleasantness during the menstrual cycle. Anesthesiology. 2006;105:120–7. doi: 10.1097/00000542-200607000-00021. [DOI] [PubMed] [Google Scholar]
- Chrisler JC, Levy KB. The media construct a menstrual monster: A content analysis of PMS articles in the popular press. Women Health. 1990;16:89–104. doi: 10.1300/J013v16n02_07. [DOI] [PubMed] [Google Scholar]
- Corpéchot C, Young J, Calvel M, Wehrey C, Veltz JN, Touyer G, et al. Neurosteroids: 3α-hydroxy-5α-pregnan-20-one and its precursors in the brain, plasma, and steroidogenic glands of male and female rats. Endocrinology. 1993;133:1003–9. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- Costa AMN, Spence KT, Smith SS, ffrench-Mullen JMH. Withdrawal from the endogenous steroid progesterone results in GABAA currents insensitive to benzodiazepine modulation in rat CA1 hippocampus. J Neurophysiol. 1995;74:464–9. doi: 10.1152/jn.1995.74.1.464. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–45. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Dazzi L, Serra M, Seu E, Cherchi G, Pisu MG, Purdy RH, et al. Progesterone enhances ethanol-induced modulation of mesocortical dopamine neurons: Antagonism by finasteride. J Neurochem. 2002;83:1103–9. doi: 10.1046/j.1471-4159.2002.01218.x. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of γ-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharmacol Exp Ther. 1996;278:510–7. [PubMed] [Google Scholar]
- Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123:349–59. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Finn DA, Beadles-Bohling AS, Beckley EH, Ford MM, Gililland KR, Gorin-Meyer RE, et al. A new look at the 5α-reductase inhibitor finasteride CNS. Drug Rev. 2006a;12:53–76. doi: 10.1111/j.1527-3458.2006.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Douglass AD, Beadles-Bohling AS, Tanchuck MA, Long SL, Crabbe JC. Selected line difference in sensitivity to a GABAergic neurosteroid during ethanol withdrawal. Genes Brain Behav. 2006b;5:53–63. doi: 10.1111/j.1601-183X.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Gee KW. The estrus cycle, sensitivity to convulsants and the anticonvulsant effect of a neuroactive steroid. J Pharmacol Exp Ther. 1994;271:164–70. [PubMed] [Google Scholar]
- Finn DA, Ford MM, Wiren KM, Roselli CE, Crabbe JC. The role of pregnane neurosteroids in ethanol withdrawal: Behavioral genetic approaches. Pharmacol Ther. 2004a;102:91–112. doi: 10.1016/j.pharmthera.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Finn DA, Long SL, Tanchuck MA, Crabbe JC. Interaction of chronic ethanol exposure and finasteride: Sex and strain differences. Pharmacol Biochem Behav. 2004b;78:435–43. doi: 10.1016/j.pbb.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Walf A, Harney J. Progesterone reduces pentylenetetrazol-induced ictal activity of wild-type mice but not those deficient in type I 5α-reductase. Epilepsia. 2002;43 (Suppl 5):14–7. doi: 10.1046/j.1528-1157.43.s.5.19.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–15. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type I 5α-reductase. Brain Res. 2004;1004:116–24. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Wide JK, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- Gilbert Evans SE, Ross LE, Sellers EM, Purdy RH, Romach MK. 3α-reduced neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecol Endocrinol. 2005;21:268–79. doi: 10.1080/09513590500361747. [DOI] [PubMed] [Google Scholar]
- Gotts G, Morse CA, Dennerstein L. Premenstrual complaints: An idiosyncratic syndrome. J Psychosom Obstet Gynaecol. 1995;16:29–35. doi: 10.3109/01674829509025654. [DOI] [PubMed] [Google Scholar]
- Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96:13512–7. doi: 10.1073/pnas.96.23.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: Sex differences and altered GABAA receptor pharmacology in adult rats. J Pharmacol Exp Ther. 2003;305:541–8. doi: 10.1124/jpet.102.045120. [DOI] [PubMed] [Google Scholar]
- Halbreich U. The etiology, biology, and evolving pathology of premenstrual syndromes. Psychoneuroendocrinology. 2003;28:55–99. doi: 10.1016/s0306-4530(03)00097-0. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Smith SS. Progesterone withdrawal produces paired-pulse inhibition in rat hippocampus: Dependence on GABAA receptor α4 subunit upregulation. J Neurophysiol. 2003;89:186–98. doi: 10.1152/jn.00195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokate TG, Cohen AL, Karp E, Rogawski MA. Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology. 1996;35:1049–56. doi: 10.1016/s0028-3908(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: Correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–9. [PubMed] [Google Scholar]
- Löfgren M, Johansson IM, Meyerson B, Lundgren P, Bäckström T. Progesterone withdrawal effects in the open field test can be predicted by elevated plus maze performance. Horm Behav. 2006;50:208–15. doi: 10.1016/j.yhbeh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–22. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- MacDonald PC, Dombroski RA, Casey ML. Recurrent secretion of progesterone in large amounts: An endocrine/metabolic disorder unique to young women? Endocr Rev. 1991;12:372–401. doi: 10.1210/edrv-12-4-372. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Neurosteroids: Endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38:379–95. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- McGrath E, Keita GP, Strickland BR, Russo NF. Women and depression: Risk Factors and treatment issues: Final report of the American Psychological Association’s national task force on women and depression. Washington, DC: American Psychological Association; 1990. [Google Scholar]
- Molina-Hernández M, Contreras CM, Téllez-Alcántar P. Antidepressant-like effects of pregnancy and progesterone in Wistar rats as measured in the differential reinforcement of the low-rate 72 s task. Psychopharmacology (Berl) 2000;151:306–11. doi: 10.1007/s002130000496. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–5. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- National Research Council of the National Academies. Guidelines for the care and use of mammals in neuroscience and behavioral research. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT uptake. Psychopharmacology (Berl) 2006;186:362–72. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90:709–14. doi: 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, et al. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry. 2006;60:704–13. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–36. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Inhibiting progesterone metabolism in the hippocampus of rats in behavioral estrus decreases anxiolytic behaviors and enhances exploratory and antinociceptive behaviors. Cogn Affect Behav Neurosci. 2001;1:287–91. doi: 10.3758/cabn.1.3.287. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Attenuating 5α-pregnane-3α-ol-20-one formation in the hippocampus of female rats increases pentylenetetrazole-induced seizures. Epilepsy Behav. 2005;6:140–6. doi: 10.1016/j.yebeh.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Landa JF, Contreras CM, Bernal-Morales B, Gutiérrez-García AG, Saavedra M. Allopregnanolone reduces immobility in the forced swimming test and increases the firing rate of lateral septal neurons through actions on the GABAA receptor in the rat. J Psychopharmacol. 2007;21:76–84. doi: 10.1177/0269881106064203. [DOI] [PubMed] [Google Scholar]
- Romeo E, Ströhle A, Spalletta G, di Michele F, Hermann B, Holsboer F, et al. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–3. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Saavedra M, Contreras CM, Azamar-Arizmendi G, Hernández-Lozano M. Differential progesterone effects on defensive burying and forced swimming tests depending upon a gradual decrease or an abrupt suppression schedules. Pharmacol Biochem Behav. 2006;83:130–5. doi: 10.1016/j.pbb.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Scmidt PJ, Purdy RH, Moore PH, Jr, Paul SM, Rubinow DH. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab. 1994;79:1256–60. doi: 10.1210/jcem.79.5.7962316. [DOI] [PubMed] [Google Scholar]
- Schüle C, Romeo E, Uzunov DP, Eser D, di Michele F, Baghai TC, et al. Influence of mirtazapine on plasma concentrations of neuroactive steroids in major depression and on 3α-hydroxysteroid dehydrogenase activity. Mol Psychiatry. 2006;11:261–72. doi: 10.1038/sj.mp.4001782. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, et al. Withdrawal from 3α-OH-5α-pregnan-20-one using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci. 1998;18:5275–84. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Ruderman Y, Frye C, Homanics G, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3α,5β-THP: A possible model of premenstrual dysphoric disorder. Psychopharmacology (Berl) 2006;186:323–333. doi: 10.1007/s00213-005-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Ruderman Y, Gong QH, Gulinello M. Effects of a low dose of ethanol in an animal model of premenstrual anxiety. Alcohol. 2004;33:41–9. doi: 10.1016/j.alcohol.2004.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M, Dunn E, Born L. Hormones and mood: From menarche to menopause and beyond. J Affect Disord. 2003;74:67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Craft RM. Ovarian hormone withdrawal-induced “depression” in female rats. Physiol Behav. 2004;83:505–13. doi: 10.1016/j.physbeh.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sampson L, Uzunov DP. Relevance of endogenous 3α-reduced neurosteroids to depression and antidepressant action. Psychopharmacology (Berl) 2006;186:351–61. doi: 10.1007/s00213-005-0201-6. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–9. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Sumida K, Frye CA. Inhibiting 5α-reductase in the amygdala attenuates antianxiety and antidepressive behavior of naturally receptive and hormone-primed ovariectomized rats. Psychopharmacology (Berl) 2006;186:302–11. doi: 10.1007/s00213-005-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


