Abstract
Hydrolysis of phosphatidylcholine by phospholipase D (PLD) leads to the generation of the versatile lipid second messenger, phosphatidic acid (PA), which is involved in fundamental cellular processes, including membrane trafficking, actin cytoskeleton remodeling, cell proliferation and cell survival. PLD activity can be dramatically stimulated by a large number of cell surface receptors and is elaborately regulated by intracellular factors, including protein kinase C isoforms, small GTPases of the ARF, Rho and Ras families and, particularly, by the phosphoinositide, phosphatidylinositol 4,5-bisphosphate (PIP2). PIP2 is well known as substrate for the generation of second messengers by phospholipase C, but is now also understood to recruit and/or activate a variety of actin regulatory proteins, ion channels and other signaling proteins, including PLD, by direct interaction. The synthesis of PIP2 by phosphoinositide 5-kinase (PIP5K) isoforms is tightly regulated by small GTPases and, interestingly, by PA as well, and the concerted formation of PIP2 and PA has been shown to mediate receptor-regulated cellular events. This review highlights the regulation of PLD by membrane receptors, and describes how the close encounter of PLD and PIP5K isoforms with small GTPases permits the execution of specific cellular functions.
Keywords: Phospholipase D, Phosphatidic acid, PIP2, Phosphoinositide 5-kinase, ARF, Rho, Ras
Introduction
The activation of membrane receptors by hormones and growth factors results in the localized generation of intracellular second messengers. The hydrolysis of membrane phospholipids and the generation of biologically active products play important roles in the regulation of cell function and cell fate. Well known is the activation of phosphoinositide-specific phospholipase C (PLC) isoforms, which hydrolyze phosphatidylinositol 4,5-bisphosphate (PIP2), a membrane phospholipid found in all eukaryotic cells (Schmidt et al. 2004). Stimulation of PLC isoforms plays a major role in many early and late cellular responses to receptor activation, including smooth muscle contraction, secretion and neuronal signaling as well as fertilization, cell growth and differentiation (Berridge 2005; Nishizuka 2003). Phospholipase D (PLD) was first described 60 years ago as a distinct, phospholipid-specific phosphodiesterase activity in cabbage leaves (Hanahan and Chaikoff 1948). This pioneering research indicated that PLD hydrolyzes phosphatidylcholine to yield phosphatidic acid (PA) and choline. The recognition that PLD is rapidly and dramatically activated in response to extracellular stimuli in cultured animal cells, now 20 years ago (Bocckino et al. 1987; Cockcroft 1984), has brought PLD signaling to the very forefront of current biological and biomedical research. Meanwhile, phosphatidylcholine-hydrolyzing PLD has been identified in bacteria, protozoa, fungi, plants and animals, and, due to this widespread distribution, is assumed to be involved in the regulation of fundamental cellular functions. Indeed, it has now been established that activation of PLD and the generation of PA by a vast number of membrane receptors modulate such a wide array of cellular responses as calcium mobilization, secretion, superoxide production, endocytosis, exocytosis, vesicle trafficking, glucose transport, rearrangements of the actin cytoskeleton, mitogenesis and survival (Cockcroft 2001; Exton 2002b; Jenkins and Frohman 2005; Liscovitch et al. 2000).
PIP2 is a critical cofactor for PLD, and profoundly affects the activity, membrane localization and receptor activation of both PLD isoforms, PLD1 and PLD2 (Brown et al. 1993; Hodgkin et al. 2000; Liscovitch et al. 1994; Pertile et al. 1995; Schmidt et al. 1996d). Thus, reduction of cellular PIP2 levels, for instance via scavenging of PIP2 by the actin-binding protein fodrin (Lukowski et al. 1998) or via forced PIP2 hydrolysis by the phosphatase synaptojanin (Chung et al. 1997), has been shown to inhibit PLD activity. Vice versa, the synthesis of PIP2 by phosphoinositide 5-kinase (PIP5K) isoforms can be directly stimulated by the PLD product PA (Jenkins et al. 1994; Moritz et al. 1992), and this regulation has also been confirmed to occur at the whole cell level (Divecha et al. 2000; Jones et al. 2000b; Skippen et al. 2002). It is now hypothesized that the reciprocal stimulation of PLD and PIP5K enzymes enables rapid feed-forward stimulation loops for a localized and explosive generation of PA and PIP2, which may then govern the recruitment and activation of proteins to execute specific cellular tasks, especially membrane trafficking, and changes in the organization of the actin cytoskeleton. The activity and localization of both PLD and PIP5K are under control of GTPases of the Arf and Rho families, which are well-defined regulators of membrane transport and actin-reorganization processes. The reciprocal stimulation of PIP5K and PLD, and the regulation of these enzymes by ARF and Rho GTPases, point to concerted mechanisms in cellular actions, involving acute, localized PIP2 and PA synthesis (Fig. 1). This review will focus on the regulation of PLD enzymes by membrane receptors and monomeric GTPases, and on how PLD signaling is organized and connected by PIP2 metabolism.
Fig. 1.
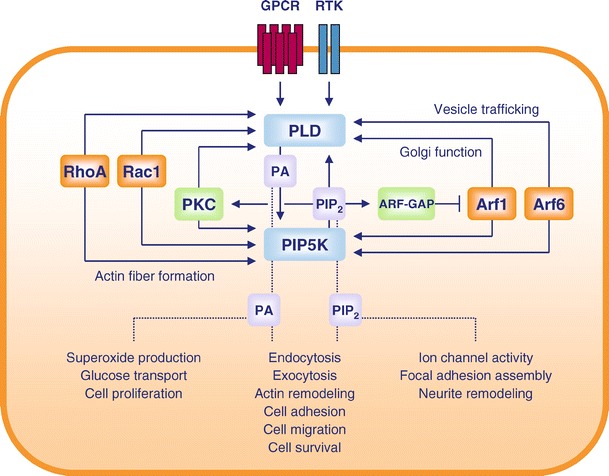
Regulation and cellular roles of PLD and PIP5K. Regulation of PLD and PIP5K by ARF and Rho family GTPases is essentially involved in the regulation of intracellular vesicle trafficking and actin cytoskeleton reorganization. Both PLD and PIP5K are stimulated by cell surface receptors and by conventional PKC isoforms, and the latter can become activated after receptor-induced hydrolysis of PIP2 by PLC. Positive feed-forward regulation is achieved by stimulation of PLD by PIP5K-derived PIP2, and of PIP5K by PLD-derived PA. Activation of ARF-GAPs by PIP2 accelerates the inactivation of ARF proteins, and may terminate a round of PA and PIP2 synthesis
Phosphatidic acid and PLD isoforms
Most cellular responses following PLD activation are probably mediated by the immediate reaction product PA. PA is a multifunctional lipid that can be further metabolized to the bioactive lipids, lysophosphatidic acid (LPA) and diacylglycerol (DAG), can by itself alter membrane curvature, and can serve as a protein attachment site and affect both cellular localization and activity of various proteins, including Raf-1 kinase, protein phosphatase 1, sphingosine kinase 1, and mTOR (mammalian target of rapamycin), a key regulator of cell growth and proliferation (Jenkins and Frohman 2005). PLD enzymes can catalyze a transphosphatidylation reaction in which the phosphatidyl moiety of phosphatidylcholine is accepted by primary alcohols, thereby producing stable phosphatidylalcohol instead of PA. This transphosphatidylation reaction is widely applied to measure PLD activity in biological samples, and quenching of PA synthesis by primary alcohols has proven extremely helpful to identify the involvement of PLD enzymes in cell physiology. In this way, a role for PLD has been demonstrated in a variety of signaling processes, such as activation of phosphoinositide (PI3K, PIP5K) and protein (Akt, ERK1/2) kinases, calcium mobilization, cytoskeleton remodeling, endocytosis, exocytosis, membrane trafficking, superoxide production, glucose transport, cell migration, cell proliferation, and survival signaling (Exton 2002a; Foster and Xu 2003).
There are two mammalian PLD genes, PLD1 and PLD2. PLD1 has a low basal activity and is extensively regulated by conventional protein kinase C (PKCα, -β, -γ) isozymes and small GTPases of the ARF (ARF1 - ARF6) and Rho (RhoA, Rac1, Cdc42) families (Henage et al. 2006). PLD2 has a higher basal activity than PLD1, but has been shown to respond to ARF and PKC as well (Chen and Exton 2004). PIP2 is recognized to be the most important cofactor for PLD, and both PLD isoforms are absolutely dependent on PIP2 for activity. Experiments utilizing inactive PLD mutants and RNA interference have discriminated isoform-specific PLD functions, and showed that PLD1 is involved in agonist-induced secretion, actin organization, and cell adhesion and migration (Exton 2002a; Iyer et al. 2006; Kim et al. 2006; Vitale et al. 2001), and PLD2 in endocytosis and recycling of membrane receptors (Du et al. 2004; Koch et al. 2006; Padrón et al. 2006).
The PLD isoforms, both with two splice variants, share an ~50% amino-acid sequence identity (Colley et al. 1997; Hammond et al. 1995, 1997; Steed et al. 1998). The catalytic core of both PLD enzymes are composed of four conserved domains (domain I-IV), and the HKD motifs in the domains II and IV probably associate together to form a catalytic centre (Xie et al. 2000). PLD1 is characterized by a 116-amino acid loop region following domain II, which has been proposed to function as a negative regulatory element (Sung et al. 1999). PLD1 and PLD2 further possess N-terminal PH (pleckstrin homology) and PX (phox homology) domains. PIP2 binds to the PH domain (Hodgkin et al. 2000), but also to a polybasic PIP2 binding motif within the catalytic core (Sciorra et al. 1999), and interaction of PIP2 with both domains has been suggested to be involved in membrane targeting of PLD as well as stimulation of PLD catalytic activity (Du et al. 2003; Hodgkin et al. 2000; Sciorra et al. 2002). The PX domain of PLD1 has been reported to preferentially bind to phosphatidylinositol-3,4,5-trisphosphate (PIP3) (Lee et al. 2005; Stahelin et al. 2004), but interaction with PI5P has been observed as well (Du et al. 2003). Recently, it was shown that the PX domain of PLD has GTPase-activating protein (GAP) activity towards dynamin, and that PLD supports EGF receptor endocytosis (Lee et al. 2006). The PH and PX domains probably contribute to the proper localization of the PLD enzymes within cells. In line with a role for PLD enzymes in different cellular tasks, PLD1 and PLD2 show a diverse subcellular distribution. PLD1 is found throughout the cell, but primarily localizes to perinuclear endosomes and the Golgi apparatus (Brown et al. 1998; Freyberg et al. 2001; Hughes and Parker 2001). PLD2 is almost exclusively present at the plasma membrane in lipid raft fractions (Czarny et al. 1999). The localization of PLD1 does not seem to be static, and regulated translocation and recycling of the enzyme between cellular compartments may be crucial to its proper functioning. In an elegant study, coordinated subcellular targeting of the lipid binding motifs has been demonstrated to drive this subcellular cycling of PLD1 (Du et al. 2003). Upon stimulation, PLD1 was found to translocate from the intracellular compartments to the plasma membrane, and this process was probably dependent on the polybasic PIP2 binding site. The PH domain then facilitated entry of PLD1 into lipid rafts, a step critical for internalization of the enzyme, whereafter interaction of the PX domain with PI5P may control the efficient return of PLD1 to the endosomes.
PIP2 and PIP5K isoforms
PIP2 is an essential and versatile factor in cellular signaling. Hydrolysis of PIP2 by PLC into the second messengers, inositol-1,4,5-trisphosphate (IP3) and DAG, is a general and well-defined answer of cells in response to stimulation of many membrane receptors (Schmidt et al. 2004). Phosphorylation of PIP2 by PI3K results in the rapid accumulation of PIP3, which recruits and activates mediators involved in actin remodeling, mitogenesis and survival (Vanhaesebroeck et al. 2001). But it is now recognized that PIP2, as well as other phosphoinositides, are signaling molecules by themselves and can, by binding to unique phosphoinositide-binding sequences, such as the PH and PX domains, affect the activity and subcellular localization of many proteins, including many actin regulatory proteins, a wide range of ion channels, and PLD (Niggli 2005; Suh and Hille 2005; Yin and Janmey 2003). In this way, PIP2 can modulate a remarkable variety of cellular processes, including cortical actin organization, membrane ruffling, vesicle trafficking, gene expression, cell migration and cell survival (Ling et al. 2006; Oude Weernink et al. 2004b; Toker 2002). Subsequent dephosphorylation of PIP2 by inositol polyphosphate 5-phosphatases, such as synaptojanin, is believed to terminate local PIP2 signaling, for instance in the process of vesicle trafficking (Majerus et al. 1999).
To execute this variety of functions, PIP2 may be organized in discrete functional pools within cells, but the existence of PIP2 clusters in the plasma membrane is currently under debate. Using green fluorescent protein-tagged PH domains or antibodies to visualize PIP2, the lipid was found to concentrate in highly dynamic, actin-rich regions (Tall et al. 2000) and lipid rafts (Laux et al. 2000; Parmryd et al. 2003) in the plasma membrane, feeding the idea that spatially organized PIP2 synthesis regulates actin polymerization and other cellular processes. The localization of PIP2 in rafts is supported by biochemical data (Pike and Casey 1996); however, specific PIP2 clustering has been disputed (van Rheenen et al. 2005).
PIP2 is generated after phosphorylation of phosphatidylinositol-4-phosphate by PIP5K. In mammals, cDNAs encoding three isoforms of PIP5K (designated Iα, Iβ and Iγ) with alternative splice variants have been cloned and characterized (Ishihara et al. 1996, 1998; Loijens and Anderson 1996). Sequence analysis has shown that PIP5K enzymes are related to PIP4K enzymes, but that they share no identity with most other lipid (PI3K and PI4K) or protein kinases. The sequence similarity between the PIP4Ks and PIP5Ks is clustered in the catalytic core of the kinases (Anderson et al. 1999; Hinchliffe et al. 1998). An activation loop spanning the catalytic domain has been shown to determine both substrate specificity and subcellular targeting of PIP5Ks, which can be swapped by substitution of a single amino acid within this loop (Kunz et al. 2002). In murine PIP5K-Iβ, two dimerization domains were identified, which may contribute to the proper subcellular localization and functioning of the enzyme (Galiano et al. 2002).
The identification of three PIP5K isoforms raised the expectation of a differential regulation of the enzymes by cellular signal transduction components, but up to now the regulatory properties of PIP5K-Iα, Iβ and Iγ appear to be remarkably similar. All PIP5K isoforms are stimulated by PA, are extensively regulated by ARF and Rho GTPases, and inhibited by protein kinase A (PKA) and PI-stimulated autophosphorylation (Oude Weernink et al. 2004b). Nevertheless, evidence has been provided that PIP5K isoforms may selectively control functional PIP2 pools, which may support particular processes in different cell types. Thus, actin reorganization down-stream of Rac1 in platelets specifically involves murine PIP5K-Iα (Tolias et al. 2000). Human PIP5K-Iα was found to localize in Rac1-induced membrane ruffles, and the LIM protein Ajuba has been identified to interact with and stimulate PIP5K-Iα in leading-edge membrane ruffles in migrating cells (Kisseleva et al. 2005). Human PIPK-Iβ was detected primarily in cytosolic vesicular structures (Doughman et al. 2003) and may synthesize the PIP2 pool involved in constitutive endocytosis (Padrón et al. 2003). The long-splice variant of PIP5K-Iγ, PIP5K-Iγ90, is enriched in neurons and is implicated in the regulation of clathrin coat recruitment, actin dynamics (Wenk et al. 2001) and focal adhesion formation (Di Paolo et al. 2002; Ling et al. 2002). In contrast, short PIP5K-Iγ87 seems to be the major producer of the PIP2 pool that supports receptor-induced IP3 generation (Wang et al. 2004).
The execution of specific PIP2-modulated processes is very probably achieved by an orchestration of appropriate signaling partners within discrete subcellular microdomains, and PLD-derived PA as well as the PLD enzymes by themselves can contribute to this organization. Indeed, both PLD1 and PLD2 interact with PIP5K-Iα, and PLD2 recruits PIP5K-Iα to a submembraneous vesicular compartment (Divecha et al. 2000). PLD2-derived PA was shown to stimulate PIP5K-Iγ splice variants, and the subsequent formation of PIP2 to drive the initial stages of integrin-mediated cellular adhesion (Powner et al. 2005). In many processes, the temporal activation and correct localization of PLD and PIP5K isoforms by monomeric GTPases appears crucial to achieve the spatially organized production of PIP2 and PA (Santarius et al. 2006).
ARF GTPases and membrane traffic
Although the direct interaction site on PLD for ARF has not yet been unequivocally defined, it is well established that ARF proteins, particularly ARF1 and ARF6, activate both PLD enzymes, but especially PLD1 (Hammond et al. 1995, 1997). ARF GTPases regulate intracellular vesicle trafficking and actin remodeling. ARF1 is localized to the Golgi complex, and is required for proper Golgi structure and function. The use of primary alcohols has also pointed to a role for PLD in vesicle transport to Golgi (Bi et al. 1997; Ktistakis et al. 1996). PLD activity has been shown to stimulate the release of nascent secretory vesicles from the trans-Golgi network (Chen et al. 1997), and to be required for maintaining the structural integrity and function of the Golgi apparatus, but the precise role for PLD in vesicle formation is still controversial. PIP5K is also a direct effector of ARF1, and an ARF1 mutant that selectively activates PIP5K, but not PLD activity, demonstrated that both PLD-derived PA and direct activation of PIP5K by ARF1 contribute to increased PIP2 synthesis (Skippen et al. 2002). In permeabilized cells, ARF1 has been shown to restore secretion by promoting PIP2 synthesis (Fensome et al. 1996), and ARF1-mediated PIP5K activation (Jones et al. 2000a) and recruitment to the Golgi complex (Godi et al. 1999) appears to be critical in Golgi functioning.
ARF6 regulates vesicular transport, secretion, and cortical actin reorganization. ARF6 activates PLD, and PA has been implicated in the mediation of the effects of ARF6 in vesicular trafficking events. A critical role for PLD1 in exocytosis has been established in different cell types, including neurons (Humeau et al. 2001), neuroendocrine cells (Vitale et al. 2001) and pancreatic β cells (Hughes et al. 2004). PLD2 has recently emerged as a mediator of ARF-dependent internalization of the μ-opioid receptor (Koch et al. 2003), and both PLD isoforms have been implicated in macrophage phagocytosis (Corrotte et al. 2006; Iyer et al. 2004). In addition, PIP5K colocalizes and interacts with, and is directly activated by ARF6 at the plasma membrane (Honda et al. 1999), and ARF6 and PIP2 colocalize on the plasma membrane and on endosomal structures (Brown et al. 2001). ARF6-organized PIP2 turnover at the plasma membrane is apparently involved in regulated secretion (Aikawa and Martin 2003; Brown et al. 2001; Lawrence and Birnbaum 2003). Focal and transient accumulation of PIP2 by PIP5K is required for phagocytosis as well (Botelho et al. 2000; Coppolino et al. 2002; Wong and Isberg 2003), and PIP2 hydrolysis probably dictates the remodeling of actin necessary for completion of phagocytosis (Scott et al. 2005). The synthesis of PIP2 is essential for priming the exocytotic apparatus, and the recruitment and activation of PLD1 by PIP2 seems the primary mechanism for the functional integration of PLD1 into the exocytotic pathway (Vitale et al. 2001; Waselle et al. 2005). Thus, CD16-induced cytolytic granule secretion mediated by ARF6 was shown to involve PIP5K-Iα membrane targeting and activation of both PIP5K and PLD (Galandrini et al. 2005). PIP2 also recruits additional proteins—for instance the endocytic proteins AP-2, epsin and AP180—to initiate clathrin-coat formation preceding endocytosis (Ford et al. 2001; Itoh et al. 2001; Padrón et al. 2003), and CAPS (Grishanin et al. 2004) to initiate dense-core vesicle exocytosis. Direct activation of PIP5K-Iγ by ARF6 has been shown to stimulate clathrin-coat recruitment to synaptic membranes to allow synaptic vesicle recycling (Krauss et al. 2003). PLD-derived PA may directly contribute to vesicle fusion in a biophysical manner, as PLD cleaves the non-fusogenic lipid, PC, to form the fusogenic lipid, PA. But PA also takes a function as an essential cofactor for PIP5K, and disruption of Golgi membranes (Sweeney et al. 2002), blockade of clathrin-coat assembly (Arneson et al. 1999) and inhibition of ARF1-reconstituted secretion (Way et al. 2000) after quenching of PA production could be attributed to inhibited PIP2 synthesis. Thus, both PLD and PIP2 synthesis seem necessary for membrane trafficking aspects in the endo- and exocytotic machinery. But PLD and PIP5K also mediate other processes down-stream of ARF6. Epidermal growth factor (EGF)-induced membrane ruffling requires ARF6-induced PIP5K-Iα translocation to the ruffles and local PIP2 production. This leads to the recruitment of PLD2, and PLD-derived PA and ARF6 may then synergistically activate PIP5K (Honda et al. 1999).
The relationship between ARF and PIP2 is also bidirectional, as phosphoinositides can regulate ARF activity by binding and activating both ARF-specific guanine nucleotide exchange factors (ARF-GEFs) (Klarlund et al. 1998; Paris et al. 1997) and ARF-GTPase-activating proteins (ARF-GAPs) (Kam et al. 2000; Nie et al. 2002) via their PH domains. The fact that ARF-GAPs bind PIP2 with high affinity and specificity offers an attractive feed-back mechanism for terminating ARF activation after a cycle of ARF-induced PIP2 synthesis.
Rho GTPases and actin dynamics
PA formation, especially by PLD1, has been reported to induce stress fibre formation in specific cell types (Cross et al. 1996; Ha and Exton 1993; Kam and Exton 2001; Porcelli et al. 2002). Rho proteins, in particular RhoA, Rac1 and Cdc42, which control actin cytoskeleton reorganization, exclusively activate PLD1 by direct interaction with its C-terminus (Exton 2002b; Powner and Wakelam 2002). Thus, PLD stimulation by RhoA may happen by direct interaction, but may involve indirect, Rho-dependent mechanisms as well. Inactivation of Rho GTPases, with Clostridium difficile toxin B or Clostridium botulinum C3 exoenzyme, reduced cellular PIP2 levels, resulting in inhibiton of receptor-mediated PIP2 hydrolysis by PLC (Schmidt et al. 1996a) as well as diminished PLD stimulation (Schmidt et al. 1996d). As the inhibition of PLD signaling after Rho inactivation could be largely rescued by the addition of PIP2, Rho proteins do seem to affect PLD via PIP5K regulation (Schmidt et al. 1996c,d). PIP2 is well-known to associate with and regulate the activity of a plethora of actin-binding proteins that organize actin dynamics (Hilpela et al. 2004; Yin and Janmey 2003), and PA and PIP2 may act in concert to mediate Rho-dependent actin cytoskeleton remodeling. PIP5K isoforms are, like PLD, under direct control of Rho GTPases. PIP5K isoforms are markedly stimulated by RhoA, Rac1, and Cdc42 (Chong et al. 1994; Hartwig et al. 1995; Oude Weernink et al. 2004a), and physically associate with both RhoA (Ren et al. 1996) and Rac1 (Tolias et al. 2000), but not with Cdc42 (Oude Weernink et al. 2004a; van Hennik et al. 2003). PIP5K isoforms are now seen as critical mediators of RhoA- and Rac1-induced actin organization and remodeling (Doughman et al. 2003; Shibasaki et al. 1997; Tolias et al. 2000). The established Rho effector Rho-kinase, a serine/threonine kinase, is apparently involved in Rho-dependent regulation of both PLD (Schmidt et al. 1999) and PIP5K activities (Oude Weernink et al. 2000), and PIP5K was found to play an essential role as down-stream effector of Rho and Rho-kinase in neurite remodeling (van Horck et al. 2002; Yamazaki et al. 2002) and platelet cytoskeleton assembly (Gratacap et al. 2001; Yang et al. 2004). But Rho may also directly signal to PIP5K independently of Rho-kinase, as RhoA-induced activation of ERM (ezrin, radixin, moesin) proteins, that cross-link actin filaments to plasma membranes, was found to be mediated by PIP5K, but not by Rho-kinase (Matsui et al. 1999). PLD and PIP5K were also demonstrated to collectively mediate Rho-induced changes in the actin cytoskeleton. Thus, myogenic differentiation induced by arginine-vasopressin, which involves actin fiber formation, is mediated by Rho proteins and PLD1, and involves PLD-induced PIP2 synthesis along the actin fibers (Komati et al. 2005). These findings suggest that PLD and PIP5K enzymes may co-operate down-stream of Rho in processes that depend on actin organization.
Another Rho effector, PKC-related protein kinase N (PKN), also directly interacts with PLD (Oishi et al. 2001) and mediates PLD activation by the α1-adrenergic receptor (Parmentier et al. 2002). Interestingly, components of the actin regulatory machinery, β-actin and α-actinin, have been found to directly associate with and inhibit the activity of PLD isoforms (Lee et al. 2001; Park et al. 2000). PLD also binds to and is stimulated by filamentous F-actin, and PLD1 in particular may act as a signal transduction component responsive to dynamic changes of the actin cytoskeleton (Kusner et al. 2002). PKN interacts with α-actinin, and PKN may modulate PLD signaling by reversing the inhibitory effect of α-actinin on PLD1, and by direct interaction with PLD1.
Regulation of PLD and PIP5K by membrane receptors
In line with the critical role of PA in cellular processes, the enzymatic activity of PLD is tightly regulated by a variety of hormones, neurotransmitters, and growth factors. Regulation of PLD enzymes by membrane receptors, including G protein-coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs), is complex and mediated by several cytosolic factors, including PKC as well as ARF, Rho and Ras GTPases (Exton 2002b; Liscovitch et al. 2000; López De Jesús et al. 2006; Powner and Wakelam 2002). Most receptors that stimulate PLD also increase PLC activity, leading to activation of the PLD regulator PKC, and it was assumed that PLD activation might be secondary to PLC activation. A physical association between PLD with PKC isoforms has been reported, resulting in strong activation of in vitro PLD1 activity, and the major interaction site was identified within the N-terminus of PLD1 (Park et al. 1998). Indeed, inhibition of PKC was shown to reduce receptor-induced PLD responses, and PLD1 mutants unresponsive to PKC did respond poorly to activation of GPCRs (Zhang et al. 1999) or to active Gαq proteins (Xie et al. 2002). However, stimulation of PLD in several receptor systems, including M3 muscarinic and α1-adrenergic receptors, was actually PKC-independent (Balboa and Insel 1998; Muthalif et al. 2000; Rümenapp et al. 1997; Schmidt et al. 1994), suggesting that PLD stimulation must not necessarily be secondary to PLC stimulation.
Brefeldin A, an inhibitor of certain ARF-GEFs, reduced receptor signaling to PLD in several cell types, indicating that ARF proteins participate in receptor-mediated PLD stimulation (Fensome et al. 1998; Mitchell et al. 1998; Rümenapp et al. 1995; Shome et al. 2000). Likewise, sequestration of ARF-GEFs by the ARF-related protein ARP inhibited M3 muscarinic receptor signaling to PLD (Schürmann et al. 1999). Clostridial toxins and enzymes that specifically inactivate Rho proteins and expression of inactive Rho mutants have been used to identify the role of Rho in signaling to PLD. Thus, Rho proteins were found to be involved in PLD stimulation by GPCRs (M3 muscarinic, bradykinin, sphingosine-1-phosphate and LPA), RTKs (PDGF, EGF), and immunoglobulin (FcεRI) receptors (Hess et al. 1997; Ojio et al. 1996; Schmidt et al. 1996c).
Stimulation of PLD by GPCRs was shown to be mediated by both pertussis toxin (PTX)-insensitive (Gosau et al. 2002; Schmidt et al. 1994) and PTX-sensitive (Cummings et al. 2002; Fensome et al. 1998) heterotrimeric G proteins. G12 family proteins can stimulate PLD (Plonk et al. 1998), and RGS (regulators of G protein signaling) proteins, that act as α subunit-specific GAPs, have been used to position G12 in PLD activation by the M3 muscarinic (Rümenapp et al. 2001), the PAR1 (Fahimi-Vahid et al. 2002), and the Ca2+-sensing receptor (Huang et al. 2004), as well as mechanical force (Ziembicki et al. 2005). As forskolin and cAMP were shown to cause activation of PLD via PKA and ERK1/2 (Ginsberg et al. 1997; Yoon et al. 2005) or, alternatively, via the cAMP-activated GEF for Ras-like GTPases, Epac and R-Ras (López De Jesús et al. 2006), Gs proteins also mediate stimulation of PLD. PLD activation is also controlled by βγ-subunits, possibly via Src and/or ARF6 (Le Stunff et al. 2000; Ushio-Fukai et al. 1999), but Gβγ can also directly interact with and inhibit PLD (Preininger et al. 2006).
As the precise mechanism of PLD stimulation in intact cells was only poorly understood, during the last 10 years our laboratory in Essen has focused on the regulation of PLD activity by membrane receptors. In HEK-293 cells, signaling to PLD by a typical GPCR, the M3 muscarinic receptor, and an RTK, the EGF receptor, was studied and shown to be executed by several distinct pathways (Fig. 2). In addition, by expressing inactive PLD mutants, the M3 muscarinic and the EGF receptors were found to signal to individual PLD isozymes and to selectively stimulate PLD1 and PLD2 respectively (Han et al. 2001). The M3 muscarinic receptor stimulates both PLC and PLD via PTX-insensitive mechanisms (Offermanns et al. 1994; Peralta et al. 1988; Schmidt et al. 1994). Interestingly, stimulation of PLD by the agonist carbachol was not affected by PKC inhibitors, suggesting that activation of PLD by the M3 muscarinic receptor was rather independent of PLC (Rümenapp et al. 1997; Schmidt et al. 1994). Expression of α-subunits of G proteins and of specific RGS proteins was used to identify the G proteins involved in these pathways, and demonstrated that whereas the M3 receptor signals to PLC via Gq proteins, activation of PLD is mediated by G12 family proteins (Rümenapp et al. 2001). PLD activation by the M3 receptor, but not by the EGF receptor, was further found to be under control of ARF (Rümenapp et al. 1995, 1997) as well as Rho proteins, particularly RhoA (Schmidt et al. 1996c,d). Likewise, regulation of mTOR by LPA, but not PDGF, involved PLD1 activation by Rho GTPases (Kam and Exton 2004). Both ARF1 and RhoA were found to become activated after M3 receptor activation (Keller et al. 1997; Rümenapp et al. 1995), and a role for Rho-kinase in RhoA-controlled PLD stimulation could be demonstrated (Schmidt et al. 1999). In further studies, it was shown that activation of PLD by RhoA and Rho-kinase is mediated by G12 and the tyrosine kinase Pyk2, whereas activation by ARF1 is mediated by G13, PI3K and the Arf-GEF ARNO (Han et al. 2003). In cardiomyocytes, Rho proteins were shown to affect signaling to PLD by both endothelin-1 and thrombin, apparently by controlling PIP2 synthesis, whereas ARF selectively affects signaling by the PAR1 receptor (Fahimi-Vahid et al. 2002).
Fig. 2.
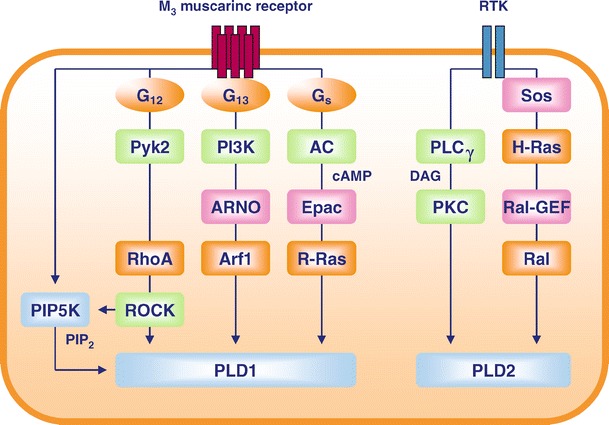
Regulation of PLD by the M3 muscarinic receptor and receptor tyrosine kinases in HEK-293 cells. In human embryonic kidney (HEK-293) cells, signaling to PLD by the M3 muscarinic receptor and by typical RTKs (EGF, PDGF, insulin) is organized into rather discrete pathways and channeled by particular heterotrimeric G proteins and small GTPases (orange), specific GEF proteins (pink) and further signaling components (green). AC, adenylyl cyclase; ROCK, Rho-kinase
PLD can directly interact with RalA, and a Ras/Ral signaling cascade was shown to regulate PLD responses. In HEK-293 cells, Ras and RalA—but not Rho proteins—were located in RTK signaling to PLD, and this Ras/Ral-dependent signaling cascade was found to be dependent on PKC-α and a Ral-specific GEF (Fig. 2) (Schmidt et al. 1998; Voss et al. 1999). RalA apparently co-operates with ARF (Kim et al. 1998; Xu et al. 2003) and Rho proteins (Frankel et al. 1999; Wilde et al. 2002) to achieve full PLD activation. Likewise, Ras proteins were found to modulate PLD responses by PDGF (Lucas et al. 2000), and RalA to affect EGF receptor signaling to PLD (Lu et al. 2000). It was recently shown that direct activation of Ras-related R-Ras by Epac is involved in PLD stimulation by the M3 muscarinic receptor, apparently by coupling to Gs proteins (López de Jesús et al. 2006), but a contribution of Ral proteins to GPCR-induced PLD activation has not been found (Meacci et al. 2002). Collectively, these data demonstrate that heterotrimeric G proteins as well as small GTPases co-ordinate PLD activation by specific membrane receptors in particular cell types, and these mechanisms probably contribute to the organization of agonist-induced PA production for the execution of diverse cellular signaling tasks.
In addition, the synthesis of PIP2 can be directly stimulated by GPCRs (thrombin, LPA, M3 muscarinic) as well as RTKs (Cochet et al. 1991; Nolan and Lapetina 1990; Pike and Eakes 1987). Receptor activation leads to increased association of PIP5K with the actin cytoskeleton (Grondin et al. 1991; Payrastre et al. 1991), and receptor-induced stimulation and cytoskeletal association of PIP5K may be directly involved in actin cytoskeletal regulation and initialize the assembly of enzymes into signaling complexes. GPCR-induced stimulation of PIP2 synthesis was found to be mediated by pertussis toxin-sensitive Gi proteins (Schmidt et al. 1996b; Stephens et al. 1993), but also by G12 and Gq proteins (Oude Weernink et al. 2003). Enhanced PIP2 synthesis is also caused by conventional PKC isoforms, which may increase PIP5K activity by stimulating PIP5K dephosphorylation by the okadaic acid-sensitive protein phosphatase 1 (Park et al. 2001).
Concluding remarks
In the last decade, PLD has taken a firm position as all-round player in cellular signaling events. It is now appreciated that PLD and PIP5K act together to execute several important cellular functions, including vesicle transport, cytoskeleton dynamics and cell adhesion. Because of the reciprocal stimulation of their activities it seems inappropriate to generally assign a conventional “upstairs-downstairs” relationship to PLD and PIP5K isozymes. The localized generation of the lipid messengers by PLD and PIP5K, PA and PIP2, is clearly co-ordinated by small GTPases of the ARF, Rho and Ras families. The following picture emerges of how PLD and PIP5K may co-operate to execute their cellular tasks. Particular small GTPases, activated by membrane receptors or cellular factors, bind to PIP5K and recruit the enzyme to specific cellular compartments. Subsequent activation of PIP5K catalytic activity triggers the localized generation of PIP2, which now serves as an anchor for specific proteins, including PLD enzymes. The sequestered PLD is activated by PIP2 and the GTPases, and PLD-derived PA now, among other tasks, contributes to the activation of PIP5K. This feed-forward regulation loop depends on both PIP5K and PLD, and quenching of PA formation (by primary alcohols) or reduction of PIP2 levels (by PLC-mediated hydrolysis or dephosphorylation by phosphatases) can interrupt the snowball from rolling. PIP2 dephosphorylation may be important in the cell as a decisive mechanism to terminate the localized reactions before a cellular avalanche develops. Attractive candidates are further specific GEFs and GAPs for the GTPases, some of which have been shown to be directly regulated by PIP2. PIP2-dependent inactivation of the organizing GTPase may then provide the final turn-off signal.
Acknowledgements
The authors’ work reported herein was supported by the Deutsche Forschungsgemeinschaft and the IFORES program of the Universitätsklinikum Essen. Martina Schmidt is Rosalind Franklin Fellow at the University of Groningen.
References
- Aikawa Y, Martin TFJ. ARF6 regulates a plasma membrane pool of phosphatidylinositol(4,5)bisphosphate required for regulated exocytosis. J Cell Biol. 2003;162:647–659. doi: 10.1083/jcb.200212142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Boronenkov IV, Doughman SD, Kunz J, Loijens JC. Phosphatidylinositol phosphate kinases, a multifaceted family of signaling enzymes. J Biol Chem. 1999;274:9907–9910. doi: 10.1074/jbc.274.15.9907. [DOI] [PubMed] [Google Scholar]
- Arneson LS, Kunz J, Anderson RA, Traub LM. Coupled inositide phosphorylation and phospholipase D activation initiates clathrin-coat assembly on lysosomes. J Biol Chem. 1999;274:17794–17805. doi: 10.1074/jbc.274.25.17794. [DOI] [PubMed] [Google Scholar]
- Balboa MA, Insel PA. Stimulation of phospholipase D via α1-adrenergic receptors in Madin-Darby canine kidney cells is independent of PKCα and -ε activation. Mol Pharmacol. 1998;53:221–227. doi: 10.1124/mol.53.2.221. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Unlocking the secrets of cell signaling. Annu Rev Physiol. 2005;67:1–21. doi: 10.1146/annurev.physiol.67.040103.152647. [DOI] [PubMed] [Google Scholar]
- Bi K, Roth MG, Ktistakis NT. Phosphatidic acid formation by phospholipase D is required for transport from the endoplasmic reticulum to the Golgi complex. Curr Biol. 1997;7:301–307. doi: 10.1016/s0960-9822(06)00153-9. [DOI] [PubMed] [Google Scholar]
- Bocckino SB, Blackmore PF, Wilson PB, Exton JH. Phosphatidate accumulation in hormone-treated hepatocytes via a phospholipase D mechanism. J Biol Chem. 1987;262:15309–15315. [PubMed] [Google Scholar]
- Botelho RJ, Teruel M, Dierckman R, Anderson R, Wells A, York JD, Meyer T, Grinstein S. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J Cell Biol. 2000;151:1353–1367. doi: 10.1083/jcb.151.7.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HA, Gutowski S, Moomaw CR, Slaughter C, Sternweis PC. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- Brown FD, Thompson N, Saqib KM, Clark JM, Powner D, Thompson NT, Solari R, Wakelam MJO. Phospholipase D1 localises to secretory granules and lysosomes and is plasma-membrane translocated on cellular stimulation. Curr Biol. 1998;8:835–838. doi: 10.1016/s0960-9822(98)70326-4. [DOI] [PubMed] [Google Scholar]
- Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol. 2001;154:1007–1017. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JS, Exton JH. Regulation of phospholipase D2 activity by protein kinase Cα. J Biol Chem. 2004;279:22076–22083. doi: 10.1074/jbc.M311033200. [DOI] [PubMed] [Google Scholar]
- Chen YG, Siddhanta A, Austin CD, Hammond SM, Sung TC, Frohman MA, Morris AJ, Shields D. Phospholipase D stimulates release of nascent secretory vesicles from the trans-Golgi network. J Cell Biol. 1997;138:495–504. doi: 10.1083/jcb.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong LD, Traynor-Kaplan A, Bokoch GM, Schwartz MA. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994;79:507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Chung JK, Sekiya F, Kang HS, Lee C, Han JS, Kim SR, Bae YS, Morris AJ, Rhee SG. Synaptojanin inhibition of phospholipase D activity by hydrolysis of phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 1997;272:15980–15985. doi: 10.1074/jbc.272.25.15980. [DOI] [PubMed] [Google Scholar]
- Cochet C, Filhol O, Payrastre B, Hunter T, Gill GN. Interaction between the epidermal growth factor receptor and phosphoinositide kinases. J Biol Chem. 1991;266:637–644. [PubMed] [Google Scholar]
- Cockcroft S. Ca2+−dependent conversion of phosphatidylinositol to phosphatidate in neutrophils stimulated with fMet-Leu-Phe or ionophore A23187. Biochim Biophys Acta. 1984;795:37–46. doi: 10.1016/0005-2760(84)90102-4. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. Signalling roles of mammalian phospholipase D1 and D2. Cell Mol Life Sci. 2001;58:1674–1687. doi: 10.1007/PL00000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley WC, Sung TC, Roll R, Jenco J, Hammond SM, Altshuller Y, Bar-Sagi D, Morris AJ, Frohman MA. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr Biol. 1997;7:191–201. doi: 10.1016/s0960-9822(97)70090-3. [DOI] [PubMed] [Google Scholar]
- Coppolino MG, Dierckman R, Loijens J, Collins RF, Pouladi M, Jongstra-Bilen J, Schreiber AD, Trimble WS, Anderson R, Grinstein S. Inhibition of phosphatidylinositol-4-phosphate 5-kinase Iα impairs localized actin remodeling and suppresses phagocytosis. J Biol Chem. 2002;277:43849–43857. doi: 10.1074/jbc.M209046200. [DOI] [PubMed] [Google Scholar]
- Corrotte M, Chasserot-Golaz S, Huang P, Du G, Ktistakis NT, Frohman MA, Vitale N, Bader MF, Grant NJ. Dynamics and function of phospholipase D and phosphatidic acid during phagocytosis. Traffic. 2006;7:365–377. doi: 10.1111/j.1600-0854.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- Cross MJ, Roberts S, Ridley AJ, Hodgkin MN, Stewart A, Claesson-Welsh L, Wakelam MJO. Stimulation of actin stress fibre formation mediated by activation of phospholipase D. Curr Biol. 1996;6:588–597. doi: 10.1016/s0960-9822(02)00545-6. [DOI] [PubMed] [Google Scholar]
- Cummings RJ, Parinandi NL, Zaiman A, Wang L, Usatyuk PV, Garcia JGN, Natarajan V. Phospholipase D activation by sphingosine 1-phosphate regulates interleukin-8 secretion in human bronchial epithelial cells. J Biol Chem. 2002;277:30227–30235. doi: 10.1074/jbc.M111078200. [DOI] [PubMed] [Google Scholar]
- Czarny M, Lavie Y, Fiucci G, Liscovitch M. Localization of phospholipase D in detergent-insoluble, caveolin-rich membrane domains. J Biol Chem. 1999;274:2717–2724. doi: 10.1074/jbc.274.5.2717. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, Pellegrini L, Letinic K, Cestra G, Zoncu R, Voronov S, Chang S, Guo J, Wenk MR, De Camilli P. Recruitment and regulation of phosphatidylinositol phosphate kinase type Iγ by the FERM domain of talin. Nature. 2002;420:85–89. doi: 10.1038/nature01147. [DOI] [PubMed] [Google Scholar]
- Divecha N, Roefs M, Halstead JR, D’Andrea S, Fernandez-Borga M, Oomen L, Saqib KM, Wakelam MJO, D’Santos C. Interaction of the type Iα PIPkinase with phospholipase D: a role for the local generation of phosphatidylinositol 4,5-bisphosphate in the regulation of PLD2 activity. EMBO J. 2000;19:5440–5449. doi: 10.1093/emboj/19.20.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughman RL, Firestone AJ, Wojtasiak ML, Bunce MW, Anderson RA. Membrane ruffling requires coordination between type Iα phosphatidylinositol phosphate kinase and rac signaling. J Biol Chem. 2003;278:23036–23045. doi: 10.1074/jbc.M211397200. [DOI] [PubMed] [Google Scholar]
- Du G, Altshuller YM, Vitale N, Huang P, Chasserot-Golaz S, Morris AJ, Bader M-F, Frohman MA. Regulation of phospholipase D1 subcellular cycling through coordination of multiple membrane association motifs. J Cell Biol. 2003;162:305–315. doi: 10.1083/jcb.200302033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G, Huang P, Liang BT, Frohman MA. Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis. Mol Biol Cell. 2004;15:1024–1030. doi: 10.1091/mbc.E03-09-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton JH. Phospholipase D-structure, regulation and function. Rev Physiol Biochem Pharmacol. 2002;144:1–94. doi: 10.1007/BFb0116585. [DOI] [PubMed] [Google Scholar]
- Exton JH. Regulation of phospholipase D. FEBS Lett. 2002;531:58–61. doi: 10.1016/s0014-5793(02)03405-1. [DOI] [PubMed] [Google Scholar]
- Fahimi-Vahid M, Gosau N, Michalek C, Han L, Jakobs KH, Schmidt M, Roberts N, Avkiran M, Wieland T. Distinct signaling pathways mediate cardiomyocyte phospholipase D stimulation by endothelin-1 and thrombin. J Mol Cell Cardiol. 2002;34:441–453. doi: 10.1006/jmcc.2002.1525. [DOI] [PubMed] [Google Scholar]
- Fensome A, Cunningham E, Prosser S, Tan SK, Swigart P, Thomas G, Hsuan J, Cockcroft S. ARF and PITP restore GTPgammaS-stimulated protein secretion from cytosol-depleted HL60 cells by promoting PIP2 synthesis. Curr Biol. 1996;6:730–738. doi: 10.1016/s0960-9822(09)00454-0. [DOI] [PubMed] [Google Scholar]
- Fensome A, Whatmore J, Morgan C, Jones D, Cockcroft S. ADP-ribosylation factor and Rho proteins mediate fMLP-dependent activation of phospholipase D in human neutrophils. J Biol Chem. 1998;273:13157–13164. doi: 10.1074/jbc.273.21.13157. [DOI] [PubMed] [Google Scholar]
- Ford MGJ, Pearse BMF, Higgins MK, Vallis Y, Owen DJ, Gibson A, Hopkins CR, Evans PR, McMahon HT. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 2001;291:1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- Foster DA, Xu L. Phospholipase D in cell proliferation and cancer. Mol Cancer Res. 2003;1:789–800. [PubMed] [Google Scholar]
- Frankel P, Ramos M, Flom J, Bychenok S, Joseph T, Kerkhoff E, Rapp UR, Feig LA, Foster DA. Ral and Rho-dependent activation of phospholipase D in v-Raf-transformed cells. Biochem Biophys Res Commun. 1999;255:502–507. doi: 10.1006/bbrc.1999.0234. [DOI] [PubMed] [Google Scholar]
- Freyberg Z, Sweeney D, Siddhanta A, Bourgoin S, Frohman M, Shields D. Intracellular localization of phospholipase D1 in mammalian cells. Mol Biol Cell. 2001;12:943–955. doi: 10.1091/mbc.12.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galandrini R, Micucci F, Tassi I, Cifone MG, Cinque B, Piccoli M, Frati L, Santoni A. Arf6: a new player in FcγRIIIA lymphocyte-mediated cytotoxicity. Blood. 2005;106:577–583. doi: 10.1182/blood-2004-10-4100. [DOI] [PubMed] [Google Scholar]
- Galiano FJ, Ulug ET, Davis JN. Overexpression of murine phosphatidylinositol 4-phosphate 5-kinase type Iβ disrupts a phosphatidylinositol 4,5 bisphosphate regulated endosomal pathway. J Cell Biochem. 2002;85:131–145. [PubMed] [Google Scholar]
- Ginsberg J, Gupta S, Matowe WC, Kline L, Brindley DN. Activation of phospholipase D in FRTL-5 thyroid cells by forskolin and dibutyryl-cyclic adenosine monophosphate. Endocrinology. 1997;138:3645–3651. doi: 10.1210/endo.138.9.5365. [DOI] [PubMed] [Google Scholar]
- Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, Luini A, Corda D, De Matteis MA. ARF mediates recruitment of PtdIns-4-OH kinase-β and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- Gosau N, Fahimi-Vahid M, Michalek C, Schmidt M, Wieland T. Signalling components involved in the coupling of α1-adrenoceptors to phospholipase D in neonatal rat cardiac myocytes. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:468–476. doi: 10.1007/s00210-002-0546-x. [DOI] [PubMed] [Google Scholar]
- Gratacap M-P, Payrastre B, Nieswandt B, Offermanns S. Differential regulation of Rho and Rac through heterotrimeric G-proteins and cyclic nucleotides. J Biol Chem. 2001;276:47906–47913. doi: 10.1074/jbc.M104442200. [DOI] [PubMed] [Google Scholar]
- Grishanin RN, Kowalchyk JA, Klenchin VA, Ann K, Earles CA, Chapman ER, Gerona RR, Martin TF. CAPS act at a prefusion step in dense-core vesicle exocytosis as a PIP2 binding protein. Neuron. 2004;43:551–562. doi: 10.1016/j.neuron.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Grondin P, Plantavid M, Sultan C, Breton M, Mauco G, Chap H. Interaction of pp60c-src, phospholipase C, inositol-lipid, and diacylglycerol kinases with the cytoskeletons of thrombin-stimulated platelets. J Biol Chem. 1991;266:15705–15709. [PubMed] [Google Scholar]
- Ha KS, Exton JH. Activation of actin polymerization by phosphatidic acid derived from phosphatidylcholine in IIc9 fibroblasts. J Cell Biol. 1993;123:1789–1796. doi: 10.1083/jcb.123.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SM, Altshuller YM, Sung TC, Rudge SA, Rose K, Engebrecht J, Morris AJ, Frohman MA. Human ADP-ribosylation factor-activated phosphatidylcholine-specific phospholipase D defines a new and highly conserved gene family. J Biol Chem. 1995;270:29640–29643. doi: 10.1074/jbc.270.50.29640. [DOI] [PubMed] [Google Scholar]
- Hammond SM, Jenco JM, Nakashima S, Cadwallader K, Gu Q, Cook S, Nozawa Y, Prestwich GD, Frohman MA, Morris AJ. Characterization of two alternately spliced forms of phospholipase D1. Activation of the purified enzymes by phosphatidylinositol 4,5-bisphosphate, ADP-ribosylation factor, and Rho family monomeric GTP-binding proteins and protein kinase C-α. J Biol Chem. 1997;272:3860–3868. doi: 10.1074/jbc.272.6.3860. [DOI] [PubMed] [Google Scholar]
- Han L, Woznicki M, Limper B, Caracciola P, Oude Weernink PA, Jakobs KH, Mizuno K, Schmidt M. Involvement of Lim-kinase in stimulation of phospholipase D (PLD) by Rho and Rho-kinase. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:R61. [Google Scholar]
- Han L, Rother T, Eicken S, Kindhäuser F, Oude Weernink PA, Schmidt M, Jakobs KH. Role of Gα12 and Gα13 in RhoA- and ARF1-mediated signalling to phospholipase D by the M3 muscarinic receptor. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:R52. [Google Scholar]
- Hanahan DJ, Chaikoff IL. On the nature of the phosphorus-containing lipides of cabbage leaves and their relation to a phospholipide-splitting enzyme contained in these leaves. J Biol Chem. 1948;172:191–198. [PubMed] [Google Scholar]
- Hartwig JH, Bokoch GM, Carpenter CL, Janmey PA, Taylor LA, Toker A, Stossel TP. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995;82:643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Henage LG, Exton JH, Brown HA. Kinetic analysis of a mammalian phospholipase D. Allosteric modulation by monomeric GTPases, protein kinase C, and polyphosphoinositides. J Biol Chem. 2006;281:3408–3417. doi: 10.1074/jbc.M508800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess JA, Ross AH, Qiu RG, Symons M, Exton JH. Role of Rho family proteins in phospholipase D activation by growth factors. J Biol Chem. 1997;272:1615–1620. doi: 10.1074/jbc.272.3.1615. [DOI] [PubMed] [Google Scholar]
- Hilpela P, Vartiainen MK, Lappalainen P. Regulation of the actin cytoskeleton by PI(4,5)P2 and PI(3,4,5)P3. Curr Top Microbiol Immunol. 2004;282:117–163. doi: 10.1007/978-3-642-18805-3_5. [DOI] [PubMed] [Google Scholar]
- Hinchliffe KA, Ciruela A, Irvine RF. PIPkins, their substrates and their products: new functions for old enzymes. Biochim Biophys Acta. 1998;1436:87–104. doi: 10.1016/s0005-2760(98)00140-4. [DOI] [PubMed] [Google Scholar]
- Hodgkin MN, Masson MR, Powner D, Saqib KM, Ponting CP, Wakelam MJO. Phospholipase D regulation and localisation is dependent upon a phosphatidylinositol 4,5-bisphosphate-specific PH domain. Curr Biol. 2000;10:43–46. doi: 10.1016/s0960-9822(99)00264-x. [DOI] [PubMed] [Google Scholar]
- Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, Kawamoto K, Nakayama K, Morris AJ, Frohman MA, Kanaho Y. Phosphatidylinositol 4-phosphate 5-kinase α is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- Huang C, Hujer KM, Wu Z, Miller RT. The Ca2+-sensing receptor couples to Gα12/13 to activate phospholipase D in Madin-Darby canine kidney cells. Am J Physiol Cell Physiol. 2004;286:C22–C30. doi: 10.1152/ajpcell.00229.2003. [DOI] [PubMed] [Google Scholar]
- Hughes WE, Parker PJ. Endosomal localization of phospholipase D 1a and 1b is defined by the C-termini of the proteins, and is independent of activity. Biochem J. 2001;356:727–736. doi: 10.1042/0264-6021:3560727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes WE, Elgundi Z, Huang P, Frohman MA, Biden TJ. Phospholipase D1 regulates secretagogue-stimulated insulin release in pancreatic beta-cells. J Biol Chem. 2004;279:27534–27541. doi: 10.1074/jbc.M403012200. [DOI] [PubMed] [Google Scholar]
- Humeau Y, Vitale N, Chasserot-Golaz S, Du Dupont JLG, Frohman MA, Bader MF, Poulain B. A role for phospholipase D1 in neurotransmitter release. Proc Natl Acad Sci USA. 2001;98:15300–15305. doi: 10.1073/pnas.261358698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H, Shibasaki Y, Kizuki N, Katagiri H, Yazaki Y, Asano T, Oka Y. Cloning of cDNAs encoding two isoforms of 68-kDa type I phosphatidylinositol-4-phosphate 5-kinase. J Biol Chem. 1996;271:23611–23614. doi: 10.1074/jbc.271.39.23611. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Shibasaki Y, Kizuki N, Wada T, Yazaki Y, Asano T, Oka Y. Type I phosphatidylinositol-4-phosphate 5-kinases. J Biol Chem. 1998;273:8741–8748. doi: 10.1074/jbc.273.15.8741. [DOI] [PubMed] [Google Scholar]
- Itoh T, Koshiba S, Kigawa T, Kikuchi A, Yokoyama S, Takenawa T. Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science. 2001;291:1047–1051. doi: 10.1126/science.291.5506.1047. [DOI] [PubMed] [Google Scholar]
- Iyer SS, Barton JA, Bourgoin S, Kusner DJ. Phospholipases D1 and D2 coordinately regulate macrophage phagocytosis. J Immunol. 2004;173:2615–2623. doi: 10.4049/jimmunol.173.4.2615. [DOI] [PubMed] [Google Scholar]
- Iyer SS, Agrawal RS, Thompson CR, Thompson S, Barton JA, Kusner DJ. Phospholipase D1 regulates phagocyte adhesion. J Immunol. 2006;176:3686–3696. doi: 10.4049/jimmunol.176.6.3686. [DOI] [PubMed] [Google Scholar]
- Jenkins GM, Frohman MA. Phospholipase D: a lipid centric review. Cell Mol Life Sci. 2005;62:2305–2316. doi: 10.1007/s00018-005-5195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins GH, Fisette PL, Anderson RA. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J Biol Chem. 1994;269:11547–11554. [PubMed] [Google Scholar]
- Jones DH, Morris JB, Morgan CP, Kondo H, Irvine RF, Cockcroft S. Type I phosphatidylinositol 4-phosphate 5-kinase directly interacts with ADP-ribosylation factor I and is responsible for phosphatidylinositol 4,5-bisphosphate synthesis in the golgi compartment. J Biol Chem. 2000;275:13962–13966. doi: 10.1074/jbc.c901019199. [DOI] [PubMed] [Google Scholar]
- Jones DR, Sanjuan MA, Mérida I. Type Iα phosphatidylinositol 4-phosphate 5-kinase is a putative target for increased intracellular phosphatidic acid. FEBS Lett. 2000;476:160–165. doi: 10.1016/s0014-5793(00)01702-6. [DOI] [PubMed] [Google Scholar]
- Kam Y, Exton JH. Phospholipase D activity is required for actin stress fiber formation in fibroblasts. Mol Cell Biol. 2001;21:4055–4066. doi: 10.1128/MCB.21.12.4055-4066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam Y, Exton JH. Role of phospholipase D1 in the regulation of mTOR activity by lysophosphatidic acid. FASEB J. 2004;18:311–319. doi: 10.1096/fj.03-0731com. [DOI] [PubMed] [Google Scholar]
- Kam JL, Miura K, Jackson TR, Gruschus J, Roller P, Stauffer S, Clark J, Aneja R, Randazzo PA. Phosphoinositide-dependent activation of the ADP-ribosylation factor GTPase-activating protein ASAP1. J Biol Chem. 2000;275:9653–9663. doi: 10.1074/jbc.275.13.9653. [DOI] [PubMed] [Google Scholar]
- Keller J, Schmidt M, Hussein B, Rümenapp U, Jakobs KH. Muscarinic receptor-stimulated cytosol-membrane translocation of RhoA. FEBS Lett. 1997;403:299–302. doi: 10.1016/s0014-5793(97)00067-7. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee SD, Han JM, Lee TG, Kim Y, Park JB, Lambeth JD, Suh PG, Ryu SH. Activation of phospholipase D1 by direct interaction with ADP-ribosylation factor 1 and RalA. FEBS Lett. 1998;430:231–235. doi: 10.1016/s0014-5793(98)00661-9. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim HW, Jeon H, Suh PG, Ryu SH. Phospholipase D1 regulates cell migration in a lipase activity-independent manner. J Biol Chem. 2006;281:15747–15756. doi: 10.1074/jbc.M509844200. [DOI] [PubMed] [Google Scholar]
- Kisseleva M, Feng Y, Ward M, Song C, Anderson RA, Longmore GD. The LIM prorein Ajuba regulates phosphatidylinositol 4,5-bisphosphate levels in migrating cells through an interaction with and activation of PIPKIα. Mol Cell Biol. 2005;25:3956–3966. doi: 10.1128/MCB.25.10.3956-3966.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarlund JK, Rameh LE, Cantley LC, Buxton JM, Holik JJ, Sakelis C, Patki V, Corvera S, Czech MP. Regulation of GRP1-catalyzed ADP ribosylation factor guanine nucleotide exchange by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:1859–1862. doi: 10.1074/jbc.273.4.1859. [DOI] [PubMed] [Google Scholar]
- Koch T, Brandenburg LO, Schulz S, Liang Y, Klein J, Höllt V. ADP-ribosylation factor-dependent phospholipase D2 activation is required for agonist-induced μ-opioid receptor endocytosis. J Biol Chem. 2003;278:9979–9985. doi: 10.1074/jbc.M206709200. [DOI] [PubMed] [Google Scholar]
- Koch T, Wu DF, Yang LQ, Brandenburg LO, Höllt V. Role of phospholipase D2 in the agonist-induced and constitutive endocytosis of G-protein coupled receptors. J Neurochem. 2006;97:365–372. doi: 10.1111/j.1471-4159.2006.03736.x. [DOI] [PubMed] [Google Scholar]
- Komati H, Naro F, Mebarek S, De Arcangelis V, Adamo S, Lagarde M, Prigent AF, Némoz G. Phospholipase D is involved in myogenic differentiation through remodeling of actin cytoskeleton. Mol Biol Cell. 2005;16:1232–1244. doi: 10.1091/mbc.E04-06-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss M, Kinuta M, Wenk MR, De Camilli P, Takei K, Haucke V. ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Iγ. J Cell Biol. 2003;162:113–124. doi: 10.1083/jcb.200301006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktistakis NT, Brown HA, Waters MG, Sternweis PC, Roth MG. Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz J, Fuelling A, Kolbe L, Anderson RA. Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a single amino acid residue. J Biol Chem. 2002;277:5611–5619. doi: 10.1074/jbc.M110775200. [DOI] [PubMed] [Google Scholar]
- Kusner DJ, Barton JA, Wen KK, Wang X, Rubenstein PA, Iyer SS. Regulation of phospholipase D activity by actin. Actin exerts bidirectional modulation of mammalian phospholipase D activity in a polymerization-dependent, isoform-specific manner. J Biol Chem. 2002;277:50683–50692. doi: 10.1074/jbc.M209221200. [DOI] [PubMed] [Google Scholar]
- Laux T, Fukami K, Thelen M, Golub T, Frey D, Caroni P. GAP43, MARCKS, and CAP23 modulate PI(4,5)P2 at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J Cell Biol. 2000;149:1455–1471. doi: 10.1083/jcb.149.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JTR, Birnbaum MJ. ADP-ribosylation factor 6 regulates insulin secretion through plasma membrane phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA. 2003;100:13320–13325. doi: 10.1073/pnas.2232129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Stunff H, Dokhac L, Bourgoin S, Bader MF, Harbon S. Phospholipase D in rat myometrium: occurrence of a membrane-bound ARF6 (ADP-ribosylation factor 6)-regulated activity controlled by βγ subunits of heterotrimeric G-proteins. Biochem J. 2000;352:491–499. doi: 10.1042/0264-6021:3520491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Park JB, Kim JH, Kim Y, Kim JH, Shin KJ, Lee JS, Ha SH, Suh PG, Ryu SH. Actin directly interacts with phospholipase D, inhibiting its activity. J Biol Chem. 2001;276:28252–28260. doi: 10.1074/jbc.M008521200. [DOI] [PubMed] [Google Scholar]
- Lee JS, Kim JH, Jang IH, Kim HS, Han JM, Kazlauskas A, Yagisawa H, Suh PG, Ryu SH. Phosphatidylinositol (3,4,5)-trisphosphate specifically interacts with the phox homology domain of phospholipase D1 and stimulates its activity. J Cell Sci. 2005;118:4405–4413. doi: 10.1242/jcs.02564. [DOI] [PubMed] [Google Scholar]
- Lee CS, Kim IS, Park JB, Lee MN, Lee HY, Suh PG, Ryu SH. The phox homology domain of phospholipase D activates dynamin GTPase activity and accelerates EGFR endocytosis. Nat Cell Biol. 2006;8:477–484. doi: 10.1038/ncb1401. [DOI] [PubMed] [Google Scholar]
- Ling K, Doughman RL, Firestone AJ, Bunce MW, Anderson RA. Type Iγ phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature. 2002;420:89–93. doi: 10.1038/nature01082. [DOI] [PubMed] [Google Scholar]
- Ling K, Schill NJ, Wagoner MP, Sun Y, Anderson RA. Movin´ on up: the role of PtdIns(4,5)P2 in cell migration. Trends Cell Biol. 2006;16:276–284. doi: 10.1016/j.tcb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Liscovitch M, Chalifa V, Pertile P, Chen CS, Cantley LC. Novel function of phosphatidylinositol 4,5-bisphosphate as a cofactor for brain membrane phospholipase D. J Biol Chem. 1994;269:21403–21406. [PubMed] [Google Scholar]
- Liscovitch M, Czarny M, Fiucci G, Tang X. Phospholipase D: molecular and cell biology of a novel gene family. Biochem J. 2000;345:401–415. [PMC free article] [PubMed] [Google Scholar]
- Loijens JC, Anderson RA. Type I phosphatidylinositol-4-phosphate 5-kinases are distinct members of this novel lipid kinase family. J Biol Chem. 1996;271:32937–32943. doi: 10.1074/jbc.271.51.32937. [DOI] [PubMed] [Google Scholar]
- López de Jesús M, Stope MB, Oude Weernink PA, Mahlke Y, Börgermann C, Ananaba VN, Rimmbach C, Rosskopf D, Michel MC, Jakobs KH, Schmidt M. Cyclic AMP-dependent and Epac-mediated activation of R-Ras by G protein-coupled receptors leads to phospholipase D stimulation. J Biol Chem. 2006;281:21837–21847. doi: 10.1074/jbc.M604156200. [DOI] [PubMed] [Google Scholar]
- Lu ZM, Hornia A, Joseph T, Sukezane T, Frankel P, Zhong M, Bychenok S, Xu LZ, Feig LA, Foster DA. Phospholipase D and RalA cooperate with the epidermal growth factor receptor to transform 3Y1 rat fibroblasts. Mol Cell Biol. 2000;20:462–467. doi: 10.1128/mcb.20.2.462-467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas L, del Peso L, Rodriguez P, Penalva V, Lacal JC. Ras protein is involved in the physiological regulation of phospholipase D by platelet-derived growth factor. Oncogene. 2000;19:431–437. doi: 10.1038/sj.onc.1203323. [DOI] [PubMed] [Google Scholar]
- Lukowski S, Mira JP, Zachowski A, Geny B. Fodrin inhibits phospholipases A2, C, and D by decreasing polyphosphoinositide cell content. Biochem Biophys Res Commun. 1998;248:278–284. doi: 10.1006/bbrc.1998.8942. [DOI] [PubMed] [Google Scholar]
- Majerus PW, Kisseleva MV, Norris FA. The role of phosphatases in inositol signaling reactions. J Biol Chem. 1999;274:10669–10672. doi: 10.1074/jbc.274.16.10669. [DOI] [PubMed] [Google Scholar]
- Matsui T, Yonemura S, Tsukita S, Tsukita S. Activation of ERM proteins in vivo by Rho involves phosphatidylinositol 4-phosphate 5-kinase and not ROCK kinases. Curr Biol. 1999;9:1259–1262. doi: 10.1016/s0960-9822(99)80508-9. [DOI] [PubMed] [Google Scholar]
- Meacci E, Becciolini L, Nuti F, Donati C, Cencetti F, Farnararo M, Bruni P. A role for calcium in sphingosine 1-phosphate-induced phospholipase D activity in C2C12 myoblasts. FEBS Lett. 2002;521:200–204. doi: 10.1016/s0014-5793(02)02866-1. [DOI] [PubMed] [Google Scholar]
- Mitchell R, McCulloch D, Lutz E, Johnson M, MacKenzie C, Fennell M, Fink G, Zhou W, Sealfon SC. Rhodopsin-family receptors associate with small G proteins to activate phospholipase D. Nature. 1998;392:411–414. doi: 10.1038/32937. [DOI] [PubMed] [Google Scholar]
- Moritz A, De Graan PNE, Gispen WH, Wirtz KWA. Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. J Biol Chem. 1992;267:7207–7210. [PubMed] [Google Scholar]
- Muthalif MM, Parmentier JH, Benter IF, Karzoun N, Ahmed A, Khandekar Z, Adl MZ, Bourgoin S, Malik KU. Ras/mitogen-activated protein kinase mediates norepinephrine-induced phospholipase D activation in rabbit aortic smooth muscle cells by a phosphorylation-dependent mechanism. J Pharmacol Exp Ther. 2000;293:268–274. [PubMed] [Google Scholar]
- Nie Z, Stanley KT, Stauffer S, Jacques KM, Hirsch DS, Takei J, Randazzo PA. AGAP1, an endosome-associated, phosphoinositide-dependent ADP-ribosylation factor GTPase-activating protein that affects actin cytoskeleton. J Biol Chem. 2002;277:48965–48975. doi: 10.1074/jbc.M202969200. [DOI] [PubMed] [Google Scholar]
- Niggli V. Regulation of protein activities by phosphoinositide phosphates. Annu Rev Cell Dev Biol. 2005;21:57–79. doi: 10.1146/annurev.cellbio.21.021704.102317. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Discovery and prospect of protein kinase C research: epilogue. J Biochem. 2003;133:155–158. doi: 10.1093/jb/mvg035. [DOI] [PubMed] [Google Scholar]
- Nolan RD, Lapetina EG. Thrombin stimulates the production of a novel polyphosphoinositide in human platelets. J Biol Chem. 1990;265:2441–2445. [PubMed] [Google Scholar]
- Offermanns S, Wieland T, Homann D, Sandmann J, Bombien E, Spicher K, Schultz G, Jakobs KH. Transfected muscarinic acetylcholine receptors selectively couple to Gi-type G proteins and Gq/11. Mol Pharmacol. 1994;45:890–898. [PubMed] [Google Scholar]
- Oishi K, Takahashi M, Mukai H, Banno Y, Nakashima S, Kanaho Y, Nozawa Y, Ono Y. PKN regulates phospholipase D1 through direct interaction. J Biol Chem. 2001;276:18096–18101. doi: 10.1074/jbc.M010646200. [DOI] [PubMed] [Google Scholar]
- Ojio K, Banno Y, Nakashima S, Kato N, Watanabe K, Lyerly DM, Miyata H, Nozawa Y. Effect of Clostridium difficile toxin B on IgE receptor-mediated signal transduction in rat basophilic leukemia cells: inhibition of phospholipase D activation. Biochem Biophys Res Commun. 1996;224:591–596. doi: 10.1006/bbrc.1996.1069. [DOI] [PubMed] [Google Scholar]
- Oude Weernink PA, Schulte P, Guo Y, Wetzel J, Amano M, Kaibuchi K, Haverland S, Voss M, Schmidt M, Mayr GW, Jakobs KH. Stimulation of phosphatidylinositol-4-phosphate 5-kinase by Rho-kinase. J Biol Chem. 2000;275:10168–10174. doi: 10.1074/jbc.275.14.10168. [DOI] [PubMed] [Google Scholar]
- Oude Weernink PA, Euteneuer S, Langemeyer K, Schmidt M, Jakobs KH. Regulation of PIP2 synthesis by muscarinic receptors and conventional protein kinase C isozymes. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:R50. [Google Scholar]
- Oude Weernink PA, Meletiadis K, Hommeltenberg S, Hinz M, Ishihara H, Schmidt M, Jakobs KH. Activation of type I phosphatidylinositol-4-phosphate 5-kinase isoforms by the Rho GTPases, RhoA, Rac1, and Cdc42. J Biol Chem. 2004;279:7840–7849. doi: 10.1074/jbc.M312737200. [DOI] [PubMed] [Google Scholar]
- Oude Weernink PA, Schmidt M, Jakobs KH. Regulation and cellular roles of phosphoinositide 5-kinases. Eur J Pharmacol. 2004;500:87–99. doi: 10.1016/j.ejphar.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Padrón D, Wang YJ, Yamamoto M, Yin H, Roth MG. Phosphatidylinositol phosphate 5-kinase Iβ recruits AP-2 to the plasma membrane and regulates rates of constitutive endocytosis. J Cell Biol. 2003;162:693–701. doi: 10.1083/jcb.200302051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrón D, Tall RD, Roth MG. Phospholipase D2 is required for efficient endocytic recycling of transferrin receptors. Mol Biol Cell. 2006;17:598–606. doi: 10.1091/mbc.E05-05-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris S, Béraud-Dufour S, Robineau S, Bigay J, Antonny B, Chabre M, Chardin P. Role of protein-phospholipid interactions in the activation of ARF1 by the guanine nucleotide exchange factor Arno. J Biol Chem. 1997;272:22221–22226. doi: 10.1074/jbc.272.35.22221. [DOI] [PubMed] [Google Scholar]
- Park SK, Min DS, Exton JH. Definition of the protein kinase C interaction site of phospholipase D. Biochem Biophys Res Commun. 1998;244:364–367. doi: 10.1006/bbrc.1998.8275. [DOI] [PubMed] [Google Scholar]
- Park JB, Kim JH, Kim Y, Ha SH, Yoo JS, Du G, Frohman MA, Suh PG, Ryu SH. Cardiac phospholipase D2 localizes to sarcolemmal membranes and is inhibited by α-actinin in an ADP-ribosylation factor-reversible manner. J Biol Chem. 2000;275:21295–21301. doi: 10.1074/jbc.M002463200. [DOI] [PubMed] [Google Scholar]
- Park SJ, Itoh T, Takenawa T. Phosphatidylinositol 4-phosphate 5-kinase type I is regulated through phosphorylation response by extracellular stimuli. J Biol Chem. 2001;276:4781–4787. doi: 10.1074/jbc.M010177200. [DOI] [PubMed] [Google Scholar]
- Parmentier JH, Ahmed A, Ruan Y, Gandhi GK, Saeed AE, Malik KU. Calcium and protein kinase C (PKC)-related kinase mediate α1A-adrenergic receptor-stimulated activation of phospholipase D in rat-1 cells, independent of PKC. J Pharmacol Exp Ther. 2002;303:1206–1215. doi: 10.1124/jpet.102.041384. [DOI] [PubMed] [Google Scholar]
- Parmryd I, Adler J, Patel R, Magee AI. Imaging metabolism of phosphatidylinositol 4,5-bisphosphate in T-cell GM1-enriched domains containing Ras proteins. Exp Cell Res. 2003;285:27–38. doi: 10.1016/s0014-4827(02)00048-4. [DOI] [PubMed] [Google Scholar]
- Payrastre B, Van Bergen en Henegouwen PMP, Breton M, den Hartigh JC, Plantavid M, Verkleij AJ, Boonstra J. Phosphoinositide kinase, diacylglycerol kinase, and phospholipase C activities associated to the cytoskeleton: effect of epidermal growth factor. J Cell Biol. 1991;115:121–128. doi: 10.1083/jcb.115.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta EG, Ashkenazi A, Winslow JW, Ramachandran J, Capon DJ. Differential regulation of PI hydrolysis and adenylyl cyclase by muscarinic receptor subtypes. Nature. 1988;334:434–437. doi: 10.1038/334434a0. [DOI] [PubMed] [Google Scholar]
- Pertile P, Liscovitch M, Chalifa V, Cantley LC. Phosphatidylinositol 4,5-bisphosphate synthesis is required for activation of phospholipase D in U937 cells. J Biol Chem. 1995;270:5130–5135. doi: 10.1074/jbc.270.10.5130. [DOI] [PubMed] [Google Scholar]
- Pike LJ, Casey L. Localization and turnover of phosphatidylinositol 4,5-bisphosphate in caveolin-enriched membrane domains. J Biol Chem. 1996;271:26453–26456. doi: 10.1074/jbc.271.43.26453. [DOI] [PubMed] [Google Scholar]
- Pike LJ, Eakes AT. Epidermal growth factor stimulates the production of phosphatidylinositol monophosphate and the breakdown of polyphosphoinositides in A431 cells. J Biol Chem. 1987;262:1644–1651. [PubMed] [Google Scholar]
- Plonk SG, Park SK, Exton JH. The α-subunit of the heterotrimeric G protein G13 activates a phospholipase D isozyme by a pathway requiring Rho family GTPases. J Biol Chem. 1998;273:4823–4826. doi: 10.1074/jbc.273.9.4823. [DOI] [PubMed] [Google Scholar]
- Porcelli AM, Ghelli A, Hrelia S, Rugolo M. Phospholipase D stimulation is required for sphingosine-1-phosphate activation of actin stress fibre assembly in human airway epithelial cells. Cell Signal. 2002;14:75–81. doi: 10.1016/s0898-6568(01)00222-4. [DOI] [PubMed] [Google Scholar]
- Powner DJ, Wakelam MJO. The regulation of phospholipase D by inositol phospholipids and small GTPases. FEBS Lett. 2002;531:62–64. doi: 10.1016/s0014-5793(02)03410-5. [DOI] [PubMed] [Google Scholar]
- Powner DJ, Payne RM, Pettitt TR, Giudici ML, Irvine RF, Wakelam MJO. Phospholipase D2 stimulates integrin-mediated adhesion via phosphatidylinositol 4-phosphate 5-kinase Iγb. J Cell Sci. 2005;118:2975–2986. doi: 10.1242/jcs.02432. [DOI] [PubMed] [Google Scholar]
- Preininger AM, Henage LG, Oldham WM, Yoon EJ, Hamm HE, Brown HA. Direct modulation of phospholipase D activity by Gβγ. Mol Pharmacol. 2006;70:311–318. doi: 10.1124/mol.105.021451. [DOI] [PubMed] [Google Scholar]
- Ren X-D, Bokoch GM, Traynor-Kaplan A, Jenkins GH, Anderson RA, Schwartz MA. Physical association of the small GTPase Rho with a 68-kDa phosphatidylinositol 4-phosphate 5-kinase in Swiss 3T3 cells. Mol Biol Cell. 1996;7:435–442. doi: 10.1091/mbc.7.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rümenapp U, Geiszt M, Wahn F, Schmidt M, Jakobs KH. Evidence for ADP-ribosylation-factor-mediated activation of phospholipase D by m3 muscarinic acetylcholine receptor. Eur J Biochem. 1995;234:240–244. doi: 10.1111/j.1432-1033.1995.240_c.x. [DOI] [PubMed] [Google Scholar]
- Rümenapp U, Schmidt M, Wahn F, Tapp E, Grannass A, Jakobs KH. Characteristics of protein-kinase-C- and ADP-ribosylation-factor-stimulated phospholipase D activities in human embryonic kidney cells. Eur J Biochem. 1997;248:407–414. doi: 10.1111/j.1432-1033.1997.00407.x. [DOI] [PubMed] [Google Scholar]
- Rümenapp U, Asmus M, Schablowski H, Woznicki M, Han L, Jakobs KH, Fahimi-Vahid M, Michalek C, Wieland T, Schmidt M. The M3 muscarinic acetylcholine receptor expressed in HEK-293 cell signals to phospholipase D via G12 but not Gq-type G proteins. Regulators of G proteins as tools to dissect pertussis toxin-resistant G proteins in receptor-effector coupling. J Biol Chem. 2001;4:2474–2479. doi: 10.1074/jbc.M004957200. [DOI] [PubMed] [Google Scholar]
- Santarius M, Lee CH, Anderson RA. Supervised membrane swimming: small G-protein lifeguards regulate PIPK signalling and monitor intracellular PtdIns(4,5)P2 pools. Biochem J. 2006;398:1–13. doi: 10.1042/BJ20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Hüwe SM, Fasselt B, Homann D, Rümenapp U, Sandmann J, Jakobs KH. Mechanisms of phospholipase D stimulation by m3 muscarinic acetylcholine receptors. Evidence for involvement of tyrosine phosphorylation. Eur J Biochem. 1994;225:667–675. doi: 10.1111/j.1432-1033.1994.00667.x. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Bienek C, Rümenapp U, Zhang C, Lümmen G, Jakobs KH, Just I, Aktories K, Moos M, von Eichel-Streiber C. A role for Rho in receptor- and G protein -stimulated phospholipase C. Reduction in phosphatidylinositol 4,5-bisphosphate by Clostridium difficile toxin B. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:87–94. doi: 10.1007/BF00178707. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Nehls C, Rümenapp U, Jakobs KH. m3 Muscarinic receptor-induced and Gi-mediated heterologous potentiation of phospholipase C stimulation: role of phosphoinositide synthesis. Mol Pharmacol. 1996;50:1038–1046. [PubMed] [Google Scholar]
- Schmidt M, Rümenapp U, Bienek C, Keller J, von Eichel-Streiber C, Jakobs KH. Inhibition of receptor signaling to phospholipase D by Clostridium difficile toxin B. Role of Rho proteins. J Biol Chem. 1996;271:2422–2426. doi: 10.1074/jbc.271.5.2422. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Rümenapp U, Nehls C, Ott S, Keller J, von Eichel-Streiber C, Jakobs KH. Restoration of Clostridium difficile toxin-B-inhibited phospholipase D by phosphatidylinositol 4,5-bisphosphate. Eur J Biochem. 1996;240:707–712. doi: 10.1111/j.1432-1033.1996.0707h.x. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Voss M, Thiel M, Bauer B, Grannass A, Tapp E, Cool RH, de Gunzburg J, von Eichel-Streiber C, Jakobs KH. Specific inhibition of phorbol ester-stimulated phospholipase D by Clostridium sordellii lethal toxin and Clostridium difficile toxin B-1470 in HEK-293 cells. J Biol Chem. 1998;273:7413–7422. doi: 10.1074/jbc.273.13.7413. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Voss M, Oude Weernink PA, Wetzel J, Amano M, Kaibuchi K, Jakobs KH. A role for Rho-kinase in Rho-controlled phospholipase D stimulation by the m3 muscarinic acetylcholine receptor. J Biol Chem. 1999;274:14648–14654. doi: 10.1074/jbc.274.21.14648. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Oude Weernink PA, vom Dorp F, Stope MB, Jakobs KH. Mammalian phospholipase C. Adv Mol Cell Biol. 2004;33:431–450. doi: 10.1016/S1569-2558(03)33021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann A, Schmidt M, Asmus M, Bayer S, Fliegert F, Koling S, Maßmann S, Schilf C, Subauste MC, Voß M, Jakobs KH, Joost HG. The ADP-ribosylation factor (ARF)-related GTPase ARF-related protein binds to the ARF-specific guanine nucleotide exchange factor cytohesin and inhibits the ARF-dependent activation of phospholipase D. J Biol Chem. 1999;274:9744–9751. doi: 10.1074/jbc.274.14.9744. [DOI] [PubMed] [Google Scholar]
- Sciorra VA, Rudge SA, Prestwich GD, Frohman MA, Engebrecht J, Morris AJ. Identification of a phosphoinositide binding motif that mediates activation of mammalian and yeast phospholipase D isoenzymes. EMBO J. 1999;20:5911–5921. doi: 10.1093/emboj/18.21.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciorra VA, Rudge SA, Wang J, McLaughlin S, Engebrecht J, Morris AJ. Dual role for phosphoinositides in regulation of yeast and mammalian phospholipase D enzymes. J Cell Biol. 2002;159:1039–1049. doi: 10.1083/jcb.200205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CC, Dobson W, Botelho RJ, Coady-Osberg N, Chavrier P, Knecht DA, Heath C, Stahl P, Grinstein S. Phosphatidylinositol-4,5-bisphosphate hydrolysis directs actin remodeling during phagocytosis. J Cell Biol. 2005;169:139–149. doi: 10.1083/jcb.200412162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki Y, Ishihara H, Kizuki N, Asano T, Oka Y, Yazaki Y. Massive actin polymerization induced by phosphatidylinositol-4-phosphate 5-kinase in vivo. J Biol Chem. 1997;272:7578–7581. doi: 10.1074/jbc.272.12.7578. [DOI] [PubMed] [Google Scholar]
- Shome K, Rizzo MA, Vasudevan C, Andresen B, Romero G. The activation of phospholipase D by endothelin-1, angiotensin II, and platelet-derived growth factor in vascular smooth muscle A10 cells is mediated by small G proteins of the ADP-ribosylation factor family. Endocrinology. 2000;141:2200–2208. doi: 10.1210/endo.141.6.7517. [DOI] [PubMed] [Google Scholar]
- Skippen A, Jones DH, Morgan CP, Li M, Cockcroft S. Mechanism of ADP ribosylation factor-stimulated phosphatidylinositol 4,5-bisphosphate synthesis in HL60 cells. J Biol Chem. 2002;277:5823–5831. doi: 10.1074/jbc.M110274200. [DOI] [PubMed] [Google Scholar]
- Stahelin RV, Ananthanarayanan B, Blatner NR, Singh S, Bruzik KS, Murray D, Cho W. Mechanism of membrane binding of the phospholipase D1 PX domain. J Biol Chem. 2004;279:54918–54926. doi: 10.1074/jbc.M407798200. [DOI] [PubMed] [Google Scholar]
- Steed PM, Clark KL, Boyar WC, Lasala DJ. Characterization of human PLD2 and the analysis of PLD isoform splice variants. FASEB J. 1998;12:1309–1317. doi: 10.1096/fasebj.12.13.1309. [DOI] [PubMed] [Google Scholar]
- Stephens L, Jackson TR, Hawkins PT. Activation of phosphatidylinositol 4,5-bisphosphate supply by agonists and non-hydrolysable GTP analogues. Biochem J. 1993;296:481–488. doi: 10.1042/bj2960481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Sung TC, Zhang Y, Morris AJ, Frohman MA. Structural analysis of human phospholipase D1. J Biol Chem. 1999;274:3659–3666. doi: 10.1074/jbc.274.6.3659. [DOI] [PubMed] [Google Scholar]
- Sweeney DA, Siddhanta A, Shields D. Fragmentation and re-assembly of the Golgi apparatus in vitro. A requirement for phosphatidic acid and phosphatidylinositol 4,5-bisphosphate synthesis. J Biol Chem. 2002;277:3030–3039. doi: 10.1074/jbc.M104639200. [DOI] [PubMed] [Google Scholar]
- Tall EG, Spector I, Pentyala SN, Bitter I, Rebecchi MJ. Dynamics of phosphatidylinositol 4,5-bisphosphate in actin-rich structures. Curr Biol. 2000;10:743–746. doi: 10.1016/s0960-9822(00)00541-8. [DOI] [PubMed] [Google Scholar]
- Toker A. Phosphoinositides and signal transduction. Cell Mol Life Sci. 2002;59:761–779. doi: 10.1007/s00018-002-8465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias KF, Hartwig JH, Ishihara H, Shibasaki Y, Cantley LC, Carpenter CL. Type Iα phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr Biol. 2000;10:153–156. doi: 10.1016/s0960-9822(00)00315-8. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Alexander RW, Akers M, Lyons PR, Lassègue B, Griendling KK (1999) Angiotensin II receptor coupling to phospholipase D is mediated by the bg subunits of heterotrimeric G proteins in vascular smooth muscle cells. Mol Pharmacol 55:142–149 [DOI] [PubMed]
- van Hennik PB, ten Klooster JP, Halstead JR, Voermans C, Anthony EC, Divecha N, Hordijk PL. The C-terminal domain of Rac1 contains two motifs that control targeting and signaling specificity. J Biol Chem. 2003;278:39166–39175. doi: 10.1074/jbc.M307001200. [DOI] [PubMed] [Google Scholar]
- van Horck FPG, Lavazais E, Eickholt BJ, Moolenaar WH, Divecha N. Essential role of type 1α phosphatidylinositol 4-phosphate 5-kinase in neurite remodeling. Curr Biol. 2002;12:241–245. doi: 10.1016/s0960-9822(01)00660-1. [DOI] [PubMed] [Google Scholar]
- van Rheenen J, Achame EM, Janssen H, Calafat J, Jalink K. PIP2 signaling in lipid domains: a critical re-evaluation. EMBO J. 2005;24:1664–1673. doi: 10.1038/sj.emboj.7600655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- Vitale N, Caumont A-S, Chasserot-Golaz S, Du G, Wu S, Sciorra VA, Morris AJ, Frohman MA, Bader M-F. Phospholipase D1: a key factor for the exocytotic machinery in neuroendocrine cells. EMBO J. 2001;20:2424–2434. doi: 10.1093/emboj/20.10.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M, Oude Weernink PA, Haupenthal S, Möller U, Cool RH, Bauer B, Camonis JH, Jakobs KH, Schmidt M. Phospholipase D stimulation by receptor tyrosine kinases mediated by protein kinase C and a Ras/Ral signaling cascade. J Biol Chem. 1999;274:34691–34698. doi: 10.1074/jbc.274.49.34691. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Li WH, Wang J, Xu K, Dong P, Luo X, Yin HL. Critical role of PIP5KIγ87 in InsP3-mediated Ca2+ signaling. J Cell Biol. 2004;167:1005–1010. doi: 10.1083/jcb.200408008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselle L, Gerona RRL, Vitale N, Martin TFJ, Bader MF, Regazzi R. Role of phosphoinositide signaling in the control of insulin exocytosis. Mol Endocrinol. 2005;19:3097–3106. doi: 10.1210/me.2004-0530. [DOI] [PubMed] [Google Scholar]
- Way G, O’Luanaigh N, Cockcroft S. Activation of exocytosis by cross-linking of the IgE receptor is dependent on ADP-ribosylation factor 1-regulated phospholipase D in RBL-2H3 mast cells: evidence that the mechanism of activation is via regulation of phosphatidylinositol 4,5-bisphosphate. Biochem J. 2000;346:63–70. [PMC free article] [PubMed] [Google Scholar]
- Wenk MR, Pellegrini L, Klenchin VA, Di Paolo G, Chang S, Daniell L, Arioka M, Martin TF, De Camilli P. PIP kinase Iγ is the major PI(4,5)P2 synthesizing enzyme at the synapse. Neuron. 2001;32:79–88. doi: 10.1016/s0896-6273(01)00456-1. [DOI] [PubMed] [Google Scholar]
- Wilde C, Barth H, Sehr P, Han L, Schmidt M, Just I, Aktories K. Interaction of the Rho-ADP-ribosylating C3 exoenzyme with RalA. J Biol Chem. 2002;277:14771–14776. doi: 10.1074/jbc.M201072200. [DOI] [PubMed] [Google Scholar]
- Wong KW, Isberg RR. Arf6 and phosphoinositol-4-phosphate-5-kinase activities permit bypass of the Rac1 requirement for β1 integrin-mediated bacterial uptake. J Exp Med. 2003;198:603–614. doi: 10.1084/jem.20021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Ho WT, Exton JH. Association of the N- and C-terminal domains of phospholipase D. J Biol Chem. 2000;275:24962–24969. doi: 10.1074/jbc.M909745199. [DOI] [PubMed] [Google Scholar]
- Xie Z, Ho WT, Spellman R, Cai S, Exton JH. Mechanisms of regulation of phospholipase D1 and D2 by the heterotrimeric G proteins G13 and Gq. J Biol Chem. 2002;277:11979–11986. doi: 10.1074/jbc.M109751200. [DOI] [PubMed] [Google Scholar]
- Xu L, Frankel P, Jackson D, Rotunda T, Boshans RL, D’Souza-Schorey C, Foster DA. Elevated phospholipase D activity in H-Ras- but not K-Ras-transformed cells by the synergistic action of RalA and ARF6. Mol Cell Biol. 2003;23:645–654. doi: 10.1128/MCB.23.2.645-654.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Miyazaki H, Watanabe H, Sasaki T, Maehama T, Frohman MA, Kanaho Y. Phosphatidylinositol 4-phosphate 5-kinase is essential for ROCK-mediated neurite remodeling. J Biol Chem. 2002;277:17226–17230. doi: 10.1074/jbc.M109795200. [DOI] [PubMed] [Google Scholar]
- Yang S-A, Carpenter CL, Abrams CS. Rho and Rho-kinase mediate thrombin-induced phosphatidylinositol 4-phosphate 5-kinase trafficking in platelets. J Biol Chem. 2004;279:42331–42336. doi: 10.1074/jbc.M404335200. [DOI] [PubMed] [Google Scholar]
- Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–789. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- Yoon MS, Koo JB, Hwang JH, Lee KS, Han JS. Activation of phospholipase D by 8-Br-cAMP occurs through novel pathway involving Src, Ras, and ERK in human endometrial stromal cells. FEBS Lett. 2005;579:5635–5642. doi: 10.1016/j.febslet.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Altshuller YM, Hammond SM, Morris AJ, Frohman MA. Loss of receptor regulation by a phospholipase D1 mutant unresponsive to protein kinase C. EMBO J. 1999;18:6339–6348. doi: 10.1093/emboj/18.22.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziembicki J, Tandon R, Schelling JR, Sedor JR, Miller RT, Huang C. Mechanical force-activated phospholipase D is mediated by Gα12/13-Rho and calmodulin-dependent kinase in renal epithelial cells. Am J Physiol Renal Physiol. 2005;289:F826–F834. doi: 10.1152/ajprenal.00412.2004. [DOI] [PubMed] [Google Scholar]


