Abstract
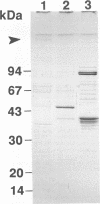
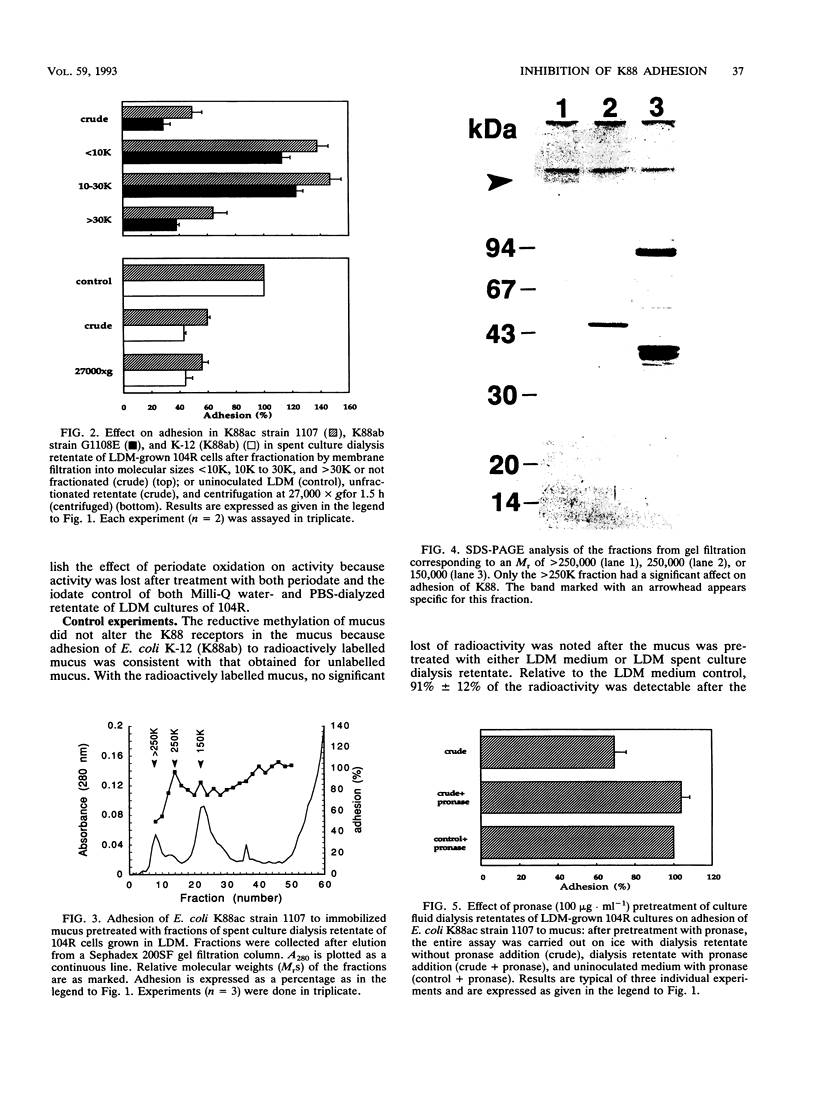
Enteropathogenic Escherichia coli K88 colonizing the piglet ileum adhere to the mucosa by K88 fimbrial appendages. A recent study in our laboratory has implicated indigenous lactobacilli in the suppression of the colonization potential of enteropathogenic E. coli as measured by adhesion to ileal mucus. The aim of this study was to investigate the effect of Lactobacillus spp. of porcine origin on the adhesion of K88 fimbriae of E. coli. With an in vitro assay, the adhesion of E. coli K88ab strain G1108E and E. coli K88ac strain 1107 to 35-day-old piglet ileal mucus was studied in the presence of spent culture fluid of Lactobacillus spp. Detailed studies focused specifically on culture fluid of Lactobacillus fermentum 104R. Subsequently, the ileal mucus was exposed to the retentate of the spent culture fluid after dialysis and fractionation. Adhesion was confirmed to be attributable to K88 fimbriae when K88-specific monoclonal antibodies and isogenic mutants of E. coli K-12 with and without the plasmid containing the K88 gene were used. The active component was characterized by pretreatment of dialysis retentate with heat, periodate, pronase, and centrifugation, as well as by growth of the lactobacillus in various media and by assays at both 0 and 37 degrees C. All three lactobacilli of porcine origin reduced adhesion of E. coli K88 by approximately 50%. Inhibition occurred when mucus was pretreated with either spent culture dialysis retentate or the void volume (fraction of > 250,000 molecular weight) after gel filtration. The activity of the dialysis retentate was sensitive to pronase, but there was still activity at 0 degrees C.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomberg L., Conway P. L. Influence of raffinose on the relative synthesis rate of K88 fimbriae and the adhesive capacity of Escherichia coli K88. Microb Pathog. 1991 Aug;11(2):143–147. doi: 10.1016/0882-4010(91)90008-x. [DOI] [PubMed] [Google Scholar]
- Chan R. C., Reid G., Irvin R. T., Bruce A. W., Costerton J. W. Competitive exclusion of uropathogens from human uroepithelial cells by Lactobacillus whole cells and cell wall fragments. Infect Immun. 1985 Jan;47(1):84–89. doi: 10.1128/iai.47.1.84-89.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway P. L., Adams R. F. Role of erythrosine in the inhibition of adhesion of Lactobacillus fermentum strain 737 to mouse stomach tissue. J Gen Microbiol. 1989 May;135(5):1167–1173. doi: 10.1099/00221287-135-5-1167. [DOI] [PubMed] [Google Scholar]
- Conway P. L., Kjelleberg S. Protein-mediated adhesion of Lactobacillus fermentum strain 737 to mouse stomach squamous epithelium. J Gen Microbiol. 1989 May;135(5):1175–1186. doi: 10.1099/00221287-135-5-1175. [DOI] [PubMed] [Google Scholar]
- Conway P. L., Welin A., Cohen P. S. Presence of K88-specific receptors in porcine ileal mucus is age dependent. Infect Immun. 1990 Oct;58(10):3178–3182. doi: 10.1128/iai.58.10.3178-3182.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson A., Szewzyk R., Conway P. L. Characteristics of the adhesive determinants of Lactobacillus fermentum 104. Appl Environ Microbiol. 1991 Feb;57(2):499–502. doi: 10.1128/aem.57.2.499-502.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- Klaenhammer T. R. Bacteriocins of lactic acid bacteria. Biochimie. 1988 Mar;70(3):337–349. doi: 10.1016/0300-9084(88)90206-4. [DOI] [PubMed] [Google Scholar]
- Kotarski S. F., Savage D. C. Models for study of the specificity by which indigenous lactobacilli adhere to murine gastric epithelia. Infect Immun. 1979 Dec;26(3):966–975. doi: 10.1128/iai.26.3.966-975.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J. W., Krogfelt K. A., Krivan H. C., Cohen P. S., Laux D. C. Characterization and identification of a porcine small intestine mucus receptor for the K88ab fimbrial adhesin. Infect Immun. 1991 Jan;59(1):91–96. doi: 10.1128/iai.59.1.91-96.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G., Bruce A. W., McGroarty J. A., Cheng K. J., Costerton J. W. Is there a role for lactobacilli in prevention of urogenital and intestinal infections? Clin Microbiol Rev. 1990 Oct;3(4):335–344. doi: 10.1128/cmr.3.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellwood R., Gibbons R. A., Jones G. W., Rutter J. M. Adhesion of enteropathogenic Escherichia coli to pig intestinal brush borders: the existence of two pig phenotypes. J Med Microbiol. 1975 Aug;8(3):405–411. doi: 10.1099/00222615-8-3-405. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Observations on the pathogenic properties of the K88, Hly and Ent plasmids of Escherichia coli with particular reference to porcine diarrhoea. J Med Microbiol. 1971 Nov;4(4):467–485. doi: 10.1099/00222615-4-4-467. [DOI] [PubMed] [Google Scholar]
- Sprunt K., Leidy G. The use of bacterial interference to prevent infection. Can J Microbiol. 1988 Mar;34(3):332–338. doi: 10.1139/m88-061. [DOI] [PubMed] [Google Scholar]
- Willemsen P. T., de Graaf F. K. Age and serotype dependent binding of K88 fimbriae to porcine intestinal receptors. Microb Pathog. 1992 May;12(5):367–375. doi: 10.1016/0882-4010(92)90099-a. [DOI] [PubMed] [Google Scholar]