Abstract.
We have previously demonstrated on human hepatocytes that apolipoprotein A-I binding to an ecto-F1-ATPase stimulates the production of extracellular ADP that activates a P2Y13-mediated high-density lipoprotein (HDL) endocytosis pathway. Therefore, we investigated the mechanisms controlling the extracellular ATP/ADP level in hepatic cell lines and primary cultures to determine their impact on HDL endocytosis. Here we show that addition of ADP to the cell culture medium induced extracellular ATP production that was due to adenylate kinase 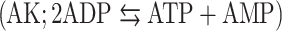 and nucleoside diphosphokinase
and nucleoside diphosphokinase 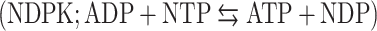 activities, but not to ATP synthase activity. We further observed that in vitro modulation of both ecto-NDPK and AK activities could regulate the ADP-dependent HDL endocytosis. But interestingly, only AK appeared to naturally participate in the pathway by consuming the ADP generated by the ecto-F1-ATPase. Thus controlling the extracellular ADP level is a potential target for reverse cholesterol transport regulation.
activities, but not to ATP synthase activity. We further observed that in vitro modulation of both ecto-NDPK and AK activities could regulate the ADP-dependent HDL endocytosis. But interestingly, only AK appeared to naturally participate in the pathway by consuming the ADP generated by the ecto-F1-ATPase. Thus controlling the extracellular ADP level is a potential target for reverse cholesterol transport regulation.
Keywords. High-density lipoprotein (HDL), apolipoprotein A-I, cholesterol, hepatocyte, nucleotide metabolism, adenylate kinase, nucleoside diphosphokinase
Footnotes
Received 13 July 2006; received after revision 29 August 2006; accepted 19 September 2006


