Abstract
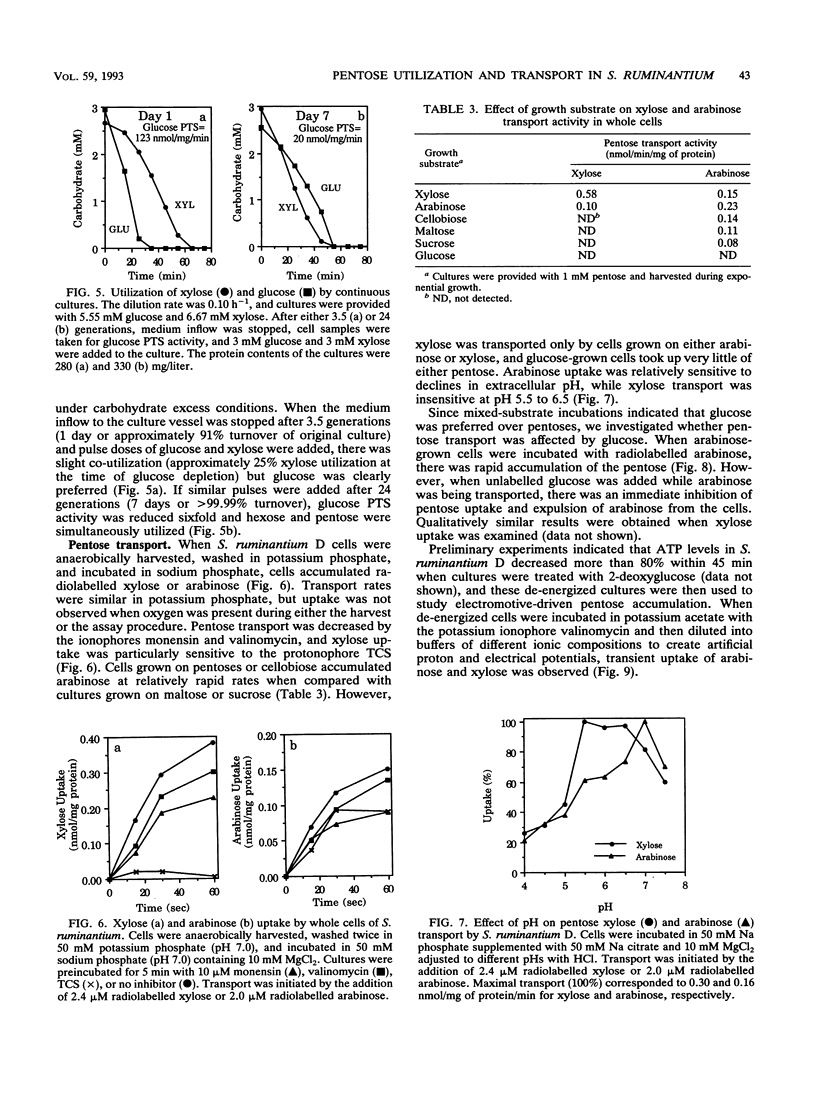
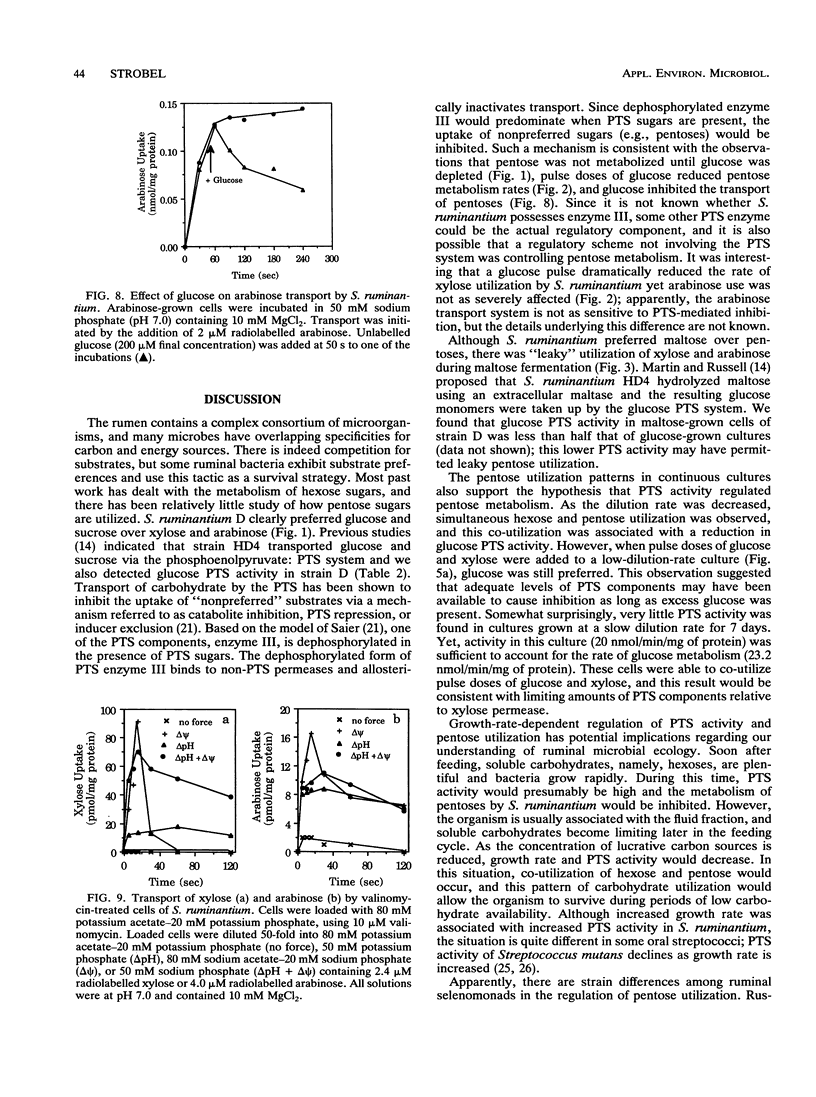
Pentose sugars can be an important energy source for ruminal bacteria, but there has been relatively little study regarding the regulation of pentose utilization and transport by these organisms. Selenomonas ruminantium, a prevalent ruminal bacterium, actively metabolizes xylose and arabinose. When strain D was incubated with a combination of glucose and xylose or arabinose, the hexose was preferentially utilized over pentoses, and similar preferences were observed for sucrose and maltose. However, there was simultaneous utilization of cellobiose and pentoses. Continuous-culture studies indicated that at a low dilution rate (0.10 h-1) the organism was able to co-utilize glucose and xylose. This co-utilization was associated with growth rate-dependent decreases in glucose phosphotransferase activity, and it appeared that inhibition of pentose utilization was due to catabolite inhibition by the glucose phosphotransferase transport system. Xylose transport activity in strain D required induction, while arabinose permease synthesis did not require inducer but was subject to repression by glucose. Since an electrical potential or a chemical gradient of protons drove xylose and arabinose uptake, pentose-proton symport systems apparently contributed to transport.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYANT M. P. The characteristics of strains of Selenomonas isolated from bovine rumen contents. J Bacteriol. 1956 Aug;72(2):162–167. doi: 10.1128/jb.72.2.162-167.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barras F., Chambost J. P., Chippaux M. Cellobiose metabolism in Erwinia: genetic study. Mol Gen Genet. 1984;197(3):486–490. doi: 10.1007/BF00329947. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John A., Isaacson H. R., Bryant M. P. Isolation and characteristics of a ureolytic strain of Selenomonas ruminatium. J Dairy Sci. 1974 Sep;57(9):1003–1014. doi: 10.3168/jds.s0022-0302(74)85001-0. [DOI] [PubMed] [Google Scholar]
- Kolodrubetz D., Schleif R. Regulation of the L-arabinose transport operons in Escherichia coli. J Mol Biol. 1981 Sep 15;151(2):215–227. doi: 10.1016/0022-2836(81)90512-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lam V. M., Daruwalla K. R., Henderson P. J., Jones-Mortimer M. C. Proton-linked D-xylose transport in Escherichia coli. J Bacteriol. 1980 Jul;143(1):396–402. doi: 10.1128/jb.143.1.396-402.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. A., Russell J. B. Phosphoenolpyruvate-dependent phosphorylation of hexoses by ruminal bacteria: evidence for the phosphotransferase transport system. Appl Environ Microbiol. 1986 Dec;52(6):1348–1352. doi: 10.1128/aem.52.6.1348-1352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. A., Russell J. B. Transport and phosphorylation of disaccharides by the ruminal bacterium Streptococcus bovis. Appl Environ Microbiol. 1987 Oct;53(10):2388–2393. doi: 10.1128/aem.53.10.2388-2393.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morehead M. C., Dawson K. A. Some growth and metabolic characteristics of monensin-sensitive and monensin-resistant strains of Prevotella (Bacteroides) ruminicola. Appl Environ Microbiol. 1992 May;58(5):1617–1623. doi: 10.1128/aem.58.5.1617-1623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny C. P., Englesberg E. The L-arabinose permease system in Escherichia coli B/r. Biochim Biophys Acta. 1966 Mar 28;117(1):217–230. doi: 10.1016/0304-4165(66)90169-3. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Substrate preferences in rumen bacteria: evidence of catabolite regulatory mechanisms. Appl Environ Microbiol. 1978 Aug;36(2):319–329. doi: 10.1128/aem.36.2.319-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Delfino F. J., Baldwin R. L. Effects of combinations of substrates on maximum growth rates of several rumen bacteria. Appl Environ Microbiol. 1979 Mar;37(3):544–549. doi: 10.1128/aem.37.3.544-549.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Dombrowski D. B. Effect of pH on the efficiency of growth by pure cultures of rumen bacteria in continuous culture. Appl Environ Microbiol. 1980 Mar;39(3):604–610. doi: 10.1128/aem.39.3.604-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr Protein phosphorylation and allosteric control of inducer exclusion and catabolite repression by the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Microbiol Rev. 1989 Mar;53(1):109–120. doi: 10.1128/mr.53.1.109-120.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheifinger C. C., Wolin M. J. Propionate formation from cellulose and soluble sugars by combined cultures of Bacteroides succinogenes and Selenomonas ruminantium. Appl Microbiol. 1973 Nov;26(5):789–795. doi: 10.1128/am.26.5.789-795.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel H. J., Russell J. B. Succinate transport by a ruminal selenomonad and its regulation by carbohydrate availability and osmotic strength. Appl Environ Microbiol. 1991 Jan;57(1):248–254. doi: 10.1128/aem.57.1.248-254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadeboncoeur C., St Martin S., Brochu D., Hamilton I. R. Effect of growth rate and pH on intracellular levels and activities of the components of the phosphoenolpyruvate: sugar phosphotransferase system in Streptococcus mutans Ingbritt. Infect Immun. 1991 Mar;59(3):900–906. doi: 10.1128/iai.59.3.900-906.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadeboncoeur C., Thibault L., Neron S., Halvorson H., Hamilton I. R. Effect of growth conditions on levels of components of the phosphoenolpyruvate:sugar phosphotransferase system in Streptococcus mutans and Streptococcus sobrinus grown in continuous culture. J Bacteriol. 1987 Dec;169(12):5686–5691. doi: 10.1128/jb.169.12.5686-5691.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. K., Martin S. A. Xylose uptake by the ruminal bacterium Selenomonas ruminantium. Appl Environ Microbiol. 1990 Jun;56(6):1683–1688. doi: 10.1128/aem.56.6.1683-1688.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



