Summary
The peroxisome proliferator activated receptor gamma (PPARγ) is a member in the nuclear receptor superfamily which mediates part of the regulatory effects of dietary fatty acids on gene expression. As PPARγ also coordinates adipocyte differentiation, it is an important component in storing the excess nutritional energy as fat. Our genes have evolved into maximizing energy storage, and PPARγ has a central role in the mismatch between our genes and our affluent western society which results in a broad range of metabolic disturbances, collectively known as the metabolic syndrome. A flurry of human and mouse studies has shed new light on the mechanisms how the commonly used insulin sensitizer drugs and PPARγ activators, thiazolidinediones, act, and which of their physiological effects are dependent of PPARγ. It is now evident that the full activation of PPARγ is less advantageous than targeted modulation of it's activity. Furthermore, new roles for PPARγ signaling have been discovered in inflammation, bone morphogenesis, endothelial function, cancer, longevity, and atherosclerosis, to mention a few. Here we draw together and discuss these recent advances in the research into PPARγ biology.
Keywords: PPARγ, mouse models, human genetic variants, longevity, bone homeostasis, metabolism
Introduction
Peroxisome proliferator-activated receptor-gamma (PPARγ, NR1C3) belongs to a nuclear receptor superfamily of transcription factors. It is mainly known to regulate adipocyte differentiation and fatty-acid uptake and storage (reviewed in [1-3]). The two distinct isoforms of PPARγ protein, PPARγ1 and PPARγ2, originate from one PPARγ gene through the use of separate promoters and 5' exons (Fig. 1), and differ by the presence of an extra 28 (human) – 30 (mouse) amino acids at the NH2-terminal end of PPARγ2 [4-10]. This extension of the ligand-independent activation domain makes PPARγ2 a better transcriptional activator relative to PPARγ1 [11]. Not only the protein structure of PPARγ1 and 2 is different but both isoforms show also a distinct expression pattern. PPARγ2 expression is mainly limited to the adipose tissue whereas PPARγ1 is ubiquitously expressed [12,13]. The transcriptional activity of PPARγ is controlled by the promiscuous binding of small lipophilic ligands into the ligand-binding pocket. Although a natural compound exhibiting specific, high-affinity binding characteristics remains unidentified, endogenous polyunsaturated fatty acids and eicosanoids, derived from nutrition or metabolic pathways, have been recognized as ligands for PPARγ [14-16]. In addition, many synthetic compounds, most particularly the thiazolidinediones (TZDs), are potent PPARγ agonists (reviewed in [17,18]).
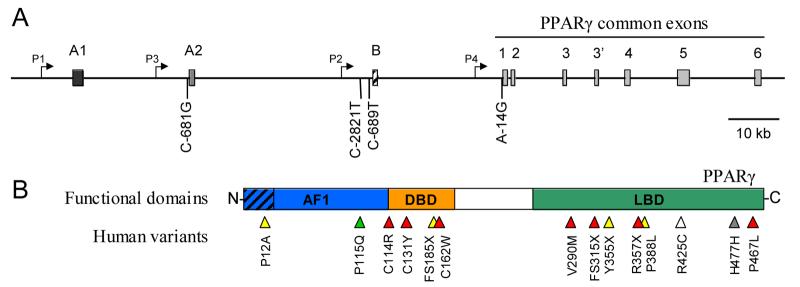
Figure 1.
Human PPARγ genetic variants. A: Genomic structure of the human PPARγ gene (>150 kb) with thus far described promoter mutants below the structural scheme. Exons are presented as boxes with identification above. Exons A1 and A2 are non-coding, while B encodes for the N-terminal addition in PPARγ2 which is missing in PPARγ1. The exon sizes are exaggerated for clarity. Note the recently identified additional exon in the intron 3 which contains an in-frame stop codon and the use of which results in a truncated protein (Kim et al, Biochem. Biophys. Res. Commun. 347 (2006) 698-706). Promoters are indicated by angled arrows. Numbering for the promoter mutants is relative to the transcriptional initiation site for each promoter. B: Functional structure of PPARγ protein with currently identified human PPARγ functional variants. Hatched section at the N-terminal end indicates the 28 amino acids specific for PPARγ2. AF1, ligand independent activation domain 1; DBD, DNA binding domain; LBD, ligand binding domain. PPARγ mutations are shown as arrowheads along the structural regions, color indicating the type of mutation as follows: green, gain of function; yellow, partial loss of function; red, loss of function, dominant-negative; grey, silent variant; white, unknown. Note that the amino acid numbering of the variants follows that of the originating publications, and thus some refer to PPARγ1, some PPARγ2. H477H is not likely to be a functional variant, but it is included here due to the fair number of papers associating it with various disease states.
Human metabolism is evolutionarily equipped to cope with pre-agricultural cycles of feast and famine, and physical activity and rest. Because of the rapid emergence of the modern westernized life-style, exposing people to chronically elevated levels of natural PPARγ ligands and positive energy balance, our genetic makeup has become ill adapted to cope with our lifestyle [19-21]. The continual PPARγ activation promotes adipogenesis and fatty-acid storage, and eventually obesity and associated metabolic diseases such as hyperlipidemia, insulin resistance, type 2 diabetes mellitus (T2DM) and cardiovascular diseases including hypertension, which constitute a heavy social and economic burden. Among the most potent current treatment strategies for T2DM are the TZDs which exert their antidiabetic effects by sensitizing the body to insulin's action. However, the clinical use of these full PPARγ agonists is limited by weight gain due to increased adiposity, fluid retention, and heart failure in up to 15% of patients [22-24]. In addition, despite their fairly wide use, the long-term adverse effects of TZDs are not very well known, and they may increase the risk for osteoporosis [25,26] and colon cancer [27,28].
In this review we summarize the studies that have shed new light on the role of PPARγ in energy homeostasis not only in the main metabolic tissues i.e. adipose tissue, liver and skeletal muscle, but also in other tissues. The reviewed studies also emphasize that insulin sensitization can be achieved without concomitant increase in fat deposition by modulating PPARγ activity. In addition to obesity, altered PPARγ activity, elicited by our westernized lifestyle, has potentially influenced bone homeostasis, longevity, cardiovascular and kidney function and cancer risk, as recent literature supports a significant role for PPARγ in these processes. Furthermore, animal models with altered PPARγ activity have elucidated the distinct roles of PPARγ in various tissues, as well as PPARγ-dependent and independent actions of TZDs therein. We can introduce here only a fraction of the existing information on this nuclear factor, and will thus mostly concentrate on the lessons learned from the study of various natural or engineered genetic variants of PPARγ that have altered PPARγ activity.
Human PPARγ genetic variants
The vital role of PPARγ in adipogenesis began to emerge more than a decade ago [12] and has remained undisputed since. Both of the processes central in adipogenesis, namely preadipocyte differentiation and fatty acid storage in mature adipocytes, are controlled by PPARγ, and particularly the PPARγ2 isoform (reviewed in [2,3,20,21]). Genetic association studies in humans underscore the role for PPARγ in adipogenesis as well as the complexity of PPARγ biology. One of the first links was the discovery that PPARγ locus on chromosome 3p25-p24 associates with obesity in Pima Indians [29]. To date, dozens of reports have revealed associations of genetic variation and population risk to T2DM or related conditions, as summarized in Table 1 for Pparγ. The most widely reproduced association is that with the PPARγ2 gene polymorphism Pro12Ala (Fig. 1B) [30,31] which has been suggested to induce a modest impairment of transcriptional activation due to decreased DNA-binding affinity [31,32]. The original reports describing a significantly reduced risk of T2DM in the normal-weight carriers of the Ala12 allele [30,31] have subsequently been confirmed by many independent studies, as reviewed in [2], and by recent meta-analyses [33-35]. For example, a meta-analysis compiling over 25,000 cases of diabetes unequivocally confirmed the association between the PPARγ Pro12 allele and T2DM, and suggested that patients who carry the Pro12 allele have a 1.27-fold higher risk for developing T2DM than Ala12 carriers [33]. This seemingly modest effect translates into a staggering 25% population-attributable risk because of the high frequency of the Pro12 allele (up to ∼80-100%), especially in Japanese and European populations [33].
Table 1.
A summary of human PPARγ genetic variants and their main characteristics.
| Variant | Main characteristics and phenotypic effects | References |
|---|---|---|
| Common polymorphisms | ||
| Pro12Ala | Specific to PPARγ2 isoform; ethnicity-dependent frequency ≤20%. Ala variant less active, protects from T2DM and weight gain in lean subjects, but with opposite effect in obese subjects. Effects on longevity, cognition, atherosclerosis, HT, birth weight, MI and cancer. Phenotypic effects modulated by environmental and genetic factors. |
[30,31,33-36,53] [41-48] [37-40,49] |
| His477His | Silent, single nucleotide polymorphism; frequency ∼14-20%. Associated with leptin levels, BMI, bone density, MI, and atherotic lipid changes. |
[64-68] |
| Dominant-negative, loss-of-function mutations | ||
| Pro467Leu Val290Met Cys114Arg Cys131Tyr Cys162Trp 315Stop Arg357X |
Mutant proteins that inhibit also the wild type protein. Either lack DNA or cofactor binding capacity. Severe insulin resistance or full diabetes, fatty liver, partial lipodystrophy, dyslipidemia, and often also HT. |
[54-59] |
| Haploinsufficient mutations | ||
| Arg425Cys Phe388Leu Tyr355X 185Stop |
Result in non-functional or missing protein without effect on the wild type variant. Severe insulin resistance or full diabetes, fatty liver, partial lipodystrophy, dyslipidemia, and often also HT, i.e. the same as for dominant-negative variants. |
[54,56,61,62] |
| Gain-of-function mutations | ||
| Pro115Gln | Constitutively active. Carriers extremely obese, but paradoxically with normal insulin sensitivity. |
[60] |
| Promoter variants | ||
| P2 C-689T | Associates with increased body weight and LDL levels; mechanism unknown. | [71] |
| P2 C-2821T | Associates with whole-body and hepatic insulin action; affects MyoD binding (?) | [69] |
| P3 C-681G | Associates with large body size and LDL levels; affects STAT5B binding. | [70] |
| P4 A-14G | Partial lipodystrophy, metabolic syndrome, no HT; reduced promoter activity. | [63] |
Notes: Frequencies are given only for the common polymorphisms as the rest are typically very rare, each found only in a hand-full of subjects. Reference list for the Pro12Ala variant is far from complete, e.g. several studies on the other variants also include this variant. T2DM, type 2 diabetes mellitus; HT, hypertension; MI, myocardial infarction; BMI, body mass index; MyoD, myogenin differentiation 1; STAT5B, signal transducer and activator of transcription 5B.
Genes do not work in a vacuum, but react to e.g. environmental stimuli. Clear demonstration of this, in the context of Pparγ, is the apparently paradoxical linkage of the Ala12 allele to higher body mass index (BMI) in obese (BMI ≥27) subjects [34]. Thus, pre-existing obesity may be required for the Ala12 allele to cause further increase in obesity, whereas in lean subjects the effect is either lacking [34] or opposite [31,36]. Furthermore, the phenotypic effects of PPARγ Pro12Ala variant, also other than those on BMI and T2DM, have been shown to be modulated by the superimposition of environmental factors like obesity, physical activity, and the dietary fatty acid composition [37-40]. For example, Ala12 allele causes an increase in muscle glucose uptake only in lean carriers and not in obese [37]. The Pro12Ala variant in itself has also been associated with such additional phenotypes as longevity, cognitive decline, atherosclerosis, hypertension, birth weight, myocardial infarction and cancer which may provide further insight into the function of this variant in vivo [41-48]. Taken together, current evidence supports a role for PPARγ at the interface between the environment and the control of metabolism [40,49].
Genes not only react to environmental signals, but also interact with each other. As most human metabolic disorders are clearly polygenic in nature, recently the combined effects of PPARγ mutations and variants of other genes have been investigated. For example, Pro12Ala variant has been shown to interactively influence insulin sensitivity and body composition with the A-376C variant of fatty acid binding protein 4 (FABP4) gene [50], representing a synergistic (1+1>2) interaction where the combined effect is more than what would be expected from simply adding up the individual effects. On the other hand, only additive (1+1=2) interaction was found among three common T2DM risk alleles (Lys23 of KCNJ11, Pro12 of PPARγ, and the T allele at rs7903146 of TCF7L2) [51]. There is also evidence for a subtractive (1−1=0) interaction, albeit within one gene, as two variants of PPARγ, Pro12Ala and C-681G were recently reported to have an opposite effect on growth in British school children [52]. Similarly, the C1431T variant (a.k.a. His477His) can negate the beneficial effects of the Pro12Ala variant on T2DM [53]. Furthermore, the effect of Pro12Ala polymorphism may be restricted to certain ethnic groups, as suggested for Caucasian subpopulations by a recent meta-analysis of pre-diabetic traits in about 32,000 nondiabetic subjects [35]. Interestingly, genetic background has also had a profound effect in certain mouse models with altered PPARγ activity, as discussed below. The above genetic interaction studies clearly indicate that the genetic makeup of an organism adds to the complex nature of PPARγ biology.
In contrast to the relatively mild effects of the common Pro12Ala variant, rare dominant negative and loss-of-function mutations affecting the ligand-binding domain of PPARγ have been identified in patients afflicted with partial lipodystrophy (loss of fat from the limbs and gluteal region), hepatic steatosis, dyslipidemia, severe insulin resistance, diabetes and hypertension. These and other currently described functional or otherwise important human PPARγ variants are depicted in Figure 1B. The first identified loss-of-function mutations were 185Stop [54], Val290Met (also called V318M) [55], Arg425Cys (also R397C) [56], and Pro467Leu (also called P495L) [55,57]. Apart from 185Stop, which results in a truncated protein, the rest of these mutant receptors retain ability to bind target DNA but exhibit severely reduced transcriptional activation via diminished capacity to recruit cofactors [55,58]. A very recent report described five new mutations, three of which (Cys114Arg, Cys131Tyr and Cys162Trp) prevent PPARγ from binding to the target DNA while the other two lead to missense (Arg357X) or truncated (315Stop) proteins [59]. Heterozygous carriers of these new mutations are severely insulin resistant and hepatosteatotic, and display partial lipodystrophy; some of the patients are also hypertensive [59]. Although these studies provide direct genetic evidence of a link between PPARγ action and the regulation of mammalian glucose homeostasis, it remains uncertain whether the profound effects on insulin resistance observed in these individuals is only a manifestation of reduced adipose tissue mass or whether other direct effects of PPARγ action on insulin signaling are impaired. Recent evidence, however, suggests that dysfunction rather than relative lack of the adipose tissue may be more significant [49].
Contrasting the loss-of-function mutations, a rare Pro115Gln substitution is a gain-of-function mutation which renders PPARγ constitutively active and results in extreme obesity while, paradoxically, normal insulin sensitivity prevails [60]. On the other hand, Phe388Leu (also F360L) [61], Tyr355X [62] and a PPARγ promoter 4 variant A-14G [63] represent cases of haploinsufficiency where the affected allele reduces PPARγ function without interfering with the wild-type PPARγ protein. However, patients carrying these variants still exhibit the stereotypical partial lipodystrophy, fatty liver, severe insulin resistance if not T2DM and hyperlipidemia. Additional human PPARγ variants include a common silent His477His polymorphism in PPARγ exon 6 (also called C1431T, C161T of exon 6, or CAC478CAT), associated originally with altered leptin levels, higher BMI and lower bone mineral density [64-66], and later with increased myocardial infarction risk [67] as well as with potential protection from atherosclerotic lipid alterations [68]; a C-2821T variant in the PPARγ2 promoter, associating with whole-body insulin action and hepatic insulin sensitivity, likely via altered binding on the overlapping E2-box, a binding consensus site for e.g. myogenin differentiation 1 (MyoD) [69]; a C-681G variant in the promoter of the shorter form of PPARγ1 (also called PPARγ3), associating with increased body size and plasma LDL levels, via reduced binding of signal transducer and activator of transcription 5B (STAT5B) onto the PPARγ promoter [70]; and a C-689T variant in the PPARγ2 promoter, associating again with increased body weight and plasma LDL levels [71]. Recently also intronic PPARγ variants, associating with body size, BMI and glucose levels, have been identified [72].
Mouse genetic variants
PPARγ exerts pleiotropic functions in a wide range of tissues and in processes beyond metabolism. Genetic manipulations in the mouse offer excellent opportunities to unravel the complex physiological effects of altered PPARγ activity in a well-controlled genetic background and environmental setting. First came the generation of a conventional PPARγ deficient mice which, unfortunately, die in utero due to major placental and cardiac defects. Although a single PPARγ−/− animal was rescued by tetraploid aggregation, it survived the severe lipodystrophy for only a few days [73]. The lipodystrophy of the rescued PPARγ−/− mouse, together with the characterization of mice chimeric for PPARγ−/− ES cells [74], showed the importance of PPARγ in adipose tissue development in vivo. Next, the physiological function of PPARγ in mice was studied in the heterozygous PPARγ+/− mice. These heterozygous PPARγ knock-out mice are a difficult model to understand, as both resistance [75] and susceptibility [76] to high-fat diet induced obesity and subsequent insulin resistance have been reported; for thorough discussion about the potential reasons, see [76]. To overcome the embryonic lethality of the germ-line PPARγ deficient mice, tissue-specific deletions of PPARγ have been generated subsequently in mice to help to elucidate the tissue specific activities of PPARγ [77] (summarized in Table 2 for main metabolic tissues as targets, and in Table 3 for other tissues).
Table 2.
A comparison of PPARγ knockout models specific to metabolic tissues.
| Targeted tissue (type of knock-out) |
Adiposity | Plasma profile | Adipokine production | Liver steatosis | Insulin resistance |
Other phenotype |
|---|---|---|---|---|---|---|
|
Adipose[26,78-83] (hypomorph[26,78]; w/ aP2- Cre[79,80]; aP2-Cre-ERT2[81]; PPARγ2-KO[82,83]) |
Congenital lipodystrophy[78] Progressive lipodystrophy[79,81] Moderate lipodystrophy[82] Normal adiposity[83] |
↑Glc & ↑Ins[78,83] Normal Glc & Ins[79,82] ↑TG, ↑FFA[78,79] ↓TG[81] Normal TG & Chol[82] or FFA[83] |
↓Leptin[78,79,82] ↑Leptin[83] ↓Adiponectin[26,78-80,82,83] |
Normal liver TG[78,82,83] Steatosis[79] |
Yes[78,79,82,83] | ↑Bone mass[26] |
|
Muscle[84,85] (w/ MCK-Cre) |
↑Adipose mass[84] (↑)Adipose mass[85] |
Normal Glc, Ins, TG & FFA[84] Normal Glc, ↑Ins, ↑TG & ↑FFA[85] |
Normal leptin[84] ↑Leptin & ↓Adiponectin[85] |
Normal liver TG[84] Steatosis[85] |
Yes[84,85] | Severely impaired HGP[84] Impaired HGP[85] |
|
Liver[86,87] (w/ Alb-Cre) |
Progressive obesity[86] Normal[87] |
↑Glc, ↑Ins, ↑TG[86] Normal Glc[87] |
↑Leptin & ↓Adiponectin[86] |
Normal liver TG[86,87] |
Yes[86] No[87] |
|
|
Pancreas[94] (w/ Insulin-Cre) |
Normal | Normal | ND | Normal liver TG | No | Pancreatic islet expansion on chow diet, but blunted response to high-fat diet |
|
Macrophage[96-98] (chimeras#,[96], w/ Mx- Cre*,[98]; Lyzs-Cre[97]) |
ND | ↓apoE, ↓LDL- cholesterol[98] |
ND | ND | ND | Increased atherosclerosis in recipients of PPARγ−/− macrophages[96,97] |
The different mouse models have various age and diet-dependent responses, thus for simplicity the above descriptions are for the adult phenotype in the postprandial state on a chow diet unless otherwise indicated. ND, not determined; Glc, glucose; Ins, insulin; TG, triglycerides; FFA, free fatty acids; Chol, cholesterol; MCK, muscle creatine kinase; HGP, hepatic glucose production; Alb, albumin; Mx, myxovirus resistance 1, interferon-inducible protein; Lyzs, lysozyme; apoE, apolipoprotein E; LDL, low density lipoprotein.
Derived from PPARγ−/− ES-cells, >90% of all macrophages PPARγ−/−.
The use of Mx-Cre also leads to PPARγ deficient liver.
Table 3.
A comparison of PPARγ knockout models specific to other tissues.
| Targeted tissue (Cre promoter) |
Main phenotypic characteristics |
|---|---|
| Vasculature, endothelial cells (Tie2)[103] |
Normal plasma profile Blood pressure normal on chow, increased on high-fat diet Hypertension unresponsive to rosiglitazone on high-fat diet |
| Heart, cardiomyocytes (αMHC)[105] |
Normal body weight and plasma glucose Cardiac hypertrophy but normal (or slightly improved) systolic function |
| Kidney, collecting ducts (Aq2)[106,107] |
Protection from thiazolidinedione-induced edema |
| Lung, conducting airway epithelium (CC10)[109] |
Impaired lung maturation (non-progressive condition) |
| Mammary epithelium (WAP, MMTV)[112] |
Normal mammary function No differences in tumorigenesis |
| B- and T-cells, and ovaries (MMTV)[112] |
Normal B- and T-cell production Partial to complete infertility (due to impaired implantation?) |
| Intestine (Villin)[117] (on ApcMin/+ background) |
Normal body weight Enhanced intestinal tumorigenesis (some gender differences) |
| Colon, epidermal cells (Villin)[119] |
Sensitization to experimental inflammatory bowel disease with similar relative protection by rosiglitazone |
| Epidermis (K14)[123] | Normal skin function |
αMHC, alpha myosin heavy chain; Aq2, aquaporin 2; WAP, whey acidic protein; MMTV, mouse mammary tumor virus; Apc, adenomatosis polyposis coli; K14, keratin 14.
PPARγ in adipose tissue
The essential role of PPARγ in adipogenesis was revealed by inactivation of both PPARγ1 and PPARγ2 in the adipose tissue [78-80]. Moreover, the vital role of adipose tissue per se was exemplified by the significant mortality rate (>40%) of the severely lipodystrophic WAT-specific hypomorphic PPARγ1 and PPARγ2 knockdown mice (PPARγhyp/hyp) [78]. When an aP2-driven Cre recombinase transgene was used to ablate PPARγ1 and PPARγ2 from the mature adipocytes, a more moderate reduction of adipose mass was observed, which was accompanied by hyperlipidemia, liver steatosis, and protection from high-fat diet induced increase in adiposity [79,80]. Interestingly, the surviving adult PPARγhyp/hyp mice did not have liver steatosis or dyslipidemia due to efficient oxidation of excess lipids in the muscle by PPARα and PPARβ/δ-driven pathways [78]. Intriguingly, all three adipose PPARγ-deficient models had close to normal glucose tolerance [78-80]. The importance of PPARγ in the survival of mature adipocytes was high-lighted by yet another independent ablation of total PPARγ function in adipocytes, this time in a temporally controlled manner in adult mice [81].
To gain more insight to the relative roles of PPARγ1 and 2, mice with selective disruption of the more adipogenic and adipose-tissue restricted PPARγ2 isoform have been generated. However, these studies have provided somewhat confusing results. On C57Bl/6 genetic background, ablation of PPARγ2 resulted in diminished adipose tissue (WAT) mass, decreased insulin sensitivity and reduced adipocyte differentiation in vitro. In addition, it provided protection against high-fat diet induced weight gain [82]. In contrast, on a 129 background, PPARγ2-deficiency led to insulin resistance without affecting adiposity in vivo, even on high-fat diet, although in vitro a defect in fat cell differentiation was clear [83]. Despite the differences in adiposity and insulin sensitivity/resistance in these two models, likely due to the different genetic backgrounds of the mice in the two studies, the authors of both papers linked the phenotype to reduced levels of plasma adiponectin [82,83]. Moreover, the fact that there still was fat in the PPARγ2-deficient mice indicates that also PPARγ1 is able to initiate at least some adipocyte differentiation.
PPARγ in skeletal muscle
Although PPARγ is predominantly expressed in adipose tissue, also skeletal muscle expresses it at low levels. Furthermore, muscle is an important tissue in glucose and fuel homeostasis. Considering the large relative mass of muscle in the body, even a small change in PPARγ activity might have significant metabolic effects. However, the role of PPARγ in the muscle is fairly minor according to the two independent reports, albeit with essentially opposite results, of muscle-specific PPARγ knock-out mouse models [84,85]. The report published first [84] described muscle-specific PPARγ knock-out mice with normal glucose homeostasis and insulin levels, but with reduced hepatic insulin sensitivity which was attributed to the increased WAT mass. The inability of PPARγ-deficient muscle to effectively use lipids as fuel explained the shunt of lipids to the adipocytes and thus the resulting adiposity. Since the beneficial effects of TZDs on glucose homeostasis prevailed in the absence of muscle PPARγ, it was concluded that the insulin-sensitizing effects of TZDs are independent of muscle PPARγ [84]. These findings are consistent with the observations in the PPARγhyp/hyp mouse model where adipose tissue PPARγ expression was shown to be crucial for the insulin-sensitizing effects of TZDs, as TZD treatment ameliorated only the glucose intolerance but not the insulin resistance of these mice [78]. In contrast to the first report, the second study [85] showed that muscle-specific PPARγ-deficient mice develop severe muscle insulin resistance, leading to hyperinsulinemia, glucose intolerance and hypertriglyceridemia. Since TZD treatment failed to enhance insulin-stimulated glucose disposal into PPARγ-deficient muscle, suggesting a lack of improvement in muscle insulin sensitivity, it was concluded that muscle PPARγ is the direct target of TZD actions [85]. According to most studies, however, it seems that PPARγ in the muscle is more responsible for coordinating the use of energy rather than directly controlling glucose homeostasis or responses to insulin [78,79,84,86,87]. Thus it is likely that the insulin-sensitizing effects of PPARγ are predominantly mediated by WAT. Therefore, in addition to the role as master regulator of adipogenesis in vivo, PPARγ in the WAT also directs glucose and lipid homeostasis.
The role of PPARγ in the adipocytes differentiation is well established. Interestingly, PPARγ seems to have a role also in muscle differentiation, at least in vitro. A recent study by Singh et al [88] shows that correct regulation of PPARγ activity is vital for proper C2C12 myocyte differentiation as both 25-30% increase and similar decrease in PPARγ protein levels resulted in virtually complete inhibition of myocyte differentiation. However, it seems that in the in vivo setting there are compensatory mechanisms at play since none of the muscle specific or heterozygous whole-body PPARγ knock-out models have been described with muscular dystrophy.
PPARγ in the liver
Like skeletal muscle, also the liver expresses low levels of PPARγ. Again, consistent with the adipose tissue and muscle-specific PPARγ knock-out models, also the liver-specific ablation of PPARγ leads to impaired lipid balance. Lack of PPARγ in the livers of two separate mouse models with pre-existing liver steatosis (leptin-deficient ob/ob or lipodystrophic A-ZIP/F-1 mice) protected the mice from the development of fatty liver by reducing liver triglyceride content [86,87]. However, it also led to elevated serum levels of FFA and lipoprotein as well as insulin resistance, illustrating PPARγ's role in liver lipogenesis. PPARγ in the liver was shown to be critical for the ability of TZDs to lower triglycerides and glucose in conditions of lipodystrophy. Indeed, in the absence of WAT, liver PPARγ regulates both fat and glucose homeostasis [86], but in the presence of WAT, the impact of PPARγ in the liver on glucose homeostasis is minimal.
An interesting alternative, especially useful for overexpression studies, is the systemic adenoviral delivery of a transgene, typically resulting in liver-specific expression. Using this methodology, in conjunction with PPARα-deficiency, the independent role of PPARγ1 in the liver has been elucidated [89,90]. In contrast to the lack of PPARγ, overexpression of PPARγ1 in the mouse liver leads to hepatic steatosis not only in the absence of PPARα but also to some degree in wild-type mice via a mechanism involving transcriptional activation of genes linked to adipogenesis, some of which are novel as PPARγ target genes [89,90]. Using classical transgenesis, the artificial Leu468Ala/Glu471Ala double variant of PPARγ has also been expressed in the mouse liver [91]. This model, resulting in suppression of both PPARα and PPARγ target genes, revealed potentially distinct roles for PPARα and PPARγ in fasting and high-fat diet induced hepatic steatosis.
The PPARγ-deficient mouse models specific to the three main metabolic tissues have helped us to realize that when PPARγ is absent in any of them, whole-body lipid homeostasis and insulin sensitivity are significantly altered. This is a fascinating observation considering that PPARγ expression levels vary greatly among these tissues. The resulting repartitioning of lipids that occurs in these mouse models has also unveiled the presence of a complex network of cross-talk between the liver, adipose and muscle that is essential to the maintenance of energy balance. This balance is in part achieved by the adaptation of PPARγ in the non-targeted tissues and by the other PPAR isoforms, PPARα and β/δ, which enhance fatty acid oxidation to minimize hyperlipidemia and the consequential insulin resistance.
PPARγ in pancreas
Based on the evidence from rat and human pancreatic islets, PPARγ is expressed by the beta-cells [92,93]. However, in contrast to adipose tissue, muscle and liver, the deletion of PPARγ in pancreatic beta-cells did not result in metabolic phenotype. Instead, it high-lighted the antiproliferative role of PPARγ because on chow diet these mice had a 2-fold increase in pancreatic islet size. Opposite to this, on a high-fat diet the normal expansion of beta-cell mass was markedly blunted in the absence of PPARγ [94]. Another study, using high-fat fed PPARγ+/− mice, identified a role for PPARγ in insulin secretion and triglyceride partitioning into the islets upon high-fat feeding, leading to protective effects against lipotoxicity [95]. However, the importance of these findings, when it comes to translating them to human biology, may be reduced by the fact that PPARγ gene expression in mouse islets [95] is very low compared to human (and rat) islets [92,93]. This difference in PPARγ expression in the pancreas is also evident in the SymAtlas gene expression data sets for mouse and human (http://symatlas.gnf.org/SymAtlas/).
PPARγ in the macrophages
As the role of PPARγ in atherosclerosis, in which macrophages are integral players, is covered by another review in this issue of BBA, we present and discuss here only those studies utilizing mice with macrophage-specific PPARγ inactivation. PPARγ has a similar function in macrophages and adipocytes as it modulates lipid homeostasis in both cell types via regulation of genes including e.g. lipoprotein lipase and CD36. Studies using macrophage-specific PPARγ-deficient mice have identified a definite antiatherogenic role for PPARγ as in these mice lipid homeostasis in the arterial wall is significantly impaired and the development of atherosclerosis enhanced [96-98]. Mechanism behind the antiatherogenic properties of PPARγ involves stimulation of cholesterol efflux from macrophages into the plasma (reduced in knock-out mice) and inhibition of monocyte recruitment into the developing atherosclerotic lesion, the latter suggested by increased CC chemokine receptor 2 gene expression [97]. Interestingly, macrophage-specific ablation of PPARγ results in exaggerated insulin resistance upon high-fat feeding, suggesting that macrophage PPARγ has a protective role in obesity [99]. Indeed, an intriguing link between macrophages, inflammation, adipose tissue and T2DM has recently emerged [100-102]. In obesity, the adipose tissue is continually under metabolic stress, leading to the activation of stress and inflammatory pathways, which in turn result in macrophage accumulation within the adipose tissue. Subsequently, adipocytes release cytokines, adipokines and free fatty acids, which may cause insulin resistance not only locally but also in the liver and/or skeletal muscle. Further research is needed to discover how macrophage PPARγ fits into the developing picture on the role of adipose-macrophages in insulin resistance.
PPARγ in cardiovascular system
In an attempt to elucidate the mechanisms by which PPARγ ligands ameliorate hypertension in humans, the role of PPARγ in vascular endothelial cells has been investigated using tissue-specific PPARγ-deficient mice (PPARγ E-null) [103]. These mice did not have apparent developmental, reproductive or biochemical abnormalities, nor changes in systolic blood pressure or heart rate on chow diet. However, upon a challenge with high-fat diet, an increase in both blood pressure and heart rate was evident in the PPARγ E-null mice, whereas only the heart rate was affected upon salt-loading [103]. Although treating the mice with rosiglitazone was clearly effective per se, as indicated by the decreased plasma insulin, it failed to lower the high-fat diet induced hypertension in PPARγ E-null mice, contrary to the effect in wild type mice. Even in the absence of mechanistic data, this study demonstrates the central role for PPARγ in mediating the antihypertensive effects of rosiglitazone, especially under conditions partly simulating human type 2 diabetes.
Due to the presence of PPARγ in cardiac muscle, combined with the fact that at super-clinical doses TZDs cause cardiac hypertrophy, the role of PPARγ in the heart has been investigated [104,105]. Initial observation on PPARγ+/− mice was that under pressure-overloaded conditions the heterozygous PPARγ-deficiency leads to cardiac hypertrophy, and that pioglitazone surprisingly provides either full (wild type) or partial protection (PPARγ+/−) against the condition, suggesting that pioglitazone action is mostly PPARγ-dependent [104]. In a more recent study [105], also the selectively absence of PPARγ in the heart muscle resulted in cardiac hypertrophy, similar to that caused by rosiglitazone, but systolic function was retained. The study of Duan et al [105] also provides deeper mechanistic insight by showing that the promotion of cardiac hypertrophy by rosiglitazone and cardiac-specific PPARγ-deficiency are mediated by different mechanisms, as evidenced by the differential effects on gene expression, NF-κB activity and the activation of the members of MAPK pathways. In this respect, the most noteworthy observation was the PPARγ-independent effect of rosiglitazone on Erk1/2 activation [105]. This study is yet another demonstration of the potential that the PPARγ cell type-specific knock-out models provide for discerning the PPARγ-dependence of e.g. pharmacological interventions.
Taken together, these studies emphasize the importance of PPARγ in the maintenance of cardiac and vascular function and shed light on the PPARγ-dependent and independent mechanisms of TZD action in this organ system.
PPARγ in kidney
Edema and fluid retention are common and serious side effects of TZD treatment. Since PPARγ is a significant target for TZDs and it is expressed in the collecting ducts of the kidneys, the specific involvement of PPARγ in fluid metabolism has been recently elucidated by two groups using mice with collecting duct-specific ablation of PPARγ [106,107]. Both studies show a critical role for PPARγ in systemic fluid retention through the regulation of renal sodium transport, and that the adverse effects of TZD in fluid metabolism are indeed PPARγ-dependent. Scnn1g, a gene encoding for the gamma subunit of the epithelial Na+ channel (ENaC) was identified as a critical PPARγ target gene in the control of fluid metabolism [107].
PPARγ in the lung
PPARγ expression has been detected in the lung, in particular the airway epithelium [108]. A specific role for PPARγ in lung maturation was recently revealed by Simon et al [109] using mice where PPARγ deletion was initiated specifically in conducting airway epithelium at late fetal to early postnatal stage. Physiological consequences of the PPARγ deletion in the airway epithelium include a persistent, but not progressive enlargement of airspaces in adult mice, which together with other phenotypic changes in the lungs point to a role for PPARγ in postnatal lung maturation [109]. Gene expression studies corroborated the physiological phenotype and suggested a role for PPARγ in differentiation as well as lipid metabolism in the lung. Although these mice were kept in pathogen-free conditions, to exclude inflammation as a confounding factor [109], it is likely that these mice are susceptible to inflammation as PPARγ is a recognized regulator of inflammatory response. For example, it has been shown that PPARγ, together with PPARα, down-regulates several immune system parameters in a murine model of human asthma [110,111]. Thus, not only PPARγ but also PPARα should be targeted for the best therapeutic effect when combating the allergic or inflammatory reactions within the airways.
PPARγ in mammary epithelium, B- and T-cells, and in ovarian cells
Cui et al generated separate knock-out lineages where PPARγ was inactivated either in mammary epithelial cells during pregnancy and lactation (WAP-Cre) or in mammary epithelial cells, salivary gland, B- and T-cells, oocytes, granulose cells and megacaryocytes (MMTV-Cre) [112]. Their results show that PPARγ is not necessary for mammary development nor function, nor does it's absence lead to increased tumorigenesis in this tissue. Also B- and T-cell development is normal in the absence of PPARγ. However, the lack of PPARγ in ovarian cells, including oocytes, granulose cells and corpora lutea, but not in the uterus, resulted in impaired female fertility, possibly due to disturbances in implantation. This points to a role for PPARγ in female reproduction, in line with other recent research (reviewed in [113]). In fact, also sperm has been shown to express PPARγ [114], and it will be interesting to see if PPARγ has a significant role also in male reproduction.
PPARγ in the gastrointestinal tract
In the mouse intestine, PPARγ regulates cell growth and differentiation. In addition, PPARγ is highly expressed in the (epithelium of) colon and cecum [28,115]. This suggests that PPARγ has potential for colon cancer association. However, the available evidence continues to be conflicting in whether PPARγ acts as a promoter or inhibitor in intestinal tumorigenesis, let alone in general. Administration of PPARγ ligands, i.e. PPARγ activation, increases tumorigenesis in C57BL/6J-APCMin/+ mice which are predisposed to intestinal neoplasia [27,28]. However, increased susceptibility to colon cancer has been shown to result also from heterozygous PPARγ-deficiency in a chemical model of tumorigenesis induced by azoxymethane administration [116]. Furthermore, when the intestinal PPARγ was fully or partly inactivated on the same C57BL/6J-APCMin/+ background as above, spontaneous tumorigenesis was significantly enhanced in the colon, and in female mice also in the small intestine, suggesting an antitumorigenic role for PPARγ in the gastrointestinal tract [117]. Intestinal PPARγ may also have a sex-specific developmental role, as some female embryonic lethality was reported. The above studies seem to suggest that an optimal level of intestinal PPARγ may exist from which any significant deviation will promote tumorigenesis. In fact, the suggested requirement for a balanced PPARγ activity in intestinal tumorigenesis resembles the effects of PPARγ in muscle cell differentiation (see above) [88]. In addition, the cellular context of PPARγ cofactors (e.g. APC (see above) and retinoblastoma protein [118]) seems to influence tumorigenesis.
Another independently generated intestine-specific PPARγ knock-out model [119] demonstrated the importance of PPARγ as a protective agent against experimental inflammatory bowel disease (IBD), as mice lacking PPARγ in the colon epithelium had increased susceptibility to dextran sodium sulphate (DSS) induced IBD and cytokine gene expression. This model recapitulates earlier results where heterozygous whole-body loss of PPARγ also rendered the mice more susceptible to experimental IBD [120]. Furthermore, the involvement of PPARγ in the anti-inflammatory effects of 5-aminosalicylic acid, which is a drug widely used to treat the human IBD, has been recently shown in PPARγ+/− mice where the drug protected only the wild type mice from IBD [121]. On the other hand, the anti-inflammatory effect of rosiglitazone in experimental IBD seems to be independent of PPARγ, as the colon-specific PPARγ knock-out mice benefited from the treatment similarly to wild-types [119]; this is in contradiction to the PPARγ-dependent anti-inflammatory role of rosiglitazone in macrophages [122].
Taken together, these results are in line with the suggested role of PPARγ as an anti-inflammatory agent. Furthermore, they suggest that the anti-inflammatory function of TZDs may act via different routes in the colon epithelium from that in macrophages. Although the tumorigenesis-related and anti-inflammatory effects of PPARγ have been clarified, to a degree, by the above studies, possible systemic effects of intestinal PPARγ remain unclear as metabolic phenotyping is lacking in the intestinal-specific PPARγ knock-out mice.
PPARγ in the epidermis
PPARγ activation stimulates keratinocyte differentiation and reduces inflammatory response in the skin. Using dermal-specific PPARγ knock-out mice, it has been possible to demonstrate that the dermal effects of TZDs on keratinocyte differentiation are indeed PPARγ-dependent but the effects on inflammation seem independent of PPARγ [123]. In basal conditions, however, all the key functions of the epidermis were normal in these mice and the only observed phenotypic effects were a patchy hair loss upon aging and a slight epidermal hyperplasia.
PPARγ in the neural tissues
High-level PPARγ expression has been detected in the mouse embryonal brain and neural stem cells, but in the adult brain in general PPARγ expression is very low [124]. The data of Wada et al [124] demonstrate a role for PPARγ in the developing brain where it promotes differentiation as the embryonal neural stem cells from heterozygous PPARγ knock-out mice have decreased growth rates. In addition, PPARγ agonists stimulate the growth of neural stem cells whereas antagonists cause apoptosis [124].
There is also a role for PPARγ in the inflammatory diseases of the brain, as heterozygous PPARγ knock-out mice have an exacerbated response in an experimental allergic encephalomyelitis model, which is a Th1 cell-mediated inflammatory demyelinating autoimmune disease resembling multiple sclerosis [125,126].
PPARγ and bone homeostasis
Physical activity is a prerequisite for food procurement, food is a prerequisite for energy storage, and stored energy is a prerequisite for physical activity. Because of this inextricable linkage, it is plausible that concomitant with the role PPARγ has in fuel storage and use, it also regulates bone mass to provide the structural strength required in procuring the next meal. Further link between energy storage and bone arises from the fact that both adipocytes, whose differentiation PPARγ promotes, and osteoblasts differentiate from the same mesenchymal progenitor cells. On the other hand, osteoclasts, mediating bone resorption, originate from hematopoietic precursors [127]. The initial suggestion that PPARγ might influence bone development and homeostasis came from an investigation of 404 Japanese menopausal women carrying a silent His477His variant of PPARγ which associated with mildly decreased bone mineral density (BMD) [65]. Although this association has been recently challenged by the lack thereof in otherwise comparable 263 Korean women [128], other evidence is aplenty and irrefutable. For example, natural and synthetic PPARγ agonists hinder bone formation in mice [129-131]. On the other hands, heterozygous PPARγ-deficient mice have enhanced osteoblastogenesis resulting in increased bone mass [132]. Similarly, the specific absence of PPARγ in fat robustly increases bone mass as it favors osteogenic rather than adipogenic differentiation of mesenchymal precursor cells [26]. The absence of PPARγ in adipocytes also limits their capacity to secrete antiosteogenic-signaling factors, including leptin, further enhancing the bone phenotype [26]. In addition, the strongly enhanced bone mass consequentially reduces the bone marrow cavity volume and suppressed hematopoiesis which is, however, compensated for by extramedullary hematopoiesis in the spleen [26]. Moreover, PPARγ haploinsufficiency (PPARγ+/−) in vitro promotes osteoblastogenesis but does not affect osteoclast differentiation [25], further strengthening the evidence for the antiosteogenic role of PPARγ.
If these data obtained in the mouse models can be extrapolated to humans, inhibition of PPARγ activity could be an interesting strategy to combat osteoporosis. It also warrants careful monitoring of T2DM patients treated with PPARγ agonists to detect the eventual development of osteoporosis. The most favorable pharmacological approaches to combat diabetes and osteoporosis via PPARγ-targeting would be those that segregate the positive effects on insulin resistance from the adverse effects on e.g. osteoporosis and cancer.
PPARγ and longevity
Obesity is accompanied by insulin resistance, hyperlipidemia and T2DM which are risk factors for many other diseases that are likely to shorten lifespan. On the other hand, also lipodystrophy is known to shorten lifespan and it results in complications surprisingly similar to those seen in obesity, both in mice and humans (reviewed in [133,134]). Conversely, caloric restriction promotes longevity and is accompanied by reduced fat mass and improved insulin sensitivity (reviewed in [135,136]). Indeed, decrease in adipose mass and alterations in the insulin/IGF-1 signaling pathway are consistently implicated as critical in mediating the extension of lifespan, as exemplified by the prolonged life of the fat-specific insulin receptor knock-out mice [137]. Among the recently identified mediators of insulin signaling are the Forkhead box ‘Other’ (FOXO) proteins (reviewed in [138]). Fittingly, the modulation of FOXO expression specifically in the adipose tissue of Drosophila or C. elegans is sufficient to mediate FOXO's effects on life span [139-141]. However, also the inhibition of PPARγ can explain the beneficial effects of caloric restriction on longevity. SIRT1, the mammalian ortholog of silent information regulator Sir2, is a NAD+-regulated protein deacetylase and a transcriptional inhibitor associating strongly with longevity. SIRT1 represses PPARγ and hence genes controlling fat storage by docking to the PPARγ corepressors NCoR and SMRT [142]. In Sirt1+/− mice, release of fatty acids from white adipocytes upon fasting is reduced, supporting Sirt1 mediated PPARγ inactivation as part of the molecular pathway connecting caloric restriction to life extension in mammals [142]. However, this model does not account for how SIRT1 mediates increased insulin sensitivity under long-term caloric restriction if it represses PPARγ. The insulin sensitizing effect of SIRT1 is, on the other hand, more likely linked to enhanced mitochondrial activity and energy expenditure as recently demonstrated [143]. Consistent with the Sirt1+/− mouse model, however, is the fact that the human Pro12Ala PPARγ variant with reduced function also associates with increased longevity [42]. Nonetheless, at present it remains unclear precisely how PPARγ influences aging and by what mechanism.
Human PPARγ mutations in the mouse
Mouse models carrying specific PPARγ mutations have been generated to further refine the information gained from the whole-body and tissue-specific deletions of PPARγ. First such model was the knock-in of alanine at position 112 (S112A) which prevents serine phosphorylation and renders PPARγ constitutively active, thus resembling but not replicating the human Pro115Gln mutation [144]. In mice this mutation preserves insulin sensitivity during diet-induced obesity due to undersized adipocytes, and elevated serum adiponectin and reduced FFA levels. This suggests that modulation in the phosphorylation state of PPARγ may serve as a pharmacological target for insulin sensitization free of weight gain. Another synthetic PPARγ variant reconstituted in mouse is the Leu466Ala variant, which in vitro is a strong dominant-negative mutant with altered coactivator (absent) and corepressor (enhanced) recruitment characteristics [145]. While this variant is lethal when homozygous, in heterozygous mice it remarkably reproduces the phenotype of adipose tissue-specific PPARγ knock-out mice [79,80] as the mice exhibit lipodystrophy and hepatosteatosis on chow diet, and insulin resistance on high-fat diet [146]. In addition, Leu466Ala female mice become hypertensive upon high-fat feeding [146], whereas in mice deficient of PPARγ in vasculature, high-fat diet induces hypertension in both males and females [103]. Unfortunately, published data is lacking on the hypertensive status of the adipose-specific PPARγ knock-outs.
Also the de facto human genetic variants of PPARγ are starting to find their way into mice. Similarly to Leu466Ala, the homozygous Pro465Leu PPARγ mutant (P467L or P495L in humans) (Fig. 1B) proved lethal [147,148]. In heterozygous state both independent versions of Pro465Leu mutant mice had, surprisingly, normal insulin sensitivity on chow diet. This is in stark contrast to the severe insulin resistance described in Pro467Leu humans [55,57]. Although the mutation in the adjacent Leu466 did lead to lipodystrophy (see above), the authors of Pro465Leu mice raised a question of how well the results obtained from PPARγ mutant mouse models actually translate to human physiology [147]. However, similarities between humans and mice with this mutation also exist, including the redistribution of fat depots [147,148], hypertension [148] and impairment in the postprandial lipid clearance [147]. Furthermore, when bred to hyperphagic ob/ob genetic background, severe insulin resistance and other metabolic disturbances became evident [147]. In mice, thermogenic capacity is also diminished due to Pro465Leu PPARγ genotype, and the brown adipocyte recruitment within the WAT is decreased [149].
Together with the vasculature-specific PPARγ-deficient mice [103], exhibiting hypertension in the absence of lipodystrophy or insulin resistance, the Pro465Leu mice [148] provide evidence against the dogma that hypertension is a consequence of the insulin resistance in the wake of PPARγ deficiency and lipodystrophy [150]. The uncoupling between lipodystrophy, insulin resistance and hypertension implies direct modulation of blood pressure by PPARγ, possibly through regulating the reninangiotensin system activity in adipose tissue [148,150]. Furthermore, this hypothesis provides an explanation for the decrease in blood pressure observed with TZD treatment and emphasizes the continued value of using natural mutations and targeted gene deficiency models to understand receptor function and pharmacology [151,152].
Conclusions and perspectives
The phenotypic effects of human PPARγ variants and various mouse models with modified PPARγ expression levels unequivocally demonstrate the highly complex nature of PPARγ biology. Despite occasionally confusing results, accumulating evidence confirms the critical role for PPARγ in adipogenesis, maintenance of glucose and lipid metabolism, bone homeostasis, and control of inflammation and blood pressure. The specific roles for PPARγ in each tissue are also emerging, as adipogenesis is regulated by PPARγ of the adipose, an inflammatory role for PPARγ is evident at least in intestinal epithelium and the brain, hypertension due to high-fat diet is modulated by endothelial PPARγ, and fluid retention is controlled by PPARγ of the kidney collecting ducts. On the other hand, concerted efforts of PPARγ in adipose tissue, liver, muscle and possibly also the macrophage are required to maintain normal insulin sensitivity. However, PPARγ is not working alone, as many of the above roles are partly complementary to those of PPARα, implicated in inflammation and fatty acid oxidation in the liver and other tissues, and the less studied, ubiquitously expressed PPARδ, also implicated in the control of inflammation as well as fat homeostasis (for review, see e.g. [153]). Taken together, in light of the adverse side effects of full PPARγ agonists, future pharmacological strategies for treatment of T2DM, metabolic syndrome and other related conditions will undoubtedly benefit both from compounds that modulate PPARγ specifically in a tissue-selective fashion [154] and compounds targeting more widely the PPAR family [155-157].
The improvement in longevity during human evolution has occurred because efficient energy conservation and storage has allowed survival through periods of famine whereas an enhanced innate immune response has protected from infections, uncontrolled cell proliferation and cancers. The fact that PPARγ has been implicated in several of these biological processes, as reviewed above, and in particular in adipogenesis and glucose homeostasis, suggests that PPARγ plays an active role in age-related pathophysiologies. It is certain that the investigation of PPARγ has already impacted on many scientific fields e.g. by revealing significant roles for PPARγ in several tissues, and not only in those directly related to fat storage. This trend is likely to continue, long into the future.
Acknowledgments
This work was supported by grants from CNRS, INSERM, Hopitaux Universitaires de Strasbourg, Academy of Finland, EU, EMBO and NIH (DK67320). We thank the members of the Auwerx Laboratory for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J. Biol. Chem. 2001;276:37731–4. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 2.Knouff C, Auwerx J. Peroxisome proliferator-activated receptor-gamma calls for activation in moderation: lessons from genetics and pharmacology. Endocr. Rev. 2004;25:899–918. doi: 10.1210/er.2003-0036. [DOI] [PubMed] [Google Scholar]
- 3.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–9. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Qi C, Korenberg JR, Chen XN, Noya D, Rao MS, Reddy JK. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc. Natl. Acad. Sci. U. S. A. 1995;92:7921–5. doi: 10.1073/pnas.92.17.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Y, Alvares K, Huang Q, Rao MS, Reddy JK. Cloning of a new member of the peroxisome proliferator-activated receptor gene family from mouse liver. J. Biol. Chem. 1993;268:26817–20. [PubMed] [Google Scholar]
- 6.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–34. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 7.Greene ME, Blumberg B, McBride OW, Yi HF, Kronquist K, Kwan K, Hsieh L, Greene G, Nimer SD. Isolation of the human peroxisome proliferator activated receptor gamma cDNA: expression in hematopoietic cells and chromosomal mapping. Gene Expr. 1995;4:281–99. [PMC free article] [PubMed] [Google Scholar]
- 8.Aperlo C, Pognonec P, Saladin R, Auwerx J, Boulukos KE. cDNA cloning and characterization of the transcriptional activities of the hamster peroxisome proliferator-activated receptor haPPAR gamma. Gene. 1995;162:297–302. doi: 10.1016/0378-1119(95)00196-d. [DOI] [PubMed] [Google Scholar]
- 9.Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R, Najib J, Laville M, Fruchart JC, Deeb S, Vidal-Puig A, Flier J, Briggs MR, Staels B, Vidal H, Auwerx J. The organization, promoter analysis, and expression of the human PPARgamma gene. J. Biol. Chem. 1997;272:18779–89. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 10.Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879–87. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 11.Werman A, Hollenberg A, Solanes G, Bjorbaek C, Vidal-Puig AJ, Flier JS. Ligand-independent activation domain in the N terminus of peroxisome proliferator-activated receptor gamma (PPARgamma). Differential activity of PPARgamma1 and −2 isoforms and influence of insulin. J. Biol. Chem. 1997;272:20230–5. doi: 10.1074/jbc.272.32.20230. [DOI] [PubMed] [Google Scholar]
- 12.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–56. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 13.Vidal-Puig AJ, Considine RV, Jimenez-Linan M, Werman A, Pories WJ, Caro JF, Flier JS. Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J. Clin. Invest. 1997;99:2416–22. doi: 10.1172/JCI119424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4312–7. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4318–23. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krey G, Braissant O, L'Horset F, Kalkhoven E, Perroud M, Parker MG, Wahli W. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol. Endocrinol. 1997;11:779–91. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 17.Picard F, Auwerx J. PPAR(gamma) and glucose homeostasis. Annu. Rev. Nutr. 2002;22:167–97. doi: 10.1146/annurev.nutr.22.010402.102808. [DOI] [PubMed] [Google Scholar]
- 18.Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J. Med. Chem. 2000;43:527–50. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 19.Mann NJ. Paleolithic nutrition: what can we learn from the past? Asia Pac. J. Clin. Nutr. 2004;13:S17. [Google Scholar]
- 20.Auwerx J. PPARgamma, the ultimate thrifty gene. Diabetologia. 1999;42:1033–49. doi: 10.1007/s001250051268. [DOI] [PubMed] [Google Scholar]
- 21.Argmann CA, Cock TA, Auwerx J. Peroxisome proliferator-activated receptor gamma: the more the merrier? Eur. J. Clin. Invest. 2005;35:82–92. doi: 10.1111/j.1365-2362.2005.01456.x. [DOI] [PubMed] [Google Scholar]
- 22.Yki-Järvinen H. Thiazolidinediones. N. Engl. J. Med. 2004;351:1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 23.Schoonjans K, Auwerx J. Thiazolidinediones: an update. Lancet. 2000;355:1008–10. doi: 10.1016/S0140-6736(00)90002-3. [DOI] [PubMed] [Google Scholar]
- 24.Mudaliar S, Chang AR, Henry RR. Thiazolidinediones, peripheral edema, and type 2 diabetes: incidence, pathophysiology, and clinical implications. Endocr. Pract. 2003;9:406–16. doi: 10.4158/EP.9.5.406. [DOI] [PubMed] [Google Scholar]
- 25.Kawaguchi H, Akune T, Yamaguchi M, Ohba S, Ogata N, Chung UI, Kubota N, Terauchi Y, Kadowaki T, Nakamura K. Distinct effects of PPARgamma insufficiency on bone marrow cells, osteoblasts, and osteoclastic cells. J. Bone Miner. Metab. 2005;23:275–9. doi: 10.1007/s00774-005-0599-2. [DOI] [PubMed] [Google Scholar]
- 26.Cock TA, Back J, Elefteriou F, Karsenty G, Kastner P, Chan S, Auwerx J. Enhanced bone formation in lipodystrophic PPARgamma(hyp/hyp) mice relocates haematopoiesis to the spleen. EMBO Rep. 2004;5:1007–12. doi: 10.1038/sj.embor.7400254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lefebvre AM, Chen I, Desreumaux P, Najib J, Fruchart JC, Geboes K, Briggs M, Heyman R, Auwerx J. Activation of the peroxisome proliferator-activated receptor gamma promotes the development of colon tumors in C57BL/6JAPCMin/+ mice. Nat. Med. 1998;4:1053–7. doi: 10.1038/2036. [DOI] [PubMed] [Google Scholar]
- 28.Saez E, Tontonoz P, Nelson MC, Alvarez JG, Ming UT, Baird SM, Thomazy VA, Evans RM. Activators of the nuclear receptor PPARgamma enhance colon polyp formation. Nat. Med. 1998;4:1058–61. doi: 10.1038/2042. [DOI] [PubMed] [Google Scholar]
- 29.Norman RA, Thompson DB, Foroud T, Garvey WT, Bennett PH, Bogardus C, Ravussin E. Genomewide search for genes influencing percent body fat in Pima Indians: suggestive linkage at chromosome 11q21-q22. Pima Diabetes Gene Group. Am. J. Hum. Genet. 1997;60:166–73. [PMC free article] [PubMed] [Google Scholar]
- 30.Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl MC, Nemesh J, Lane CR, Schaffner SF, Bolk S, Brewer C, Tuomi T, Gaudet D, Hudson TJ, Daly M, Groop L, Lander ES. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat. Genet. 2000;26:76–80. doi: 10.1038/79216. [DOI] [PubMed] [Google Scholar]
- 31.Deeb SS, Fajas L, Nemoto M, Pihlajamäki J, Mykkänen L, Kuusisto J, Laakso M, Fujimoto W, Auwerx J. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat. Genet. 1998;20:284–7. doi: 10.1038/3099. [DOI] [PubMed] [Google Scholar]
- 32.Masugi J, Tamori Y, Mori H, Koike T, Kasuga M. Inhibitory effect of a prolineto-alanine substitution at codon 12 of peroxisome proliferator-activated receptor-gamma 2 on thiazolidinedione-induced adipogenesis. Biochem. Biophys. Res. Commun. 2000;268:178–82. doi: 10.1006/bbrc.2000.2096. [DOI] [PubMed] [Google Scholar]
- 33.Florez JC, Hirschhorn J, Altshuler D. The inherited basis of diabetes mellitus: implications for the genetic analysis of complex traits. Annu. Rev. Genomics. Hum. Genet. 2003;4:257–91. doi: 10.1146/annurev.genom.4.070802.110436. [DOI] [PubMed] [Google Scholar]
- 34.Masud S, Ye S. Effect of the peroxisome proliferator activated receptor-gamma gene Pro12Ala variant on body mass index: a meta-analysis. J. Med. Genet. 2003;40:773–80. doi: 10.1136/jmg.40.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tönjes A, Scholz M, Loeffler M, Stumvoll M. Association of Pro12Ala Polymorphism in Peroxisome Proliferator-Activated Receptor {gamma} With Pre-Diabetic Phenotypes: Meta-analysis of 57 studies on nondiabetic individuals. Diabetes Care. 2006;29:2489–97. doi: 10.2337/dc06-0513. [DOI] [PubMed] [Google Scholar]
- 36.Ek J, Urhammer SA, Sorensen TI, Andersen T, Auwerx J, Pedersen O. Homozygosity of the Pro12Ala variant of the peroxisome proliferation-activated receptor-gamma2 (PPAR-gamma2): divergent modulating effects on body mass index in obese and lean Caucasian men. Diabetologia. 1999;42:892–5. doi: 10.1007/s001250051243. [DOI] [PubMed] [Google Scholar]
- 37.Vänttinen M, Nuutila P, Pihlajamäki J, Hallsten K, Virtanen KA, Lautamäki R, Peltoniemi P, Kemppainen J, Takala T, Viljanen AP, Knuuti J, Laakso M. The effect of the Ala12 allele of the peroxisome proliferator-activated receptor-gamma2 gene on skeletal muscle glucose uptake depends on obesity: a positron emission tomography study. J. Clin. Endocrinol. Metab. 2005;90:4249–54. doi: 10.1210/jc.2005-0101. [DOI] [PubMed] [Google Scholar]
- 38.Soriguer F, Morcillo S, Cardona F, Rojo-Martinez G, de la Cruz Almaraz M, Ruiz de Adana Mde L, Olveira G, Tinahones F, Esteva I. Pro12Ala polymorphism of the PPARG2 gene is associated with type 2 diabetes mellitus and peripheral insulin sensitivity in a population with a high intake of oleic acid. J. Nutr. 2006;136:2325–30. doi: 10.1093/jn/136.9.2325. [DOI] [PubMed] [Google Scholar]
- 39.Franks PW, Luan J, Browne PO, Harding AH, O'Rahilly S, Chatterjee VK, Wareham NJ. Does peroxisome proliferator-activated receptor gamma genotype (Pro12ala) modify the association of physical activity and dietary fat with fasting insulin level? Metabolism. 2004;53:11–6. doi: 10.1016/j.metabol.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Luan J, Browne PO, Harding AH, Halsall DJ, O'Rahilly S, Chatterjee VK, Wareham NJ. Evidence for gene-nutrient interaction at the PPARgamma locus. Diabetes. 2001;50:686–9. doi: 10.2337/diabetes.50.3.686. [DOI] [PubMed] [Google Scholar]
- 41.Al-Shali KZ, House AA, Hanley AJ, Khan HM, Harris SB, Zinman B, Mamakeesick M, Fenster A, Spence JD, Hegele RA. Genetic variation in PPARG encoding peroxisome proliferator-activated receptor gamma associated with carotid atherosclerosis. Stroke. 2004;35:2036–40. doi: 10.1161/01.STR.0000138784.68159.a5. [DOI] [PubMed] [Google Scholar]
- 42.Barbieri M, Bonafe M, Rizzo MR, Ragno E, Olivieri F, Marchegiani F, Franceschi C, Paolisso G. Gender specific association of genetic variation in peroxisome proliferator-activated receptor (PPAR)gamma-2 with longevity. Exp. Gerontol. 2004;39:1095–100. doi: 10.1016/j.exger.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 43.Ridker PM, Cook NR, Cheng S, Erlich HA, Lindpaintner K, Plutzky J, Zee RY. Alanine for proline substitution in the peroxisome proliferator-activated receptor gamma-2 (PPARG2) gene and the risk of incident myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2003;23:859–63. doi: 10.1161/01.ATV.0000068680.19521.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Temelkova-Kurktschiev T, Hanefeld M, Chinetti G, Zawadzki C, Haulon S, Kubaszek A, Koehler C, Leonhardt W, Staels B, Laakso M. Ala12Ala genotype of the peroxisome proliferator-activated receptor gamma2 protects against atherosclerosis. J. Clin. Endocrinol. Metab. 2004;89:4238–42. doi: 10.1210/jc.2003-032120. [DOI] [PubMed] [Google Scholar]
- 45.Ylihärsilä H, Eriksson JG, Forsen T, Laakso M, Uusitupa M, Osmond C, Barker DJ. Interactions between peroxisome proliferator-activated receptor-gamma 2 gene polymorphisms and size at birth on blood pressure and the use of antihypertensive medication. J. Hypertens. 2004;22:1283–7. doi: 10.1097/01.hjh.0000125438.28861.a4. [DOI] [PubMed] [Google Scholar]
- 46.Zmuda JM, Modugno F, Weissfeld JL, Cauley JA, Trump DL, Moffett SP, Ferrell RE. Peroxisome proliferator-activated receptor-gamma polymorphism, body mass and prostate cancer risk: evidence for gene-environment interaction. Oncology. 2006;70:185–9. doi: 10.1159/000093805. [DOI] [PubMed] [Google Scholar]
- 47.Pihlajamäki J, Vanhala M, Vanhala P, Laakso M. The Pro12Ala polymorphism of the PPAR gamma 2 gene regulates weight from birth to adulthood. Obes. Res. 2004;12:187–90. doi: 10.1038/oby.2004.25. [DOI] [PubMed] [Google Scholar]
- 48.Yaffe K, Kanaya AM, Lindquist K, Hsueh WC, Cummings SR, Beamer B, Newman A, Rosano C, Li R, Harris T. PPAR-gamma Pro12Ala genotype and risk of cognitive decline in elders. Neurobiol. Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.09.010. doi:10.1016/j.neurobiolaging.2006.09.010. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Semple RK, Chatterjee VK, O'Rahilly S. PPAR gamma and human metabolic disease. J. Clin. Invest. 2006;116:581–9. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Damcott CM, Moffett SP, Feingold E, Barmada MM, Marshall JA, Hamman RF, Ferrell RE. Genetic variation in fatty acid-binding protein-4 and peroxisome proliferator-activated receptor gamma interactively influence insulin sensitivity and body composition in males. Metabolism. 2004;53:303–9. doi: 10.1016/j.metabol.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Weedon MN, McCarthy MI, Hitman G, Walker M, Groves CJ, Zeggini E, Rayner NW, Shields B, Owen KR, Hattersley AT, Frayling TM. Combining Information from Common Type 2 Diabetes Risk Polymorphisms Improves Disease Prediction. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cecil JE, Fischer B, Doney AS, Hetherington M, Watt P, Wrieden W, Bolton-Smith C, Palmer CN. The Pro12Ala and C-681G variants of the PPARG locus are associated with opposing growth phenotypes in young schoolchildren. Diabetologia. 2005;48:1496–502. doi: 10.1007/s00125-005-1817-0. [DOI] [PubMed] [Google Scholar]
- 53.Doney AS, Fischer B, Cecil JE, Boylan K, McGuigan FE, Ralston SH, Morris AD, Palmer CN. Association of the Pro12Ala and C1431T variants of PPARG and their haplotypes with susceptibility to Type 2 diabetes. Diabetologia. 2004;47:555–8. doi: 10.1007/s00125-003-1323-1. [DOI] [PubMed] [Google Scholar]
- 54.Savage DB, Agostini M, Barroso I, Gurnell M, Luan J, Meirhaeghe A, Harding AH, Ihrke G, Rajanayagam O, Soos MA, George S, Berger D, Thomas EL, Bell JD, Meeran K, Ross RJ, Vidal-Puig A, Wareham NJ, O'Rahilly S, Chatterjee VK, Schafer AJ. Digenic inheritance of severe insulin resistance in a human pedigree. Nat. Genet. 2002;31:379–84. doi: 10.1038/ng926. [DOI] [PubMed] [Google Scholar]
- 55.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O'Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–3. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 56.Agarwal AK, Garg A. A novel heterozygous mutation in peroxisome proliferator-activated receptor-gamma gene in a patient with familial partial lipodystrophy. J. Clin. Endocrinol. Metab. 2002;87:408–11. doi: 10.1210/jcem.87.1.8290. [DOI] [PubMed] [Google Scholar]
- 57.Savage DB, Tan GD, Acerini CL, Jebb SA, Agostini M, Gurnell M, Williams RL, Umpleby AM, Thomas EL, Bell JD, Dixon AK, Dunne F, Boiani R, Cinti S, Vidal-Puig A, Karpe F, Chatterjee VK, O'Rahilly S. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes. 2003;52:910–7. doi: 10.2337/diabetes.52.4.910. [DOI] [PubMed] [Google Scholar]
- 58.Agostini M, Gurnell M, Savage DB, Wood EM, Smith AG, Rajanayagam O, Garnes KT, Levinson SH, Xu HE, Schwabe JW, Willson TM, O'Rahilly S, Chatterjee VK. Tyrosine agonists reverse the molecular defects associated with dominant-negative mutations in human peroxisome proliferator-activated receptor gamma. Endocrinology. 2004;145:1527–38. doi: 10.1210/en.2003-1271. [DOI] [PubMed] [Google Scholar]
- 59.Agostini M, Schoenmakers E, Mitchell C, Szatmari I, Savage D, Smith A, Rajanayagam O, Semple R, Luan J, Bath L, Zalin A, Labib M, Kumar S, Simpson H, Blom D, Marais D, Schwabe J, Barroso I, Trembath R, Wareham N, Nagy L, Gurnell M, O'Rahilly S, Chatterjee K. Non-DNA binding, dominant-negative, human PPARgamma mutations cause lipodystrophic insulin resistance. Cell Metab. 2006;4:303–11. doi: 10.1016/j.cmet.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ristow M, Muller-Wieland D, Pfeiffer A, Krone W, Kahn CR. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N. Engl. J. Med. 1998;339:953–9. doi: 10.1056/NEJM199810013391403. [DOI] [PubMed] [Google Scholar]
- 61.Hegele RA, Cao H, Frankowski C, Mathews ST, Leff T. PPARG F388L, a transactivation-deficient mutant, in familial partial lipodystrophy. Diabetes. 2002;51:3586–90. doi: 10.2337/diabetes.51.12.3586. [DOI] [PubMed] [Google Scholar]
- 62.Francis GA, Li G, Casey R, Wang J, Cao H, Leff T, Hegele RA. Peroxisomal proliferator activated receptor-gamma deficiency in a Canadian kindred with familial partial lipodystrophy type 3 (FPLD3) BMC Med. Genet. 2006;7:3. doi: 10.1186/1471-2350-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Shali K, Cao H, Knoers N, Hermus AR, Tack CJ, Hegele RA. A single-base mutation in the peroxisome proliferator-activated receptor gamma4 promoter associated with altered in vitro expression and partial lipodystrophy. J. Clin. Endocrinol. Metab. 2004;89:5655–60. doi: 10.1210/jc.2004-0280. [DOI] [PubMed] [Google Scholar]
- 64.Meirhaeghe A, Fajas L, Helbecque N, Cottel D, Lebel P, Dallongeville J, Deeb S, Auwerx J, Amouyel P. A genetic polymorphism of the peroxisome proliferator-activated receptor gamma gene influences plasma leptin levels in obese humans. Hum. Mol. Genet. 1998;7:435–40. doi: 10.1093/hmg/7.3.435. [DOI] [PubMed] [Google Scholar]
- 65.Ogawa S, Urano T, Hosoi T, Miyao M, Hoshino S, Fujita M, Shiraki M, Orimo H, Ouchi Y, Inoue S. Association of bone mineral density with a polymorphism of the peroxisome proliferator-activated receptor gamma gene: PPARgamma expression in osteoblasts. Biochem. Biophys. Res. Commun. 1999;260:122–6. doi: 10.1006/bbrc.1999.0896. [DOI] [PubMed] [Google Scholar]
- 66.Valve R, Sivenius K, Miettinen R, Pihlajamäki J, Rissanen A, Deeb SS, Auwerx J, Uusitupa M, Laakso M. Two polymorphisms in the peroxisome proliferator-activated receptor-gamma gene are associated with severe overweight among obese women. J. Clin. Endocrinol. Metab. 1999;84:3708–12. doi: 10.1210/jcem.84.10.6061. [DOI] [PubMed] [Google Scholar]
- 67.Doney AS, Fischer B, Leese G, Morris AD, Palmer CN. Cardiovascular risk in type 2 diabetes is associated with variation at the PPARG locus: a Go-DARTS study. Arterioscler. Thromb. Vasc. Biol. 2004;24:2403–7. doi: 10.1161/01.ATV.0000147897.57527.e4. [DOI] [PubMed] [Google Scholar]
- 68.Rhee EJ, Oh KW, Lee WY, Kim SY, Oh ES, Baek KH, Kang MI, Kim SW. Effects of two common polymorphisms of peroxisome proliferator-activated receptor-gamma gene on metabolic syndrome. Arch. Med. Res. 2006;37:86–94. doi: 10.1016/j.arcmed.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 69.Muller YL, Bogardus C, Beamer BA, Shuldiner AR, Baier LJ. A functional variant in the peroxisome proliferator-activated receptor gamma2 promoter is associated with predictors of obesity and type 2 diabetes in Pima Indians. Diabetes. 2003;52:1864–71. doi: 10.2337/diabetes.52.7.1864. [DOI] [PubMed] [Google Scholar]
- 70.Meirhaeghe A, Fajas L, Gouilleux F, Cottel D, Helbecque N, Auwerx J, Amouyel P. A functional polymorphism in a STAT5B site of the human PPAR gamma 3 gene promoter affects height and lipid metabolism in a French population. Arterioscler. Thromb. Vasc. Biol. 2003;23:289–94. doi: 10.1161/01.atv.0000051382.28752.fe. [DOI] [PubMed] [Google Scholar]
- 71.Meirhaeghe A, Tanck MW, Fajas L, Janot C, Helbecque N, Cottel D, Auwerx J, Amouyel P, Dallongeville J. Study of a new PPARgamma2 promoter polymorphism and haplotype analysis in a French population. Mol. Genet. Metab. 2005;85:140–8. doi: 10.1016/j.ymgme.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 72.Wei Q, Jacobs DR, Jr., Schreiner PJ, Siscovick DS, Steffes MW, Fornage M. Patterns of association between PPARgamma genetic variation and indices of adiposity and insulin action in African-Americans and whites: the CARDIA Study. J. Mol. Med. 2006;84:955–65. doi: 10.1007/s00109-006-0088-7. [DOI] [PubMed] [Google Scholar]
- 73.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell. 1999;4:585–95. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 74.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell. 1999;4:611–7. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 75.Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Kadowaki T, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 76.Miles PD, Barak Y, Evans RM, Olefsky JM. Effect of heterozygous PPARgamma deficiency and TZD treatment on insulin resistance associated with age and high-fat feeding. Am. J. Physiol. Endocrinol. Metab. 2003;284:E618–26. doi: 10.1152/ajpendo.00312.2002. [DOI] [PubMed] [Google Scholar]
- 77.Jones JR, Shelton KD, Guan Y, Breyer MD, Magnuson MA. Generation and functional confirmation of a conditional null PPARgamma allele in mice. Genesis. 2002;32:134–7. doi: 10.1002/gene.10042. [DOI] [PubMed] [Google Scholar]
- 78.Koutnikova H, Cock TA, Watanabe M, Houten SM, Champy MF, Dierich A, Auwerx J. Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPAR gamma hypomorphic mice. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14457–62. doi: 10.1073/pnas.2336090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15712–7. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, Shiota M, Kesterson RA, Kahn BB, Magnuson MA. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. U. S. A. 2005;102:6207–12. doi: 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Imai T, Takakuwa R, Marchand S, Dentz E, Bornert JM, Messaddeq N, Wendling O, Mark M, Desvergne B, Wahli W, Chambon P, Metzger D. Peroxisome proliferator-activated receptor gamma is required in mature white and brown adipocytes for their survival in the mouse. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4543–7. doi: 10.1073/pnas.0400356101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang J, Fu M, Cui T, Xiong C, Xu K, Zhong W, Xiao Y, Floyd D, Liang J, Li E, Song Q, Chen YE. Selective disruption of PPARgamma 2 impairs the development of adipose tissue and insulin sensitivity. Proc. Natl. Acad. Sci. U. S. A. 2004;101:10703–8. doi: 10.1073/pnas.0403652101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Medina-Gomez G, Virtue S, Lelliott C, Boiani R, Campbell M, Christodoulides C, Perrin C, Jimenez-Linan M, Blount M, Dixon J, Zahn D, Thresher RR, Aparicio S, Carlton M, Colledge WH, Kettunen MI, Seppanen-Laakso T, Sethi JK, O'Rahilly S, Brindle K, Cinti S, Oresic M, Burcelin R, Vidal-Puig A. The link between nutritional status and insulin sensitivity is dependent on the adipocyte-specific peroxisome proliferator-activated receptor-gamma2 isoform. Diabetes. 2005;54:1706–16. doi: 10.2337/diabetes.54.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Norris AW, Chen L, Fisher SJ, Szanto I, Ristow M, Jozsi AC, Hirshman MF, Rosen ED, Goodyear LJ, Gonzalez FJ, Spiegelman BM, Kahn CR. Muscle-specific PPAR{gamma}-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J. Clin. Invest. 2003;112:608–618. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hevener AL, He W, Barak Y, Le J, Bandyopadhyay G, Olson P, Wilkes J, Evans RM, Olefsky J. Muscle-specific Pparg deletion causes insulin resistance. Nat. Med. 2003;9:1491–7. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- 86.Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, Nicol CJ, Vinson C, Gonzalez FJ, Reitman ML. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J. Biol. Chem. 2003;278:34268–76. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 87.Matsusue K, Haluzik M, Lambert G, Yim SH, Gavrilova O, Ward JM, Brewer B, Jr., Reitman ML, Gonzalez FJ. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J. Clin. Invest. 2003;111:737–47. doi: 10.1172/JCI17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Singh J, Verma NK, Kansagra SM, Kate BN, Dey CS. Altered PPARgamma expression inhibits myogenic differentiation in C2C12 skeletal muscle cells. Mol. Cell. Biochem. 2007;294:163–71. doi: 10.1007/s11010-006-9256-x. [DOI] [PubMed] [Google Scholar]
- 89.Yu S, Matsusue K, Kashireddy P, Cao WQ, Yeldandi V, Yeldandi AV, Rao MS, Gonzalez FJ, Reddy JK. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J. Biol. Chem. 2003;278:498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]
- 90.Yu S, Viswakarma N, Batra SK, Sambasiva Rao M, Reddy JK. Identification of promethin and PGLP as two novel up-regulated genes in PPARgamma1-induced adipogenic mouse liver. Biochimie. 2004;86:743–61. doi: 10.1016/j.biochi.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 91.Tanaka T, Masuzaki H, Ebihara K, Ogawa Y, Yasue S, Yukioka H, Chusho H, Miyanaga F, Miyazawa T, Fujimoto M, Kusakabe T, Kobayashi N, Hayashi T, Hosoda K, Nakao K. Transgenic expression of mutant peroxisome proliferator-activated receptor gamma in liver precipitates fasting-induced steatosis but protects against high-fat diet-induced steatosis in mice. Metabolism. 2005;54:1490–8. doi: 10.1016/j.metabol.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 92.Welters HJ, McBain SC, Tadayyon M, Scarpello JH, Smith SA, Morgan NG. Expression and functional activity of PPARgamma in pancreatic beta cells. Br. J. Pharmacol. 2004;142:1162–70. doi: 10.1038/sj.bjp.0705844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dubois M, Pattou F, Kerr-Conte J, Gmyr V, Vandewalle B, Desreumaux P, Auwerx J, Schoonjans K, Lefebvre J. Expression of peroxisome proliferator-activated receptor gamma (PPARgamma) in normal human pancreatic islet cells. Diabetologia. 2000;43:1165–9. doi: 10.1007/s001250051508. [DOI] [PubMed] [Google Scholar]
- 94.Rosen ED, Kulkarni RN, Sarraf P, Ozcan U, Okada T, Hsu CH, Eisenman D, Magnuson MA, Gonzalez FJ, Kahn CR, Spiegelman BM. Targeted elimination of peroxisome proliferator-activated receptor gamma in beta cells leads to abnormalities in islet mass without compromising glucose homeostasis. Mol. Cell. Biol. 2003;23:7222–9. doi: 10.1128/MCB.23.20.7222-7229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matsui J, Terauchi Y, Kubota N, Takamoto I, Eto K, Yamashita T, Komeda K, Yamauchi T, Kamon J, Kita S, Noda M, Kadowaki T. Pioglitazone reduces islet triglyceride content and restores impaired glucose-stimulated insulin secretion in heterozygous peroxisome proliferator-activated receptor-gamma-deficient mice on a high-fat diet. Diabetes. 2004;53:2844–54. doi: 10.2337/diabetes.53.11.2844. [DOI] [PubMed] [Google Scholar]
- 96.Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell. 2001;7:161–71. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 97.Babaev VR, Yancey PG, Ryzhov SV, Kon V, Breyer MD, Magnuson MA, Fazio S, Linton MF. Conditional knockout of macrophage PPARgamma increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005;25:1647–53. doi: 10.1161/01.ATV.0000173413.31789.1a. [DOI] [PubMed] [Google Scholar]
- 98.Akiyama TE, Sakai S, Lambert G, Nicol CJ, Matsusue K, Pimprale S, Lee YH, Ricote M, Glass CK, Brewer HB, Jr., Gonzalez FJ. Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol. Cell. Biol. 2002;22:2607–19. doi: 10.1128/MCB.22.8.2607-2619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Luca C, Olefsky JM. Stressed out about obesity and insulin resistance. Nat. Med. 2006;12:41–2. doi: 10.1038/nm0106-41. [DOI] [PubMed] [Google Scholar]
- 100.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 101.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nicol CJ, Adachi M, Akiyama TE, Gonzalez FJ. PPARgamma in endothelial cells influences high fat diet-induced hypertension. Am. J. Hypertens. 2005;18:549–56. doi: 10.1016/j.amjhyper.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 104.Asakawa M, Takano H, Nagai T, Uozumi H, Hasegawa H, Kubota N, Saito T, Masuda Y, Kadowaki T, Komuro I. Peroxisome proliferator-activated receptor gamma plays a critical role in inhibition of cardiac hypertrophy in vitro and in vivo. Circulation. 2002;105:1240–6. doi: 10.1161/hc1002.105225. [DOI] [PubMed] [Google Scholar]
- 105.Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ. Res. 2005;97:372–9. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- 106.Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ, Yang T. Collecting duct-specific deletion of peroxisome proliferator-activated receptor gamma blocks thiazolidinedione-induced fluid retention. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9406–11. doi: 10.1073/pnas.0501744102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat. Med. 2005;11:861–6. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- 108.Wang AC, Dai X, Luu B, Conrad DJ. Peroxisome proliferator-activated receptor-gamma regulates airway epithelial cell activation. Am. J. Respir. Cell. Mol. Biol. 2001;24:688–93. doi: 10.1165/ajrcmb.24.6.4376. [DOI] [PubMed] [Google Scholar]
- 109.Simon DM, Arikan MC, Srisuma S, Bhattacharya S, Tsai LW, Ingenito EP, Gonzalez F, Shapiro SD, Mariani TJ. Epithelial cell PPAR[gamma] contributes to normal lung maturation. Faseb J. 2006;20:1507–9. doi: 10.1096/fj.05-5410fje. [DOI] [PubMed] [Google Scholar]
- 110.Woerly G, Honda K, Loyens M, Papin JP, Auwerx J, Staels B, Capron M, Dombrowicz D. Peroxisome proliferator-activated receptors alpha and gamma down-regulate allergic inflammation and eosinophil activation. J. Exp. Med. 2003;198:411–21. doi: 10.1084/jem.20021384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Delayre-Orthez C, Becker J, Guenon I, Lagente V, Auwerx J, Frossard N, Pons F. PPARalpha downregulates airway inflammation induced by lipopolysaccharide in the mouse. Respir. Res. 2005;6:91. doi: 10.1186/1465-9921-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cui Y, Miyoshi K, Claudio E, Siebenlist UK, Gonzalez FJ, Flaws J, Wagner KU, Hennighausen L. Loss of the peroxisome proliferation-activated receptor gamma (PPARgamma ) does not affect mammary development and propensity for tumor formation but leads to reduced fertility. J. Biol. Chem. 2002;277:17830–5. doi: 10.1074/jbc.M200186200. [DOI] [PubMed] [Google Scholar]
- 113.Schaiff WT, Barak Y, Sadovsky Y. The pleiotropic function of PPAR gamma in the placenta. Mol. Cell. Endocrinol. 2006;249:10–5. doi: 10.1016/j.mce.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 114.Aquila S, Bonofiglio D, Gentile M, Middea E, Gabriele S, Belmonte M, Catalano S, Pellegrino M, Ando S. Peroxisome proliferator-activated receptor (PPAR)gamma is expressed by human spermatozoa: Its potential role on the sperm physiology. J. Cell Physiol. 2006;209:977–86. doi: 10.1002/jcp.20807. [DOI] [PubMed] [Google Scholar]
- 115.Lefebvre M, Paulweber B, Fajas L, Woods J, McCrary C, Colombel JF, Najib J, Fruchart JC, Datz C, Vidal H, Desreumaux P, Auwerx J. Peroxisome proliferator-activated receptor gamma is induced during differentiation of colon epithelium cells. J. Endocrinol. 1999;162:331–40. doi: 10.1677/joe.0.1620331. [DOI] [PubMed] [Google Scholar]
- 116.Girnun GD, Smith WM, Drori S, Sarraf P, Mueller E, Eng C, Nambiar P, Rosenberg DW, Bronson RT, Edelmann W, Kucherlapati R, Gonzalez FJ, Spiegelman BM. APC-dependent suppression of colon carcinogenesis by PPARgamma. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13771–6. doi: 10.1073/pnas.162480299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McAlpine CA, Barak Y, Matise I, Cormier RT. Intestinal-specific PPARgamma deficiency enhances tumorigenesis in Apc(Min/+) mice. Int. J. Cancer. 2006;119:2339–46. doi: 10.1002/ijc.22115. [DOI] [PubMed] [Google Scholar]
- 118.Fajas L, Egler V, Reiter R, Miard S, Lefebvre AM, Auwerx J. PPARgamma controls cell proliferation and apoptosis in an RB-dependent manner. Oncogene. 2003;22:4186–93. doi: 10.1038/sj.onc.1206530. [DOI] [PubMed] [Google Scholar]
- 119.Adachi M, Kurotani R, Morimura K, Shah Y, Sanford M, Madison BB, Gumucio DL, Marin HE, Peters JM, Young HA, Gonzalez FJ. Peroxisome proliferator activated receptor gamma in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut. 2006;55:1104–13. doi: 10.1136/gut.2005.081745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Desreumaux P, Dubuquoy L, Nutten S, Peuchmaur M, Englaro W, Schoonjans K, Derijard B, Desvergne B, Wahli W, Chambon P, Leibowitz MD, Colombel JF, Auwerx J. Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor gamma (PPARgamma) heterodimer. A basis for new therapeutic strategies. J. Exp. Med. 2001;193:827–38. doi: 10.1084/jem.193.7.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rousseaux C, Lefebvre B, Dubuquoy L, Lefebvre P, Romano O, Auwerx J, Metzger D, Wahli W, Desvergne B, Naccari GC, Chavatte P, Farce A, Bulois P, Cortot A, Colombel JF, Desreumaux P. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J. Exp. Med. 2005;201:1205–15. doi: 10.1084/jem.20041948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK. PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6712–7. doi: 10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mao-Qiang M, Fowler AJ, Schmuth M, Lau P, Chang S, Brown BE, Moser AH, Michalik L, Desvergne B, Wahli W, Li M, Metzger D, Chambon PH, Elias PM, Feingold KR. Peroxisome-proliferator-activated receptor (PPAR)-gamma activation stimulates keratinocyte differentiation. J. Invest. Dermatol. 2004;123:305–12. doi: 10.1111/j.0022-202X.2004.23235.x. [DOI] [PubMed] [Google Scholar]
- 124.Wada K, Nakajima A, Katayama K, Kudo C, Shibuya A, Kubota N, Terauchi Y, Tachibana M, Miyoshi H, Kamisaki Y, Mayumi T, Kadowaki T, Blumberg RS. Peroxisome proliferator-activated receptor gamma-mediated regulation of neural stem cell proliferation and differentiation. J. Biol. Chem. 2006;281:12673–81. doi: 10.1074/jbc.M513786200. [DOI] [PubMed] [Google Scholar]
- 125.Raikwar HP, Muthian G, Rajasingh J, Johnson CN, Bright JJ. PPARgamma antagonists reverse the inhibition of neural antigen-specific Th(1) response and experimental allergic encephalomyelitis by Ciglitazone and 15-Deoxy-Delta(12,14)-Prostaglandin J(2) J. Neuroimmunol. 2006;178:76–86. doi: 10.1016/j.jneuroim.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 126.Natarajan C, Muthian G, Barak Y, Evans RM, Bright JJ. Peroxisome proliferator-activated receptor-gamma-deficient heterozygous mice develop an exacerbated neural antigen-induced Th1 response and experimental allergic encephalomyelitis. J. Immunol. 2003;171:5743–50. doi: 10.4049/jimmunol.171.11.5743. [DOI] [PubMed] [Google Scholar]
- 127.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 128.Rhee EJ, Oh KW, Lee WY, Kim SY, Oh ES, Baek KH, Kang MI, Kim SW. The effects of C161-->T polymorphisms in exon 6 of peroxisome proliferator-activated receptor-gamma gene on bone mineral metabolism and serum osteoprotegerin levels in healthy middle-aged women. Am. J. Obstet. Gynecol. 2005;192:1087–93. doi: 10.1016/j.ajog.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 129.Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004;145:401–6. doi: 10.1210/en.2003-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Klein RF, Allard J, Avnur Z, Nikolcheva T, Rotstein D, Carlos AS, Shea M, Waters RV, Belknap JK, Peltz G, Orwoll ES. Regulation of bone mass in mice by the lipoxygenase gene Alox15. Science. 2004;303:229–32. doi: 10.1126/science.1090985. [DOI] [PubMed] [Google Scholar]
- 131.Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146:1226–35. doi: 10.1210/en.2004-0735. [DOI] [PubMed] [Google Scholar]
- 132.Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Invest. 2004;113:846–55. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reue K, Phan J. Metabolic consequences of lipodystrophy in mouse models. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:436–41. doi: 10.1097/01.mco.0000232904.82038.db. [DOI] [PubMed] [Google Scholar]
- 134.Garg A. Acquired and inherited lipodystrophies. N. Engl. J. Med. 2004;350:1220–34. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 135.Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17:313–21. doi: 10.1101/gad.1052903. [DOI] [PubMed] [Google Scholar]
- 136.Wolf G. Calorie restriction increases life span: a molecular mechanism. Nutr. Rev. 2006;64:89–92. doi: 10.1301/nr.2006.feb.89-92. [DOI] [PubMed] [Google Scholar]
- 137.Blüher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–4. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 138.Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol. Metab. 2005;16:183–9. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 139.Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science. 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- 140.Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–6. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- 141.Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- 142.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–6. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 144.Rangwala SM, Rhoades B, Shapiro JS, Rich AS, Kim JK, Shulman GI, Kaestner KH, Lazar MA. Genetic modulation of PPARgamma phosphorylation regulates insulin sensitivity. Dev. Cell. 2003;5:657–63. doi: 10.1016/s1534-5807(03)00274-0. [DOI] [PubMed] [Google Scholar]
- 145.Park Y, Freedman BD, Lee EJ, Park S, Jameson JL. A dominant negative PPARgamma mutant shows altered cofactor recruitment and inhibits adipogenesis in 3T3-L1 cells. Diabetologia. 2003;46:365–77. doi: 10.1007/s00125-003-1037-4. [DOI] [PubMed] [Google Scholar]
- 146.Freedman BD, Lee EJ, Park Y, Jameson JL. A dominant negative peroxisome proliferator-activated receptor-gamma knock-in mouse exhibits features of the metabolic syndrome. J. Biol. Chem. 2005;280:17118–25. doi: 10.1074/jbc.M407539200. [DOI] [PubMed] [Google Scholar]
- 147.Gray SL, Dalla Nora E, Grosse J, Manieri M, Stoeger T, Medina-Gomez G, Burling K, Wattler S, Russ A, Yeo GS, Chatterjee VK, O'Rahilly S, Voshol PJ, Cinti S, Vidal-Puig A. Leptin Deficiency Unmasks the Deleterious Effects of Impaired Peroxisome Proliferator-Activated Receptor {gamma} Function (P465L PPAR{gamma}) in Mice. Diabetes. 2006;55:2669–2677. doi: 10.2337/db06-0389. [DOI] [PubMed] [Google Scholar]
- 148.Tsai YS, Kim HJ, Takahashi N, Kim HS, Hagaman JR, Kim JK, Maeda N. Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARgamma. J. Clin. Invest. 2004;114:240–9. doi: 10.1172/JCI20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gray SL, Dalla Nora E, Backlund EC, Manieri M, Virtue S, Noland RC, O'Rahilly S, Cortright RN, Cinti S, Cannon B, Vidal-Puig A. Decreased brown adipocyte recruitment and thermogenic capacity in mice with impaired peroxisome proliferator-activated receptor (P465L PPARgamma) function. Endocrinology. 2006;147:5708–14. doi: 10.1210/en.2006-0684. [DOI] [PubMed] [Google Scholar]
- 150.Hegele RA, Leff T. Unbuckling lipodystrophy from insulin resistance and hypertension. J. Clin. Invest. 2004;114:163–5. doi: 10.1172/JCI22382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. PPAR(gamma) agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension. 2004;43:661–6. doi: 10.1161/01.HYP.0000116303.71408.c2. [DOI] [PubMed] [Google Scholar]
- 152.Dobrian AD, Schriver SD, Khraibi AA, Prewitt RL. Pioglitazone prevents hypertension and reduces oxidative stress in diet-induced obesity. Hypertension. 2004;43:48–56. doi: 10.1161/01.HYP.0000103629.01745.59. [DOI] [PubMed] [Google Scholar]
- 153.Blaschke F, Takata Y, Caglayan E, Law RE, Hsueh WA. Obesity, peroxisome proliferator-activated receptor, and atherosclerosis in type 2 diabetes. Arterioscler. Thromb. Vasc. Biol. 2006;26:28–40. doi: 10.1161/01.ATV.0000191663.12164.77. [DOI] [PubMed] [Google Scholar]
- 154.Rocchi S, Picard F, Vamecq J, Gelman L, Potier N, Zeyer D, Dubuquoy L, Bac P, Champy MF, Plunket KD, Leesnitzer LM, Blanchard SG, Desreumaux P, Moras D, Renaud JP, Auwerx J. A unique PPARgamma ligand with potent insulin-sensitizing yet weak adipogenic activity. Mol. Cell. 2001;8:737–47. doi: 10.1016/s1097-2765(01)00353-7. [DOI] [PubMed] [Google Scholar]
- 155.Tenenbaum A, Motro M, Fisman EZ, Schwammenthal E, Adler Y, Goldenberg I, Leor J, Boyko V, Mandelzweig L, Behar S. Peroxisome proliferator-activated receptor ligand bezafibrate for prevention of type 2 diabetes mellitus in patients with coronary artery disease. Circulation. 2004;109:2197–202. doi: 10.1161/01.CIR.0000126824.12785.B6. [DOI] [PubMed] [Google Scholar]
- 156.Fiávet C, Fruchart JC, Staels B. PPARalpha and PPARgamma dual agonists for the treatment of type 2 diabetes and the metabolic syndrome. Curr. Opin. Pharmacol. 2006;6:606–14. doi: 10.1016/j.coph.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 157.Xu Y, Etgen GJ, Broderick CL, Canada E, Gonzalez I, Lamar J, MontroseRafizadeh C, Oldham BA, Osborne JJ, Xie C, Shi Q, Winneroski LL, York J, Yumibe N, Zink R, Mantlo N. Design and synthesis of dual peroxisome proliferator-activated receptors gamma and delta agonists as novel euglycemic agents with a reduced weight gain profile. J. Med. Chem. 2006;49:5649–52. doi: 10.1021/jm060617c. [DOI] [PubMed] [Google Scholar]