Abstract
Background
Post-hepatectomy liver insufficiency is one of the most serious postoperative problems and its prevention is important after major hepatic resection, especially in the cirrhotic liver. Some growth factors and cytokines appear to play important roles in liver regeneration. In the present study we have investigated the effects of granulocyte-macrophage colony-stimulating factor (GM-CSF) on hepatic regeneration after 70% partial hepatectomy (PH) in cirrhotic and non-cirrhotic rats.
Methods
A rat model of liver cirrhosis was prepared using thioacetamide (TAA) (a dose of 20 mg/100 g body w, intra-peritoneally) on three days a week for 12 weeks. Adult male rats were divided into four groups:Group 1 (n=10) no cirrhosis and no GM-CSF; Group 2 (n=10) no cirrhosis and GM-CSF; Group 3 (n=10) cirrhosis and no GM-CSF; and Group 4 (n=10) cirrhosis and GM-CSF. All the rats underwent a 70% hepatectomy, and GM-CSF was administrated immediately after operation in Groups 2 and 4. On postoperative days 2 and 7, fresh samples from the remnant liver were obtained to evaluate its regenerative capacity.The liver regenerative process was estimated by DNA synthesis, using flow cytometry.
Results
Proliferation index (PI) of hepatocytes at 48 h was higher in Group 4 rats than Group 3 rats (p<0.05). On postoperative day 7, PI was elevated in Group 3 rats compared with Group 4 rats, but this difference was not statistically significant. In non-cirrhotic rats given GM-CSF, PI was increased compared with Group 1 rats at day 2 (p<0.05), but not at day 7.
Conclusions
The findings suggest that the proliferative capacity of liver cells is impaired and delayed after 70% PH in cirrhotic rat liver. GM-CSF administration might enhance the liver PI in both normal and TAA-induced cirrhotic rats.
Keywords: cirrhosis, GM-CSF, liver resection, liver regeneration
Introduction
Liver regeneration is an essential component of the reparative process following liver injury and surgical resection in man 1. In its absence, morbidity and mortality rates are often increased. The capacity for hepatic regeneration after hepatectomy is important for allowing surgeons to determine the appropriate extent of resection. Post-hepatectomy liver insufficiency is one of the most serious problems associated with liver surgery, especially in the cirrhotic liver, which has less function reserve than the normal liver 2.
Since hepatocellular carcinoma (HCC) is often seen in cirrhotic livers, the morbidity and mortality rates after hepatectomy are higher in these patients 3. Insufficient regeneration and dysfunction of cirrhotic liver following partial hepatectomy (PH) often make the patients vulnerable to postoperative liver failure, which frequently leads to multiple organ failure. In operations for HCC, the degree of underlying liver cirrhosis limits the extent of safe hepatic resection.
Experimental hepatic cirrhosis has been induced in rats with chronic administration of hepatotoxins such as carbon tetrachloride (CCI4), dimethylnitrosamine (DMN) or thioacetamide (TAA) 1,4,5,6. These animal models mimic, to various degrees, the pathological processes observed in human hepatic fibrosis. TAA is a well known hepatotoxin and has been used in studying liver pathology.
It causes hepatic centrilobular necrosis after acute administration, while its chronic administration induces liver cirrhosis and bile duct carcinoma in a rat model 5,6,7,8,9.
Prevention of postoperative hepatic failure is important after liver resection. Hepatic regeneration is a physiological mechanism that leads to restoration of remnant hepatic parenchyma after partial hepatectomy (PH). This process is mediated by a variety of cytokines and growth factors 1,2,7,10,11,12,13,14,15. Hepatocyte growth factor (HGF) was originally identified as the most potent stimulator of DNA synthesis in primary hepatocytes 16.
The liver is the target organ for many cytokines, and some cytokines are known to affect the proliferation of hepatocytes 1,17,18. Many growth factors and cytokines, including HGF, epidermal growth factor, transforming growth factor-β interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α), insulin and noradrenaline (norepinephrine), appear to play important roles in liver regenerative capacity in response to loss of hepatic parenchyma.
Granulocyte-macrophage colonystimulating factor (GM-CSF) is a cytokine able to regulate a number of functions. It was first identified as the most potent mitogen for bone marrow 19, but also has been used in local treatment of impaired wound healing 20. It not only influences the proliferation and differentiation of stem cells, but also regulates some other cells involved in acute and chronic inflammation 20,21. We have previously demonstrated that GM-CSF therapy improves radiation-impaired wound healing in rats 22. To our knowledge, there has been no previous report on the effect of GM-CSF on hepatic regeneration after PH. We have therefore developed an animal model and investigated its effect on hepatic regeneration after 70% PH in normal and TAA-induced cirrhotic rats.
Material and methods
Male Wistar rats (n=85) weighing 240–285 g were obtained from the Refik Saydam Central Institute of Hygiene, Drug and Cosmetic Research Department, Ankara, Turkey for use in this study. The study was approved by the local ethics committee. The rats were randomly selected and assigned to experimental groups. Animals were kept in an air-conditioned room at 21 °C, received humane care and were given a standard rat diet and water ad libitum under standard environmental conditions.
Liver cirrhosis was induced in 60 rats by administrating TAA (Sigma Chemical Co. St. Louis, MO). Rats in the cirrhosis groups received intraperitoneal injections of a sterile solution of TAA dissolved in 0.15 mol/liter NaCI (40 mg/ml) at a dose of 20 mg/100 g body wt and administered three times per week for up to 12 weeks. Animals were then rested for 2 weeks to allow TAA washout before operation. They had no additional treatment during the study period. Animals were fasted for 12 h before 70% PH (70% hepatectomy comprised removal of the left lateral and median lobes).
The animals were anaesthetised with an intramuscular injection of ketamine hydrochloride (100 mg/kg) (Ketalar, Parke-Davis, Morris Plains, NJ, USA). All the normal and TAA-induced cirrhotic rats were subjected to 70% PH, according to the technique of Higgins and Anderson 23. Under anaesthesia, a midline incision was made in the sub-xiphoid area, the abdomen was opened and the liver was mobilised. The median and lateral lobes were then removed.
Rats were divided into four groups: Group 1 had no cirrhosis and no GM-CSF; Group 2 had no cirrhosis but received GM-CSF; Group 3 had cirrhosis but no GM-CSF; and Group 4 had both cirrhosis and GM-CSF. Recombinant human GM-CSF (rh-GM-CSF) was kindly provided by Novartis (Ankara, Turkey) in the form of Leucomax (lyophilized powder with reconstitution fluid). rh-GM-CSF (40 µ/ml) was injected subcutaneously immediately after hepatectomy in Group 2 and 4 rats.
Hepatic regeneration was documented by determining DNA synthesis by flow cytometry at various time points after PH. On postoperative day 2 and 7, fresh biopsy specimens were obtained from the rats. The specimens were stored at −30°C until flow-cytometric analysis of DNA synthesis.
Separation of nuclei
Liver tissues were mechanically divided into millimetre pieces, which were digested with 1 mg/ml protease (Sigma type XXIV) at 37°C for 30 minutes with manual shaking every 5–10 minutes. Following digestion, 2 ml cold PBS was added to the solution. The solution was filtered through a 37-µm nylon mesh. After two washings with cold PBS, cell suspensions (with 5×105 to 1×106 nuclei per ml) were prepared with trypsin buffer and were centrifuged. The nuclei were then incubated with RNAse solution for 10 minutes. Finally, the nuclear suspensions were stained with propidium iodide and were kept in the dark for at least 10 minutes at 4°C. Samples were filtered through a 37-nm nylon mesh before flow cytometric analysis. Since we could not purify the liver cells separately, the nuclei obtained from digested liver tissues included Kupffer cells as well as hepatocytes.
Flow cytometry
DNA analyses were performed with a FacSort flow cytometry (Becton & Dickinson) equipped with a 2 W argon-laser. Excitation of propidium iodid occurred at 488 nm. At least 15,000–20,000 nuclei from each specimen were analysed.
DNA histograms having only one G0/G1 peak with a coefficient of variation (CV) of less than 5% were defined as diploid 24. DNA peaks with a CV of more than 9% were not evaluated. The histograms with CV between 5% and 9% were accepted as ‘wide-diploid’ and were then collected in the same group with diploid ones. The liver tissues were classified as aneuploid if there were at least two distinct G0/G1 peaks; the latter having at least 10% of total counts. Aneuploid tissues with a DNA index (DI) between 1.9 and 2.1 were classified as tetraploid, and those with a DI >2.1 as hypertetrapooid. Cell cycle analyses were carried out with MODFIT software (Becton & Dickinson). DNA histograms having a CV of more than 8% were not evaluated for s-phase fraction (SPF) measurement 25.
The liver proliferation index (PI) expressed as G2 + SPF/M was compared between the groups. The Wilcoxon test for unpaired measurements was used to analyse the data. Differences between samples were considered significant when p value was less than 0.05. The statistical analysis was performed by Statistical Package for Social Sciences (SPSS for MS Windows Release 7.0, Chicago, IL, USA).
Results
TAA was given to 60 rats, 18 of which died during the period of administration. Because TAA induces anorexia, pair-fed control rats were given the same amount of laboratory chow as that consumed by the TAA-treated rats on the previous day, to equalise the nutritional status between groups. After the designated period of TAA injection, animals were kept for two weeks to allow the washout of TAA. During the 12-week period of TAA injection, the mean body weight of TAA-treated rats was officially lower than that of controls.
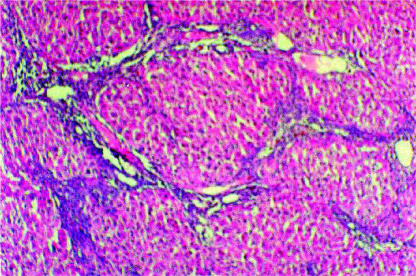
The livers of the surviving rats were shown to have the pathological criteria of cirrhosis microscopically. Cirrhosis was evaluated before the start of GM-CSF treatment. The cirrhotic liver was deformed and had a relatively small lobe. The liver was pale, with irregular large regeneration nodules. The histological appearance of liver exposed to TAA for 12 weeks is shown in Figure 1. The lobular architecture was disrupted, and pseudonodular formation was observed with wide collagen bands extending between the portal areas. Portal fibrosis became more advanced, and bridging fibrosis was also seen.
Figure 1. .
Section of liver tissue from a rat adminsteredTAA for 12 weeks (H&E; original magnification×100).
The PI of hepatocytes at 48 h after 70% PH was measured in normal and TAA-induced cirrhotic rats. The PI in normal rats treated with GM-CSF (Group 2) was higher than that of the normal controls (Table 1). However, there was no statistically significant difference between the groups at postoperative day 7. In cirrhotic rats give GM-CSF (Group 4), PI was increased compared to Group 3 at postoperative day 2 (p <0.01) (Table 2). The mean PI on day 7 in the GM-CSF-treated and control cirrhotic rats were 6.5% and 13.7%, respectively. Although this PI was higher in Group 3, the difference was not statistically significant (p >0.05). In addition, in GM-CSF-treated cirrhotic rats the PI on postoperative days 2 and 7 was 18.9% and 6.5%, respectively (p=0.004). There was no significant difference between the groups for DNA histograms.
Table 1. Proliferation index (PI) in Group I and Group 2.
| PI | Group 1 | Group 2 | p value |
|---|---|---|---|
| Postoperative day 2 | 7.6% (2.9–12.3)* | 11.2% (2.5–20.7) | 0.04 |
| Postoperative day 7 | 7.1% (2.4–20.7) | 9.6% (2.9–22.3) | NS |
* values in parentheses are range
NS: not significant
Table 2. Proliferation index (PI) in Group 3 and Group 4.
| PI | Group 3 | Group 4 | p value |
|---|---|---|---|
| Postoperative day 2 | 8.8% (1.3–19.5)* | 18.9% (12.6–26.7) | 0.02 |
| Postoperative day 7 | 13.7% (1.1–20.9) | 6.5% (2.7–13.9) | NS |
* values in parentheses are range
NS: not significant
Eleven rats submitted to a 70% hepatectomy died within 48 h. Four rats in Group 4 died shortly after the procedure and seven in Group 3. Although the mortality rate was higher in Group 3 than Group 4, this difference was not statistically significant (Pearson chi-square test, p >0.05).
Postmortem examination of these animals revealed severe ascites and portal congestion, suggesting that sequestration of blood in the splanchnic bed was the cause of death. The data show that the GM-CSF administration not only improved hepatocellular regenerative capacity, but also might suppress the onset of postoperative liver failure.
Discussion
Cirrhotic livers are thought to display less regenerative capacity than normal livers after hepatic resection 1,2,3. In patients with cirrhosis, impaired liver function and regenerative capacity after major hepatic resection are associated with increased morbidity and mortality rates 1,2,3,26. In a cirrhotic liver, the regenerative ability and the specific functions are so impaired that excessive resection can easily produce postoperative liver dysfunction, which frequently leads to life-threatening multiple organ failure.
Liver cell proliferation after PH in healthy rats has been thoroughly investigated 1,27. PH is a good model of compensatory cellular growth, and 70% resection has been widely used to initiate a proliferative hepatocyte response 28. Liver regeneration is impaired and delayed after 70% PH in a cirrhotic liver; the process is prolonged and the functional capacity is impaired 3.
In the present study cirrhosis was established by the administration of TAA. This well-known hepatotoxin has been used widely in studying liver pathology 5,6,7,8,9. Several reports have shown that liver damage caused in rats by chronic TAA administration resembles human cirrhosis in both biological and morphological aspects 5,8,29. Acute and chronic administration of TAA leads to liver necrosis, cirrhosis and carcinoma in rats 5,6,7,8,9,29.
The liver has a marked regenerative capacity 1. Regeneration is mainly a result of hyperplasia and has a rapid onset. The onset of DNA synthesis in hepatocytes occurs 12–16 h posthepatectomy with a peak level at 24 h, followed by a peak of mitosis at 32-34 h; the mass of the liver is restored within 7–10 days. Following PH the major wave of DNA synthesis starts 14 h after operation and reaches a peak by 24 h 30. As previously demonstrated, the liver mass is restored in rats with extraordinary rapidity, the residual lobes nearly doubling in size by 48 h and approaching the original liver weight by 7 days 31. We estimated PI from remnant liver tissue samples on postoperative days 2 and 7.
Many tissue-based and serum-based methods are presently employed in clinical and experimental animals to evaluate liver regeneration 27,32, but a ‘gold standard’ has yet to be identified. DNA synthesis can be measured by a variety of techniques. Flow cytometry is an accurate and objective method of monitoring hepatic regenerative activity 29,33,34. The S phase refers to the DNA synthesis phase of the cell cycle. Following the S phase is the G2 phase, during which the cell prepared for mitosis or physical cell division 35. The principal advantage of this method is that the information obtained is objective and represents measurements on large numbers of nuclei and/or cells, but disadvantages include the cost of the sophisticated equipment required that is not generally available in many laboratories and the need to disrupt tissue resulting in the loss of relationships between cell subpopulation 36.
Several cytokines including GM-CSF, IL-2, IL-3 and IL-6 have been shown to induce proliferation of macro-phages or their precursors in bone marrow and various other organs, both in vivo and in vitro37,38,39. Hemato-poietic cytokines such as granulocyte colony-stimulating factor (G-CSF) and GM-CSF can enhance the immune response in several ways 38,39,40,41. These agents stimulate proliferation and differentiation of granulocytes, macro-phages, monocytes and eosinophils from pluripotent stem cells.
Previously Sakamoto and colleagues demonstrated that cultured murine parenchymal liver cells produce GM-CSF 42. They also suggested that hepatocytes might play a role in the regional haematolymphoid system of the liver. Some authors have shown that treatments such as GM-CSF have antiviral and immunoregulatory effects in patients with chronic hepatitis B 43. Hoedemakers & associates indicated that GM-CSF plays an important role in the in-vitro proliferation of rat liver macrophages 39; more recently, Theocharis and co-workers have reported that G-CSF can augment liver regenerative capacity 7. Based on these reports, GM-CSF was used as a hepatotrophic factor in an attempt to stimulate DNA synthesis after 70% PH in the rat model.
The effect of GM-CSF on hepatic regeneration after 70% PH was evaluated in normal and TAA-induced cirrhotic rats. To our knowledge, this is the first report testing the effect of GM-CSF on liver regeneration via flow cytometric analysis of DNA content. On postoperative day 2, the PI of hepatocytes in the remaining cirrhotic liver was higher in the GM-CSF-treated rats than the controls. A similar finding was seen in non-cirrhotic rats. These results indicate that exogenous GM-CSF administration promotes the hepatocellular DNA synthesis.
It is well known that GM-CSF acts as a potent growth factor, stimulating both the proliferation and maturation of myeloid progenitor cells. Yet the mechanism of action of GM-CSF on hepatocellular proliferation remains unclear. It could exert a direct as well as an indirect effect on the liver, through the cytokine cascade. Sakamoto and colleagues studied whether the liver could function as one of the haematolymphoid organs during postresectional regeneration 44. They found proliferation of the intrahepatic lymphocyte-rich fraction with GM-CSF. The proliferative response to the cytokines was augmented, while liver regeneration affected the systemic haematolymphoid system.
Liver regeneration after PH involves proliferation of cells in the remaining organ including hepatocytes, Kupffer cells, biliary epithelial cells, Ito cells and fenestrated endothelial cells 1,2,27. Colony-stimulating factors such as GM-CSF can induce the proliferation of Kupffer cells 45,46. Thus the number of Kupffer cells increased four-to-six-fold after stimulation with GM-CSF 45. Although we could not separate hepatocytes from Kupffer cells, GM-CSF administration in TAA-induced cirrhotic rats enhanced liver cell proliferation and ameliorated their suppressed regenerative capacity. Based on previously published data and our findings, it is suggested that administration of GM-CSF as an exogenous hepatotrophic factor may play a useful role in the liver regeneration after cirrhotic liver resection. However, further studies will be necessary to determine both its clinical impact and its exact mechanism of action.
References
- 1.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–6. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 2.Moser MAJ, Kneteman NM, Minuk GY. Research toward safer resection of the cirrhotic liver. HPB Surgery. 2000;11:285–97. doi: 10.1155/2000/31945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagasue N, Yukaya H, Ogawa Y, Kohno H, Nakamura T. Human liver regeneration after major hepatic resection: a study of normal liver and livers with chronic hepatitis and cirrhosis. Ann Surg. 1987;206:30–9. doi: 10.1097/00000658-198707000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashimoto M, Watanabe G. Functional capacity of the cirrhotic liver after partial hepatectomy in the rat. Surgery. 1999;126:541–7. [PubMed] [Google Scholar]
- 5.Nozu F, Takeyama N, Tanaka T. Changes of hepatic fatty acid metabolism produced by chronic thioacetamide administration in rats. Hepatology. 1992;15:1099–106. doi: 10.1002/hep.1840150621. [DOI] [PubMed] [Google Scholar]
- 6.Usami M, Furuchi K, Shiroiwa H, Saitoh Y. Effect of repeated portal-triad cross clamping during partial hepatectomy on hepatic regeneration in normal and cirrhotic rats. J Surg Res. 1994;57:541–8. doi: 10.1006/jsre.1994.1180. [DOI] [PubMed] [Google Scholar]
- 7.Theocharis SE, Margeli AP, Kittas CN. Effect of granulocyte colony stimulating factor administration on tissue regeneration due to thioacetamide-induced liver injury in rats. DigDis Sci. 1999;44:626–8. doi: 10.1023/a:1026657931829. [DOI] [PubMed] [Google Scholar]
- 8.Fitzhugh OG, Nelson AA. Liver tumors in rats fed thiourea or thioacetamide. Science. 1948;108:626–8. doi: 10.1126/science.108.2814.626. [DOI] [PubMed] [Google Scholar]
- 9.Trennery PN, Waring RH. Early changes in thioacetamide-induced liver damage. Toxicol Lett. 1983;19:299–307. doi: 10.1016/0378-4274(83)90134-0. [DOI] [PubMed] [Google Scholar]
- 10.Kaido T, Yoshikawa A, Seto S, et al. Portal branch ligation with a continuous hepatocyte growth factor supply makes extensive hepatectomy possible in cirrhotic rats. Hepatology. 1998;28:756–60. doi: 10.1002/hep.510280323. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto K, Takenaka K, Matsumata T, Shimada M, Sugimachi K. The effect of octreotide on morphological hepatic regeneration and hepatic functional recovery after a two-thirds hepatectomy in rats. Hepatogastroenterology. 1999;46:1880–4. [PubMed] [Google Scholar]
- 12.Theocharis SE, Agapitos EB, Margeli AP, et al. Effect of two forms of granulocyte-colony-stimulating factor on hepatic regeneration after 70% partial hepatectomy in rats. Clin Sci. 1997;92:315–20. doi: 10.1042/cs0920315. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto M, Kothary PC, Eckhauser FE, Raper SE. Treatment of cirrhotic rats with epidermal growth factor and insulin accelerates liver DNA synthesis after partial hepatectomy. J Gastroenterol Hepatol. 1998;13:1259–65. [PubMed] [Google Scholar]
- 14.Shimada M, Matsumata T, Yamamoto K, et al. The role of growth hormone, somatostatin and glucagon in hepatic resection. Hepatogastroenterology. 1998;45:178–83. [PubMed] [Google Scholar]
- 15.Fujiwara K, Nagoshi S, Ohno A, et al. Stimulation of liver growth by exogenous human hepatocyte growth factor in normal and partially hepatectomized rats. Hepatology. 1993;18:1443–9. [PubMed] [Google Scholar]
- 16.Rubin JS, Bottaro DP, Aaronso SA. Hepatocyte growth factor/scatter factor and its receptor, the c-met protooncogen product. Biochim Biophy Ada. 1993;1155:357–71. doi: 10.1016/0304-419x(93)90015-5. [DOI] [PubMed] [Google Scholar]
- 17.Fausto N, Laird AD, Webber EM. Liver regeneration 2. Role of growth factors and cytokines in hepatic regeneration. FASEB J. 1995;9:1527–36. doi: 10.1096/fasebj.9.15.8529831. [DOI] [PubMed] [Google Scholar]
- 18.Diehl AM, Rai RM. Liver regeneration 3: Regulation of signal transduction during liver regeneration. FASEB J. 1996;10:215–27. doi: 10.1096/fasebj.10.2.8641555. [DOI] [PubMed] [Google Scholar]
- 19.Metcalf D, Burgerss AW. Clonal analysis of progenitor cell commitment to granulocyte or macrophage production. J Cell Physiol. 1982;111:275–83. doi: 10.1002/jcp.1041110308. [DOI] [PubMed] [Google Scholar]
- 20.Arnold F, O'Bnen J, Cherry G. Granulocyte monocyte-colony stimulating factor as an agent or wound healing. J Wound Care. 1995;4:400–02. doi: 10.12968/jowc.1995.4.9.400. [DOI] [PubMed] [Google Scholar]
- 21.da Costa RM, Aniceto C, Jesus FM, Mendes M. Quick healing of leg ulcers after molgramostim. Lancet. 1994;344:481–2. doi: 10.1016/s0140-6736(94)91819-8. [DOI] [PubMed] [Google Scholar]
- 22.Eroğlu A, Kurtman C, Ayyıldız A, Karadayı K, Demirci S. Effect of granulocyte-macrophage colony-stimulating factor on wound nitrite level in normal and irradiated rats. Med Sci Res. 1999;27:685–8. [Google Scholar]
- 23.Higgins GM, Anderson RM. Experimental pathology of the liver. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–201. [Google Scholar]
- 24.Benson NA, Bray Ian RC. Evaluation of sensitivity in DNA aneuploidy detection using a mathematical model. Cytometry. 1994;15:53–8. doi: 10.1002/cyto.990150109. [DOI] [PubMed] [Google Scholar]
- 25.Shankey TV, Rabinovitch PS, Bagwell B. Guidelines for implementation of clinical DNA cytometry. Cytometry. 1993;14:472–7. doi: 10.1002/cyto.990140503. [DOI] [PubMed] [Google Scholar]
- 26.Kawasaki S, Imamura H, Bandai Y, Sanjo K, Idezuki Y. Direct evidence for the intact hepatocyte theory in patients with liver cirrhosis. Gastroenterology. 1992;102:1351–5. [PubMed] [Google Scholar]
- 27.Assay N, Minuk GY. Liver regeneration: methods for monitoring and their applications. J Hepatology. 1997;26:945–52. doi: 10.1016/s0168-8278(97)80266-8. [DOI] [PubMed] [Google Scholar]
- 28.Michalopoulos GK. Liver regeneration: Molecular mechanisms of growth control. FASEB J. 1990;4:176–87. [PubMed] [Google Scholar]
- 29.Zimmermann T, Franke H, Dargel R. Studies on lipid and lipoprotein metabolism in rat liver cirrhosis induced by differ ent regimens of thioacetamide administration. Exp Pathol. 1986;30:109–17. doi: 10.1016/s0232-1513(86)80069-x. [DOI] [PubMed] [Google Scholar]
- 30.Fausto N, Mead J. Regulation of liver growth: protooncogenes and transforming growth factors. Lab Invest. 1989;60:4–13. [PubMed] [Google Scholar]
- 31.Buchner NLR, Malt RA. Boston Little, Brown & Co; 1971. Regeneration of Liver and Kidney; pp. 23–53. [Google Scholar]
- 32.Theocharis SE, Skopelitou AS, Margeli AP, Pavlaki KJ, Kittas C. Proliferating cell nuclear antigen (PCNA) expression in regenerating rat liver after partial hepatectomy. Dig Dis Sci. 1994;39:245–52. doi: 10.1007/BF02090193. [DOI] [PubMed] [Google Scholar]
- 33.Garcia RL, Coltrera MD, Gown AM. Analysis of proliferative grade using anti-PCNA/cyclin monoclonal antibodies in fixed, embedded tissues. Comparison with flow cytometric analysis. Am J Pathol. 1989;134:733–9. [PMC free article] [PubMed] [Google Scholar]
- 34.Braylan RC. Atttibutes and applications of flow cytometry. Ann Clin Lab Sci. 1983;13:379–84. [PubMed] [Google Scholar]
- 35.Vemuru RP, Aragona E, Gupta S. Analysis of hepatocellular proliferation: study of the archival liver tissue is facilitated by an endogenous marker of DNA replication. Hepatology. 1992;16:968–73. doi: 10.1002/hep.1840160419. [DOI] [PubMed] [Google Scholar]
- 36.Larsen JK. Cell proliferation: analysis by flow cytometry. Nouv Rev Fr Hematol. 1992;34:317–35. [PubMed] [Google Scholar]
- 37.Metcalf D. The molecular control of cell division, differentiation, commitment and maturation in haemopoietic cells. Nature. 1989;339:27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- 38.Chen BD, Clark CR, Chou TH. Granulocyte/macrophages colony-stimulating factor stimulates monocyte and tissue macrophage proliferation and enhances their responsiveness to macrophage colony-stimulating factor. Blood. 1988;71:997–1002. [PubMed] [Google Scholar]
- 39.Hoedemakers RMJ, Scherphof GL, Daemen T. Proliferation of rat liver macrophages in vitro: influence of hemopoietic growth factors. Hepatology. 1994;19:666–74. doi: 10.1002/hep.1840190318. [DOI] [PubMed] [Google Scholar]
- 40.Metcalf D, Begley CG, Johnson GR, et al. Biologic properties in vitro of a recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1986;67:37–45. [PubMed] [Google Scholar]
- 41.Groopman JE, Molina JM, Scadden DT. Hematopoietic growth factors. Biology and clinical applications N Engl J Med. 1989;321:1449–59. doi: 10.1056/NEJM198911233212106. [DOI] [PubMed] [Google Scholar]
- 42.Sakamoto T, Mabuchi A, Kuriya S. Production of granulocyte-macrophage colony-stimulating factor by adult murine parenchymal liver cells (hepatocytes) Reg Immunol -91. 1990;3:260–7. [PubMed] [Google Scholar]
- 43.Kountouras J, Boura P, Tsapas G. In vivo effect of granulocyte- macrophage colony-stimulating factor and interferon combination on monocyte-macrophage and T-lymphocyte functions in chronic hepatitis B leukocytopenic patients. Hepatogastroenterology. 1998;45:2295–302. [PubMed] [Google Scholar]
- 44.Sakamoto T, Saizawa T, Mabuchi A, et al. The liver as a potential hematolyphoid organ examined from modifications occurring in the systemic and intrahepatic hematolymphoid system during liver regeneration afte partial hepatectomy. Reg Immunol. 1992;4:1–11. [PubMed] [Google Scholar]
- 45.Hashimoto S, Yamada M, Yanai N, et al. Phenotypic change and proliferation of murine Kupffer cells by colony stimulating factors. J Interferan Cytokine Res. 1996;16:237–43. doi: 10.1089/jir.1996.16.237. [DOI] [PubMed] [Google Scholar]
- 46.Schuurman B, Heuff G, Beelen RH, Meyer S. Enhanced killing capacity of human Kupffer cells after activation with human granulocyte/macrophage-colony-stimulating factor and interferon gamma. Cancer Immunol Immunother. 1994;39:179–84. doi: 10.1007/BF01533384. [DOI] [PMC free article] [PubMed] [Google Scholar]