Abstract
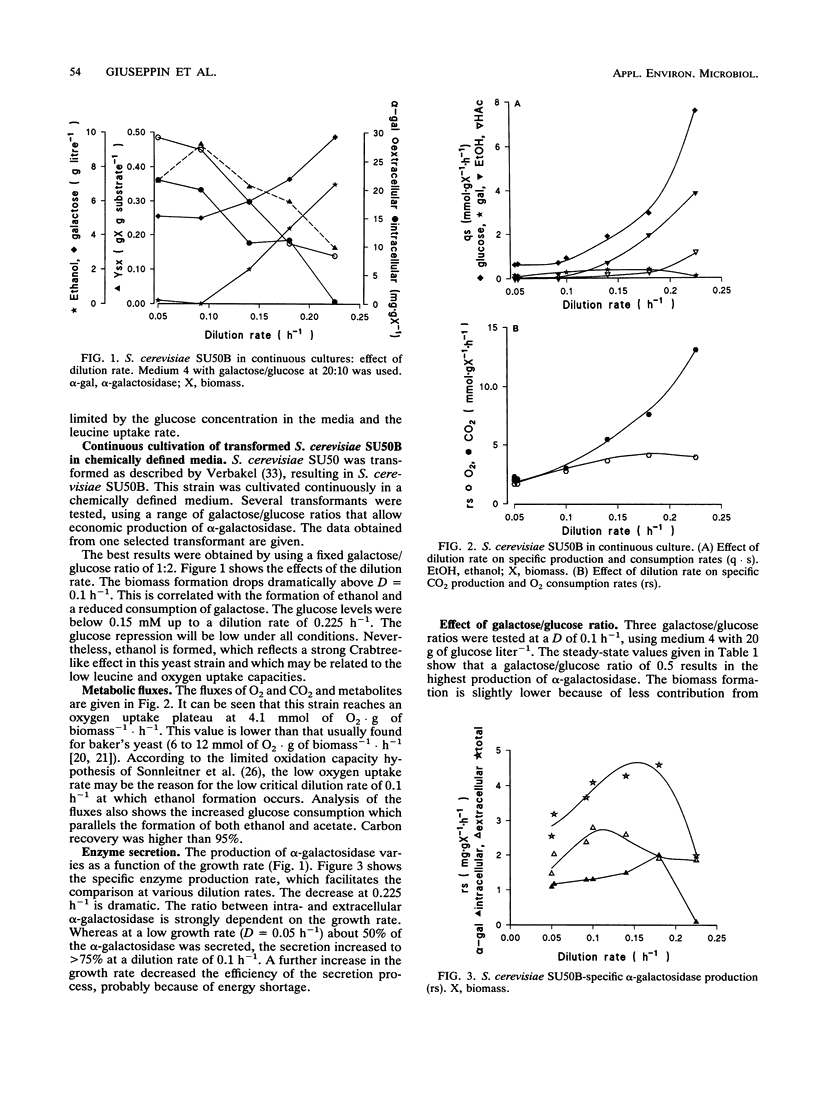
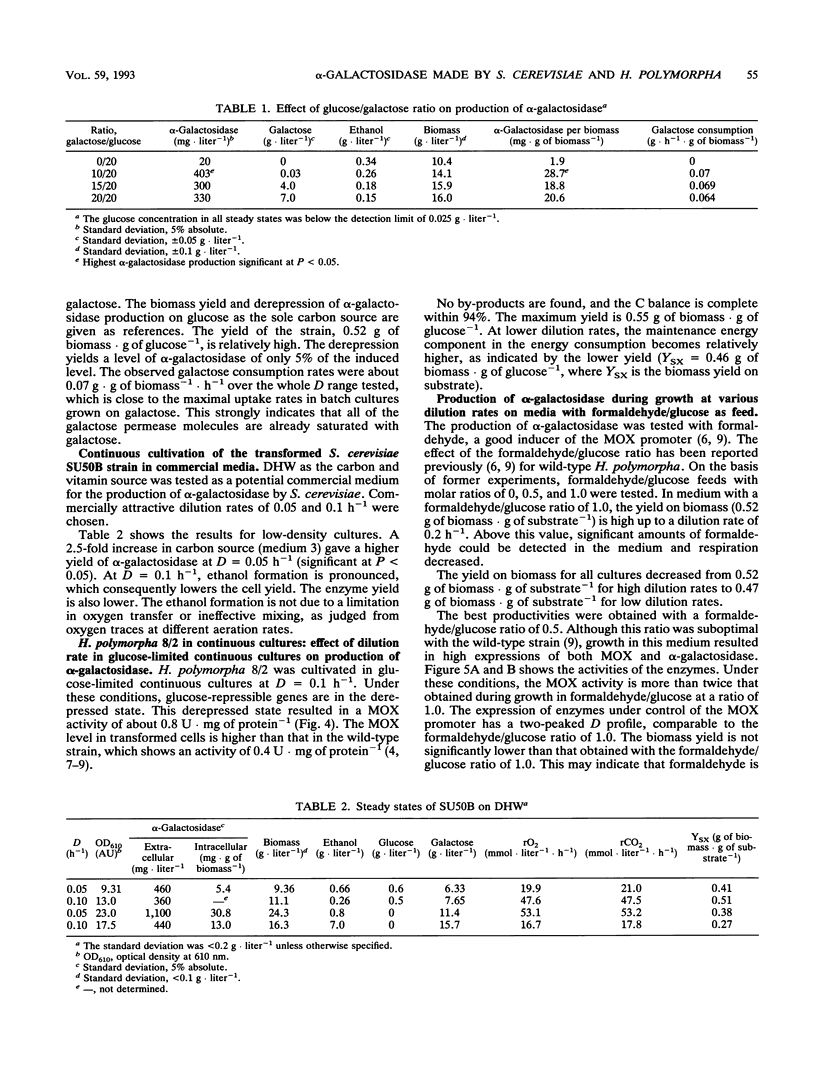
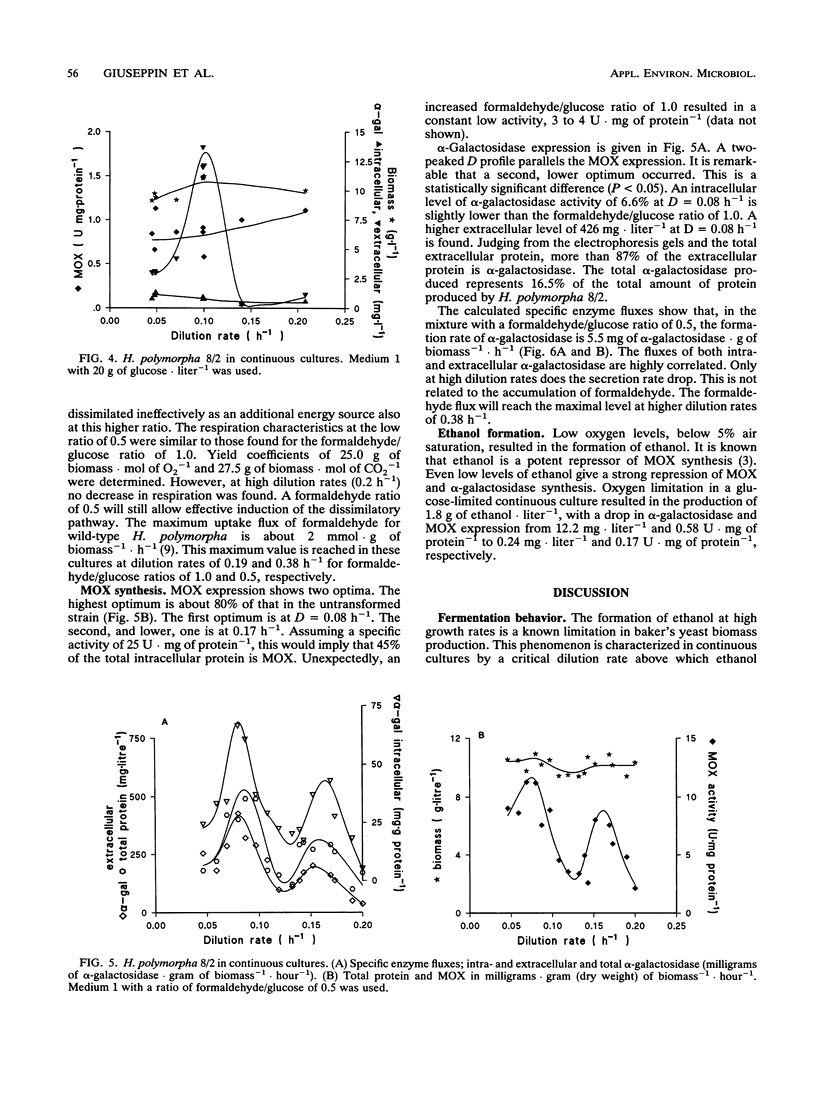
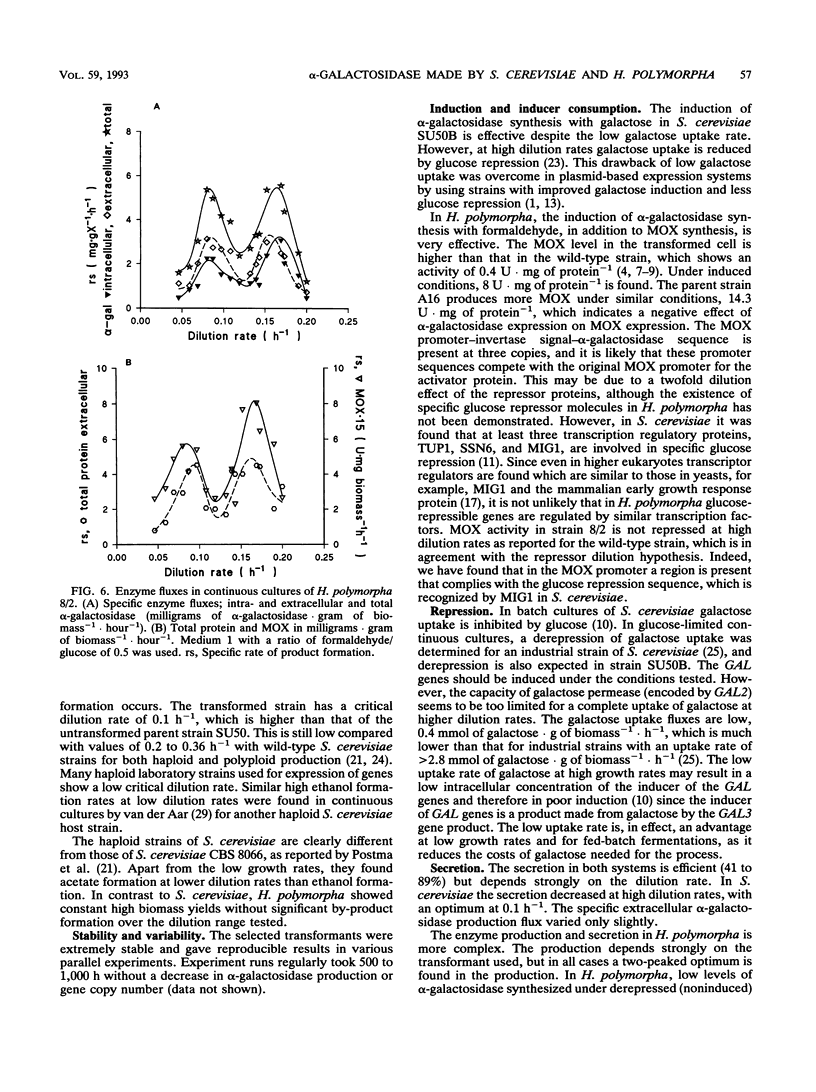
Saccharomyces cerevisiae SU50B and Hansenula polymorpha 8/2, both carrying a multicopy integrated guar alpha-galactosidase, have been cultivated in continuous cultures, using various mixtures of carbon sources and cultivation conditions. Both S. cerevisiae SU50B and H. polymorpha 8/2 are stable and produce high levels of extracellular alpha-galactosidase in continuous cultures for more than 500 h. For these expression systems the strong inducible promoter systems GAL7 and methanol oxidase, respectively, were used. The induction of alpha-galactosidase synthesis by galactose in SU50B is limited by the low galactose uptake. Apart from that, at high dilution rates, the glucose repression is substantial, and a maximal expression level of 28.6 mg of extracellular alpha-galactosidase.g (dry weight) of biomass-1 could be obtained. In H. polymorpha, the induction of alpha-galactosidase synthesis, in addition to methanol oxidase synthesis using formaldehyde, is very effective up to 42 mg of extracellular alpha-galactosidase.g (dry weight) of biomass-1. Productivities in terms of specific production rate enable a good comparison with those of other heterologous expression systems in the literature. The productivities found with S. cerevisiae SU50B and H. polymorpha, 3.25 and 5.5 mg of alpha-galactosidase.g of biomass-1.liter-1.h-1, respectively, rank among the highest reported in the literature. Enzyme production and secretion in H. polymorpha are more complex. A two-peaked optimum is found in enzyme production. No clear explanation of this phenomenon can be given.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberghina L., Porro D., Martegani E., Ranzi B. M. Efficient production of recombinant DNA proteins in Saccharomyces cerevisiae by controlled high-cell-density fermentation. Biotechnol Appl Biochem. 1991 Aug;14(1):82–92. [PubMed] [Google Scholar]
- Fellinger A. J., Verbakel J. M., Veale R. A., Sudbery P. E., Bom I. J., Overbeeke N., Verrips C. T. Expression of the alpha-galactosidase from Cyamopsis tetragonoloba (guar) by Hansenula polymorpha. Yeast. 1991 Jul;7(5):463–473. doi: 10.1002/yea.320070505. [DOI] [PubMed] [Google Scholar]
- Johnston M. A model fungal gene regulatory mechanism: the GAL genes of Saccharomyces cerevisiae. Microbiol Rev. 1987 Dec;51(4):458–476. doi: 10.1128/mr.51.4.458-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleher C. A., Redd M. J., Schultz J., Carlson M., Johnson A. D. Ssn6-Tup1 is a general repressor of transcription in yeast. Cell. 1992 Feb 21;68(4):709–719. doi: 10.1016/0092-8674(92)90146-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ledeboer A. M., Edens L., Maat J., Visser C., Bos J. W., Verrips C. T., Janowicz Z., Eckart M., Roggenkamp R., Hollenberg C. P. Molecular cloning and characterization of a gene coding for methanol oxidase in Hansenula polymorpha. Nucleic Acids Res. 1985 May 10;13(9):3063–3082. doi: 10.1093/nar/13.9.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlin J. O., Ronne H. Yeast MIG1 repressor is related to the mammalian early growth response and Wilms' tumour finger proteins. EMBO J. 1990 Sep;9(9):2891–2898. doi: 10.1002/j.1460-2075.1990.tb07479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeeke N., Fellinger A. J., Toonen M. Y., van Wassenaar D., Verrips C. T. Cloning and nucleotide sequence of the alpha-galactosidase cDNA from Cyamopsis tetragonoloba (guar). Plant Mol Biol. 1989 Nov;13(5):541–550. doi: 10.1007/BF00027314. [DOI] [PubMed] [Google Scholar]
- Overbeeke N., Termorshuizen G. H., Giuseppin M. L., Underwood D. R., Verrips C. T. Secretion of the alpha-galactosidase from Cyamopsis tetragonoloba (guar) by Bacillus subtilis. Appl Environ Microbiol. 1990 May;56(5):1429–1434. doi: 10.1128/aem.56.5.1429-1434.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma E., Verduyn C., Scheffers W. A., Van Dijken J. P. Enzymic analysis of the crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol. 1989 Feb;55(2):468–477. doi: 10.1128/aem.55.2.468-477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekrishna K., Potenz R. H., Cruze J. A., McCombie W. R., Parker K. A., Nelles L., Mazzaferro P. K., Holden K. A., Harrison R. G., Wood P. J. High level expression of heterologous proteins in methylotrophic yeast Pichia pastoris. J Basic Microbiol. 1988;28(4):265–278. doi: 10.1002/jobm.3620280410. [DOI] [PubMed] [Google Scholar]
- Veale R. A., Giuseppin M. L., van Eijk H. M., Sudbery P. E., Verrips C. T. Development of a strain of Hansenula polymorpha for the efficient expression of guar alpha-galactosidase. Yeast. 1992 May;8(5):361–372. doi: 10.1002/yea.320080504. [DOI] [PubMed] [Google Scholar]
- van Dijken J. P., Otto R., Harder W. Growth of Hansenula polymorpha in a methanol-limited chemostat. Physiological responses due to the involvement of methanol oxidase as a key enzyme in methanol metabolism. Arch Microbiol. 1976 Dec 1;111(1-2):137–144. doi: 10.1007/BF00446560. [DOI] [PubMed] [Google Scholar]



