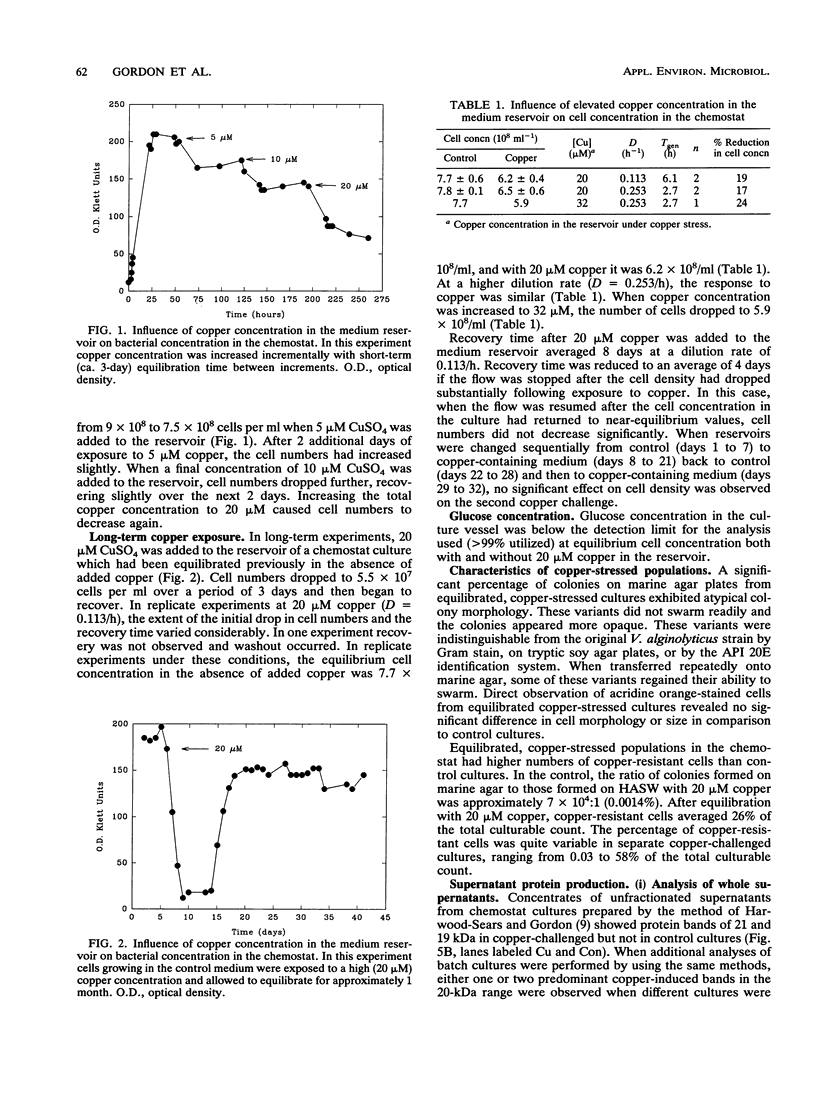
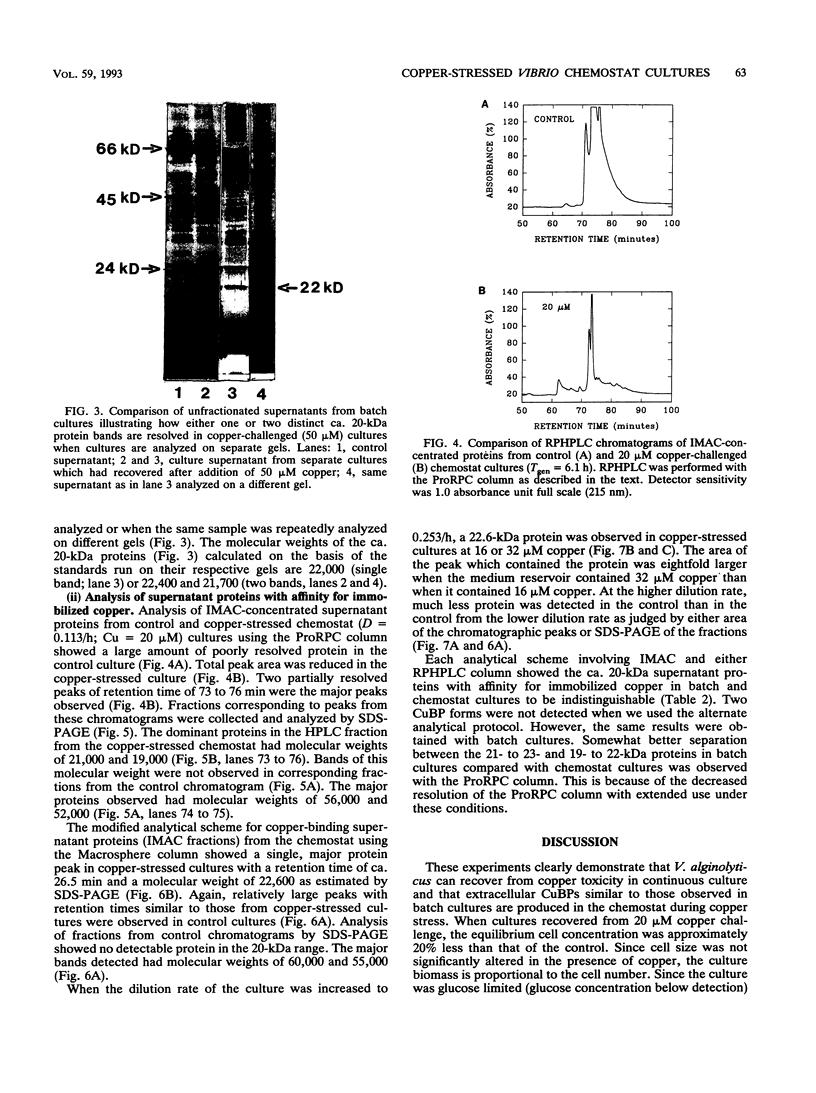
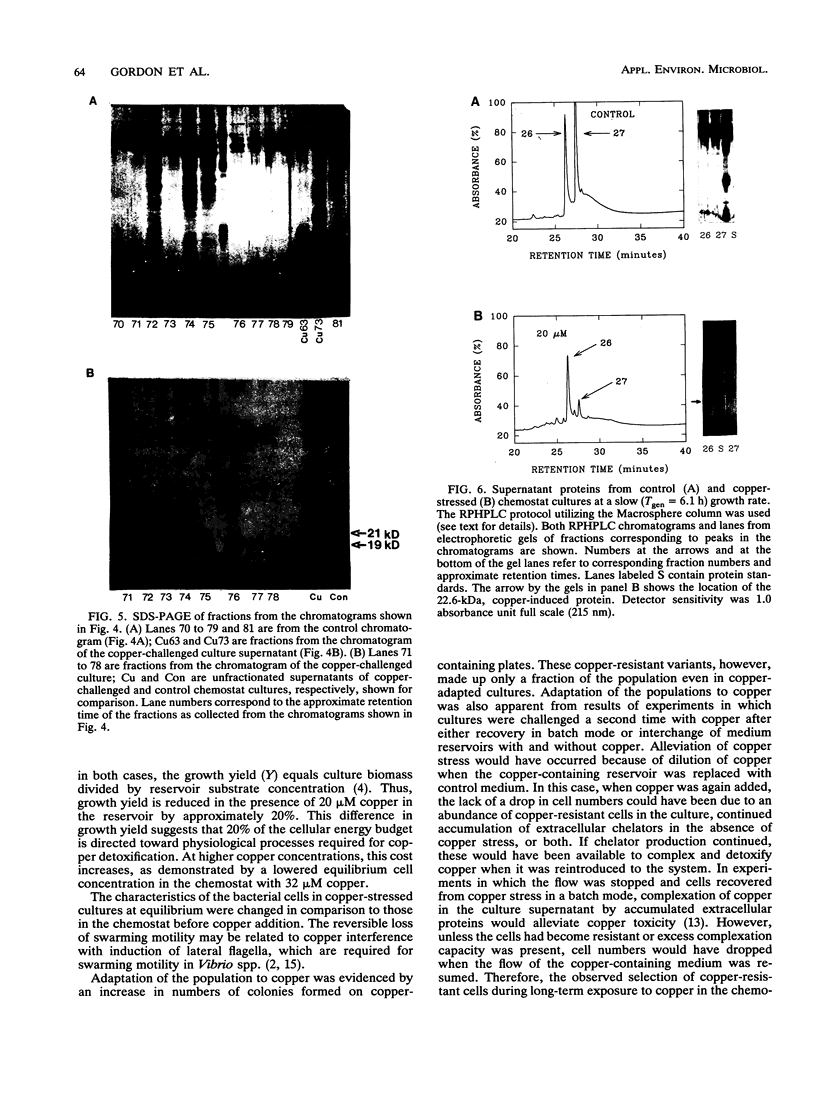
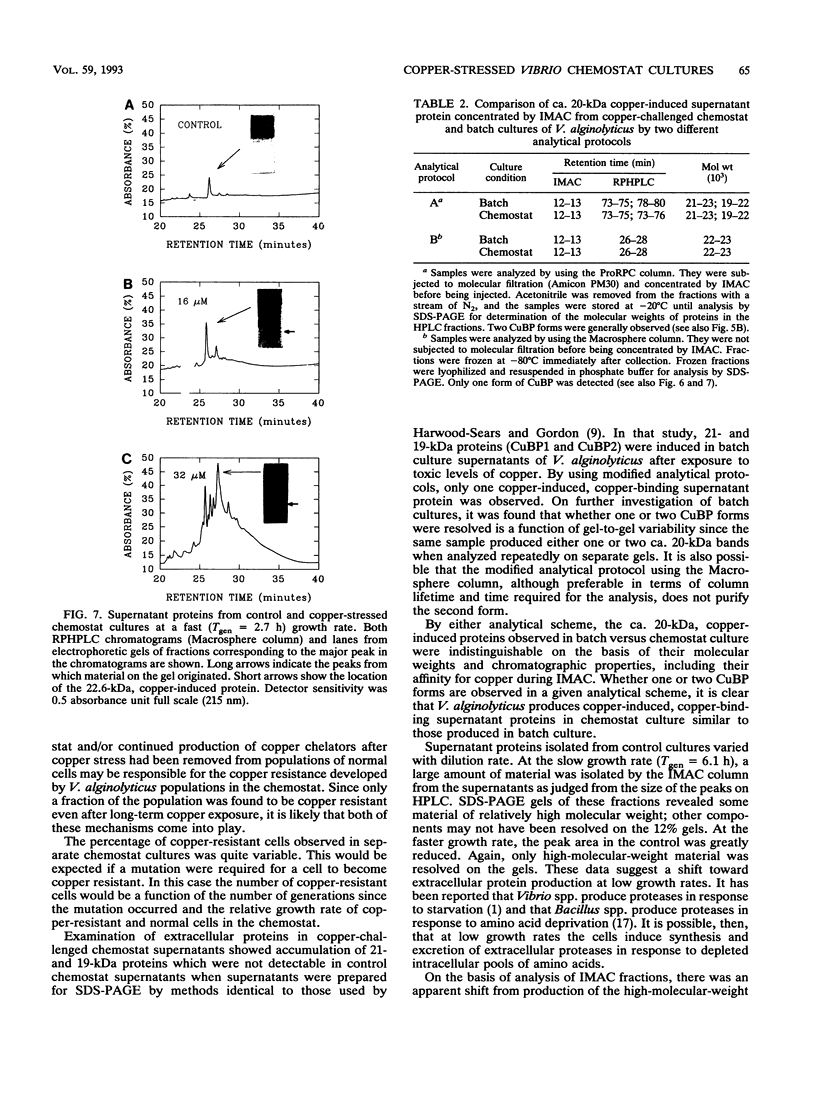
Abstract
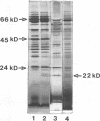
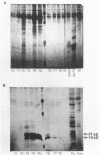
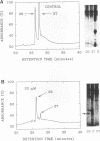
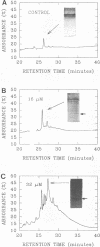
The influence of elevated copper concentrations on cell numbers and extracellular protein production was investigated in chemostat cultures of Vibrio alginolyticus. High (20 microM) copper in the medium reservoir resulted in a dramatic drop in cell numbers which was overcome with time. The copper-stressed cultures established a new equilibrium cell concentration slightly (ca. 20%) lower than control cultures. Copper-stressed chemostat populations contained an increased number of copper-resistant cells, but these averaged only 26% of the copper-adapted population. Previously copper-stressed populations exhibited resistance to a second challenge with copper. Proteins with properties identical to those of copper-induced, copper-binding proteins (CuBPs) observed in batch cultures of V. alginolyticus were observed in the supernatants of copper-stressed chemostat cultures and not in controls. CuBPs from batch and chemostat cultures were identical in terms of their induction by copper, molecular weight, and retention volumes on both immobilized copper ion-affinity chromatography and reverse-phase high-performance liquid chromatography columns. The concentration of CuBP in the chemostat was dependent on copper concentration in the medium reservoir. Either one or two forms of CuBP were observed in various analyses from both batch and chemostat cultures. Gel-to-gel variability was implicated as a factor determining whether one or two forms were resolved in a given analysis.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertson N. H., Nyström T., Kjelleberg S. Exoprotease Activity of Two Marine Bacteria during Starvation. Appl Environ Microbiol. 1990 Jan;56(1):218–223. doi: 10.1128/aem.56.1.218-223.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. D., Baumann P. Structure and arrangement of flagella in species of the genus Beneckea and Photobacterium fischeri. J Bacteriol. 1971 Jul;107(1):295–302. doi: 10.1128/jb.107.1.295-302.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender C. L., Cooksey D. A. Indigenous plasmids in Pseudomonas syringae pv. tomato: conjugative transfer and role in copper resistance. J Bacteriol. 1986 Feb;165(2):534–541. doi: 10.1128/jb.165.2.534-541.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J. S., Cooksey D. A. Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8915–8919. doi: 10.1073/pnas.88.20.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood-Sears V., Gordon A. S. Copper-induced production of copper-binding supernatant proteins by the marine bacterium Vibrio alginolyticus. Appl Environ Microbiol. 1990 May;56(5):1327–1332. doi: 10.1128/aem.56.5.1327-1332.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda S., Okamoto K. Formation and function of Vibrio parahaemolyticus lateral flagella. J Bacteriol. 1977 Mar;129(3):1266–1271. doi: 10.1128/jb.129.3.1266-1271.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]