Abstract
Background
Commensurate with the advances in diagnostic and therapeutic radiology in the past two decades, percutaneous needle aspiration and catheter drainage have replaced open operation as the first choice of treatment for both single and multiple pyogenic liver abscesses. There has been little written on the place of surgical resection in the treatment of pyogenic liver abscess due to underlying hepatobiliary pathology or after failure of non-operative management.
Methods
The medical records of patients who underwent resection for pyogenic liver abscess over a 15-year period were retrospectively reviewed. The demographics, time from onset of symptoms to medical treatment and operation, site of abscess, organisms cultured, aetiology, reason for operation, type of resection and outcome were analysed. There were 49 patients in whom the abscesses were either single (19), single but multiloculated (11) or multiple (19). The median time from onset of symptoms to medical treatment was 21 days and from treatment to operation was 12 days. The indications for operation were underlying hepatobiliary pathology in 20% and failed non-operative treatment in 76%. Two patients presented with peritonitis from a ruptured abscess.
Results
The resections performed were anatomic (44) and non-anatomic (5). No patient suffered a recurrent abscess or required surgical or radiological intervention for any abdominal collection. Antibiotics were ceased within 5 days of operation in all but one patient. The median postoperative stay was 10 days. There were two deaths (4%), both following rupture of the abscess.
Discussion
Except for an initial presentation with intraperitoneal rupture and, possibly, cases of hepatobiliary pathology causing multiple abscesses above an obstructed duct system that cannot be negotiated non-operatively, primary surgical treatment of pyogenic liver abscess is not indicated. Non-operative management with antibiotics and percutaneous aspiration/drainage will be successful in most patients. If non-operative treatment fails, different physical characteristics of the abscesses are likely to be present and partial hepatectomy of the involved portion of liver is good treatment when performed by an experienced surgeon.
Keywords: liver abscess, pyogenic, hepatectomy
Introduction
The evolution of diagnostic and interventional radiology over the past two decades has allowed earlier diagnosis and treatment of pyogenic liver abscess (PLA). The combination of antibiotics and closed percutaneous drainage has been shown to be safe and effective in a high proportion of cases, and a non-operative approach has become the first choice of treatment for both single and multiple abscesses. Open operation is now confined to patients who fail non-operative management or in whom operation is necessary for the underlying cause of the PLA. Partial hepatectomy for PLA has been rarely reported. The aim of the present study was to evaluate the results of hepatic resection for PLA in a tertiary referral unit.
Patients and methods
All patients who underwent resection for pyogenic liver abscess from 1987 to 2001 were identified from a prospective data base of patients under the care of the HPB Unit. The medical records were retrospectively reviewed, and the demographics, time from onset of symptoms to medical treatment and operation, site of abscess (es), organisms cultured, aetiology, reason for operation, type of resection and outcome were recorded and analysed. Patients who underwent resection for hepatic necrosis secondary to blunt liver trauma or for hepatic artery thrombosis after liver transplantation but without true evidence of progress to a PLA, were excluded from the analysis.
In all, 49 patients (25 women, 22 men) with a median age of 56 years (range 5–92) underwent hepatic resection for PLA. The abscesses were single (19), single but multi-loculated (11) or multiple (19) and were located in the right hemiliver (29) or left hemiliver (20), with five extending across the principal plane. Two patients presented with intraperitoneal rupture before any medical treatment. Three patients with PLAs associated with iatrogenic bile duct with or without hepatic artery injury and two patients with malignancy were referred for management of the combined pathology. In the remaining 42 patients, a period of medical therapy preceded referral for operation. The time from onset of symptoms to commencement of medical treatment ranged from 3 to 56 days (median 21) and the time from onset of medical treatment to operation ranged from 4 to 150 days (median 12).
The indications for operation were (i) failed medical treatment in 37 patients (76%), including intraperitoneal rupture during treatment (n = 1) and complications of percutaneous drainage (n = 2) or (ii) underlying hepatobiliary pathology in 10 patients (20%), in whom the operation included management of the primary disease process and the PLA. The hepatobiliary pathology was hepatolithiasis (n = 5), iatrogenic bile duct with or without hepatic artery injury (n = 3), cholangiocarcinoma (n = 1) or gallbladder cancer (n = 1). The five patients with hepatolithiasis had multiple abscesses above a duct obstruction. Two of the three patients with bile duct injuries had multiple abscesses in an atrophied hemiliver with an obstructed duct.
The organisms identified from culture are shown in Table 1. Apart from the 10 patients with primary hepatobiliary pathology, the aetiology of the PLA was not delineated in most of the cases. Diverticulitis of the sigmoid colon (3), banding of haemorrhoids 7 days before onset of symptoms (1), carcinoma of the stomach (1) and fish bone perforation of the duodenum into the liver (1) were the only identifiable potential sources. One patient with inherited immunodeficiency disease developed multiple PLAs with Staphylococcus aureus and one liver transplant recipient of 7 years developed a PLA from Eikenella corrodens.
Table 1. Microbiology of pyogenic liver Abscesses.
| Organism | n |
|---|---|
| No growth | 5 |
| Multiple organisms (each included Escherichia coli and Bacteroides sp.) | 4 |
| Single organisms | (40) |
| Streptococcus milleri | 18 |
| Klebsiella pneumoniae | 10 |
| Fusobacterium sporogenase | 3 |
| Staphylococcus aureus | 2 |
| Pseudomonas aeruginosa | 2 |
| Escherichia coli | 1 |
| Mycobacterium fortuitum | 1 |
| Serratia marcescens | 1 |
| Eikinella corrodens | 1 |
| Burkholderia pseudomallei | 1 |
Results
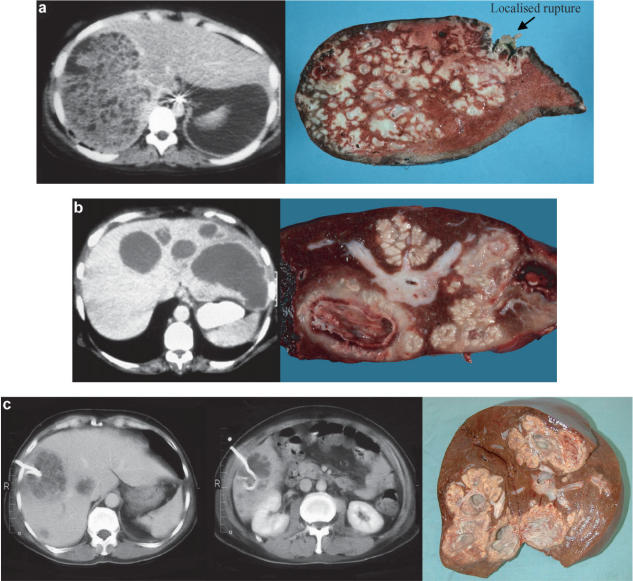
The resections that were performed are outlined in Table 2. The type of resection was determined by the site of the PLA(s), the extent of liver involvement and the ability to encompass the abscess and preserve as much parenchyma as possible. When the majority of a hemiliver was destroyed by PLA, a hemihepatectomy was carried out, but if the abscess could be removed with a mono- or bi-segmentectomy then this approach was selected. In five cases, the abscess was removed in toto with a non-anatomically based resection. Three patients had pre-operative CT evidence of portal vein thrombosis to the hemiliver in which the PLA was situated, as confirmed at operation. Six other patients had occlusive thrombi in large veins in juxtaposition to the PLA on pathological examination of the removed specimen. The three cases shown in Figure 1 are typical of those patients in whom resection was performed for failed medical treatment and demonstrate the solid granulomatous/gelatinous substance of the PLA with limited liquefaction. Histologically, these abscesses showed mostly solid walls with stellate necrosis and surrounding fibrosis.
Table 2. Liver resections performed for pyogenic abscess.
| Procedure | n |
|---|---|
| Right hemihepatectomy | 18 |
| Left hemihepatectomy | 7 |
| Left lateral sectorectomy | 7 |
| Segment-orientated resection | 12 |
| Non-anatomical | 5 |
| Resection combined with | |
| Roux-en-Y biliary reconstruction | |
| Iatrogenic bile duct injury | 3 |
| Hepatolithiasis | 2 |
| Cholangiocarcinoma | 1 |
| Gallbladder cancer | 1 |
Figure 1. .
CT scans and open resection specimens of three patients treated by open operation following failed non-operative treatment. (a) Right hemihepatectomy for multiple abscesses caused by Staphylococcus aureus. (b) Left hemihepatectomy for multiple abscesses caused by Klebsiella pneumoniae. (c) Right hemihepatectomy for multiple abscesses caused by Streptococcus milleri
Two patients who were admitted with generalised peritonitis from a ruptured PLA required ventilation and inotropic support both before and after operation. While mobilising on the surgical ward, one of these patients collapsed and died of a pulmonary embolus 12 days after operation. Four of the five patients transferred from the Intensive Care Unit of other institutions were ventilated and three were on inotropic support. One of these latter three had been on long-term steroids and developed generalised peritonitis from rupture of the PLA after transfer. Following resection of the ruptured PLA there was no evidence of ongoing abdominal sepsis, but the patient could not be weaned from ventilation and died 24 days postoperatively. In addition to the three patients who developed generalised peritonitis from a ruptured abscess, seven other patients had localised rupture with contained subphrenic or subhepatic abscesses outside the liver, in juxtaposition to the PLA. It could not be ascertained whether these abscesses were spontaneous or were related to percutaneous intervention.
Antibiotics were ceased within 5 days of resection in all but the one patient with melioidosis, where there had been 6 months of antibiotic therapy before referral which was continued for 1 month after resection. The postoperative hospital stay ranged from 5 to 90 days (median 10). No patient required reoperation on the abdomen or radiological intervention for any intra-abdominal collection, and no patient suffered from a recurrent abscess. Two patients required an intercostal catheter for drainage of a right pleural effusion. One patient developed a wound infection with a different organism to that causing the PLA. There were four patients who had an extended postoperative stay. Two ventilated patients transferred from another institution on inotropic support required ventilation for 5 and 7 days after operation and were discharged from hospital at 21 and 39 days, respectively. One patient with severe chronic obstructive airways disease who had had 5 weeks of medical treatment for PLA before referral for operation required prolonged treatment for his respiratory disease and was discharged 43 days postoperatively. One patient who was on long-term steroids for a medical condition developed an empyema of the right thorax which necessitated decortication, and she remained in hospital for 90 days after her liver operation. There were two deaths (4%), both following rupture of the PLA (as outlined previously).
Discussion
Before the development of ultrasound (US) and computed tomography (CT) scans, open surgical drainage was the mainstay of treatment for PLA, but it was hampered by the frequent late diagnosis and the relative inaccuracy associated with palpation in identifying induration or fluctuation as the site of the lesion. Percutaneous drainage of a PLA was first proposed in 1953, when the authors reported the successful treatment of 14 patients without a death 1. The major shift from open surgical treatment to percutaneous needle aspiration (PNA) or catheter drainage (PCD), which accompanied the advances in radiology over the past two decades, has made a non-surgical approach the first choice of treatment for both single and multiple abscesses. Baek and colleagues 2 found PNA to be successful in 64% of cases, and Lambiase and co-workers 3 reported success with PCD in 69% of cases. Seeto and Rockey 4 found a cure rate of 58% with PNA and 77% for PCD, while Chu and associates 5 had rates of 89% for both PNA and PCD. Giorgino and colleagues 6 were successful in 98.3% of 115 patients using PNA but suggested that their particularly high success rate was related to the patient population that they treated rather than to any greater technical expertise. There have been no reports of prospective randomised controlled trials comparing PNA and PCD for PLAs, but there has been one trial which included amoebic and pyogenic abscesses 7. This trial showed a 100% success rate for PCD and 60% for PNA, with the failures successfully treated by PCD.
The role of open operation in the treatment of PLA is limited to patients who fail non-operative treatment or in whom operation is necessary for the cause of the abscess. The treatment of primary abdominal pathology outside the hepatobiliary area may be delayed until percutaneous intervention of the PLA has been carried out and defervescence has occurred 8. Surgical intervention as a primary mode of therapy may be indicated when there is a complication such as a ruptured abscess, when there are multiple abscesses from hepatolithiasis or multiple abscesses secondary to an iatrogenic bile duct injury.
Abscesses that contain homogeneous purulent material should first be treated with antibiotics and PNA/PCD, and this is likely to be successful in 75–90% of patients 9. The limited size of the PCD tubes, especially when there is viscid pus and necrotic debris, may be associated with failure of this first-line therapy 8. If non-operative treatment has failed and open operation is required, what is the most appropriate procedure? The vast majority of the PLAs in the present series failed to respond to PCD because of the inability to drain inspissated pus, which was of limited volume and associated with granulomatous or necrotic tissue that had not or could not liquefy. While open drainage may improve the aspiration of viscid pus, the physical characteristics of the non-collapsible thick wall and septae are as much an obstacle to resolution as with closed drainage and indicate the need for excision of the abscess cavity.
There have been isolated references to the use of hepatic resection in the treatment of PLA 10,11,12,13, usually with the caveat that they were multiple non-drainable abscesses above a biliary obstruction or had a thick-walled non-collapsing structure. In a 1981 report on the management of 125 PLAs, Balasegaram performed resection in 23 patients with three deaths (13%) 14. This experience came in an era in which there was little in the way of radiological imaging, and a number of the patients presented with abdominal complications of the abscess. Chu and associates 5 reported six liver resections for PLA, and all were related to hepatolithiasis. In a series of 483 PLAs, Chou and co-workers 15 performed resection in 27 patients with one death (3.7%), but it was not clear how many of these cases were related to hepatolithiasis. They concluded that liver resection should be considered for single or multiple abscess in the presence of severe hepatic destruction.
In a series of patients with chronic granulomatous disease from inherited immunodeficiency, Lublin and colleagues 16 described the unique characteristics of the PLAs associated with the condition. The abscesses were dense, septate masses with a fibrous pseudocapsule and thick inspissated pus. They advocated aggressive surgical excision, preserving as much parenchyma as possible, because 58% of their patients required more than one operation on the liver for recurrent disease. There was only one patient in the present series with primary inherited immunodeficiency but many of the patients had PLAs not dissimilar to those of chronic granulomatous disease (Figure 1). In particular, PLAs caused by the Streptococcus milleri group of organisms and some of those caused by Klebsiella pneumoniae manifested this type of abscess; these two organisms accounted for 57% of the cases in this series.
There is a profound bias in cases referred to a specialised unit such as the one staffed by the authors, and the present series could give a false impression as to the need for open operation. The patients referred for operation following failed non-operative management were not assumed to be surgical candidates but were assessed as to whether treatment had been adequate or maximised. Surgical intervention was avoided in approximately 40% of patients who received further percutaneous procedures that resulted in a successful outcome. However, this was rarely achieved when the causative organisms belonged to the Streptococcus milleri group. Overall, the cohort of patients who came to open operation after unsuccessful non-operative management were extremely unwell, and a proportion were frankly moribund. Nevertheless, the mortality and morbidity rates after resection were low, and prolonged antibiotic therapy was not required.
In conclusion, surgical intervention is not advocated as primary treatment for PLA, except when the initial presentation is intraperitoneal rupture or possibly in cases of hepatobiliary pathology causing multiple abscesses above an obstructed duct system. Non-operative treatment with PNA/PCD and appropriate antibiotics should be the primary treatment and will be successful in most patients. If it fails to bring about resolution and open operation is required, different physical characteristics of PLA are to be expected and partial hepatectomy of the involved portion of destroyed liver is good treatment when performed by an experienced surgeon.
References
- 1.McFadzean AJ, Chang KP, Wong CC. Solitary pyogenic abscess of the liver treated by closed aspiration and antibiotics. Br J Surg. 1953;41:141–52. doi: 10.1002/bjs.18004116606. [DOI] [PubMed] [Google Scholar]
- 2.Baek SY, Lee M, Cho KS, Lee SC, Subg KB, Auh YO. Therapeutic percutaneous aspiration of hepatic abscesses: effectiveness in 25 patients. Am J Roent. 1993;160:799–802. doi: 10.2214/ajr.160.4.8456667. [DOI] [PubMed] [Google Scholar]
- 3.Lambiase RE, Deyoe L, Cronan JJ, Dorfman GS. Percutaneous drainage of 335 consecutive abscesses: results of primary drainage with 1-year follow-up. Radiology. 1992;184:167–79. doi: 10.1148/radiology.184.1.1376932. [DOI] [PubMed] [Google Scholar]
- 4.Seeto RK, Rockey DC. Pyogenic liver abscess. Changes in aetiology, management and outcome. Medicine. 1996;75:99–113. doi: 10.1097/00005792-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Chu KM, Fan ST, Lai ECS, Lo CM, Wong J. Pyogenic liver abscess. An audit of experience over the past decade. Arch Surg. 1996;131:148–52. doi: 10.1001/archsurg.1996.01430140038009. [DOI] [PubMed] [Google Scholar]
- 6.Giorgino A, Tarantino L, Mariniello N, et al. Pyogenic liver abscesses: 13 years of experience in percutaneous aspiration with US guidance. Radiology. 1995;195:122–4. doi: 10.1148/radiology.195.1.7892451. [DOI] [PubMed] [Google Scholar]
- 7.Rajak CL, Gupta S, Jain S, Chawla Y, Gulati M, Suri S. Percutaneous treatment of liver abscesses: needle aspiration versus catheter drainage. Am J Roent. 1998;170:1035–9. doi: 10.2214/ajr.170.4.9530055. [DOI] [PubMed] [Google Scholar]
- 8.Farges O, Leese T, Bismuth H. Pyogenic liver abscess: an improvement in prognosis. Br J Surg. 1988;75:862–5. doi: 10.1002/bjs.1800750910. [DOI] [PubMed] [Google Scholar]
- 9.Pitt HA. Surgical management of hepatic abscesses. World J Surg. 1990;14:498–504. doi: 10.1007/BF01658675. [DOI] [PubMed] [Google Scholar]
- 10.Rubin RH, Swartz MN, Malt R. Hepatic abscess: changes in clinical, bacteriologic and therapeutic aspects. Am J Med. 1974;57:601–10. doi: 10.1016/0002-9343(74)90012-6. [DOI] [PubMed] [Google Scholar]
- 11.Hanks JB, Meyers WC, Filston HC, Killenberg PG, Scott Jones R. Surgical resection for benign and malignant liver disease. Ann Surg. 1980;191:584–90. doi: 10.1097/00000658-198005000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klatchko BA, Schwartz SI. Diagnostic and therapeutic approaches to pyogenic abscess of the liver. Surg Gynecol Obstet. 1989;168:332–6. [PubMed] [Google Scholar]
- 13.Frey CF, Zhu Y, Suzuki M, Isagi S. Liver abscesses. Surg Clin North Am. 1989;69:259–71. doi: 10.1016/s0039-6109(16)44784-5. [DOI] [PubMed] [Google Scholar]
- 14.Balasegaram M. Management of hepatic abscess. Curr Prob Surg. 1981;18:282–340. [PubMed] [Google Scholar]
- 15.Chou FF, Sheen-Chen SM, Chen YS, Chen MC. Single and multiple pyogenic liver abscesses: clinical course, etiology and results of treatment. World J Surg. 1997;21:384–9. doi: 10.1007/pl00012258. [DOI] [PubMed] [Google Scholar]
- 16.Lublin M, Bartlett DL, Danforth DW, et al. Hepatic abscesses in patients with chronic granulomatous disease. Ann Surg. 2002;235:383–91. doi: 10.1097/00000658-200203000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]