Abstract
Background
Acinar cell carcinoma (ACC) is a rare pancreatic neoplasm, and its presentation with acute pancreatitis has not been reported previously.
Case outline
A 70-year-old man presented with acute pancreatitis, and a spiral CT scan showed a 5-cm tumour in the body of the pancreas. Distal pancreatectomy was performed, and histological examination showed an ACC.
Discussion
This is a newly reported mode of presentation for a rare pancreatic tumour
Keywords: pancreas, acinar cell carcinoma, acute pancreatitis
Introduction
Acinar cell carcinoma (ACC) of the pancreas is a rare condition, representing approximately 1% of pancreatic exocrine tumours 1. Pancreatic tumours are a rare cause of acute pancreatitis 2 and, to our knowledge, this is the first reported case of ACC presenting as acute pancreatitis.
Case report
A 70-year-old man presented with an 11-day history of left upper quadrant abdominal pain, exacerbated by eating. He was a moderate consumer of alcohol, a heavy smoker, and was being treated for hypothyroidism and hypertension. Medication included losartan and thyroxine. A diagnosis of acute pancreatitis was made on admission, with a serum amylase of 2378 IU/L (normal range 12–100) and a CRP of 195 mg/L. The Glasgow Severity Score for acute pancreatitis was two. There was no history of weight loss, jaundice or acute heavy consumption of alcohol.
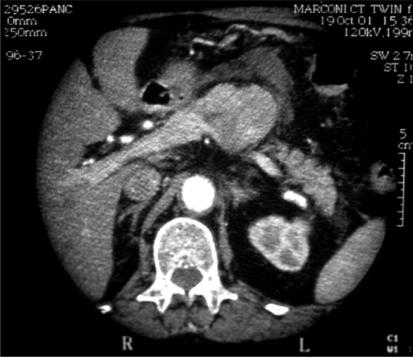
Ultrasound scan showed a 45-mm poorly echogenic lesion related to the tail of pancreas. There were no gallstones, and the biliary tree was normal. A contrast enhanced CT scan (Figure 1) confirmed a solid 5-cm lesion within the body of pancreas, with adjacent fluid collections. The lesion lay adjacent to the superior mesenteric and splenic vein confluence and demonstrated a small amount of central necrosis. There was moderate pancreatic ascites.
Figure 1. .
CT scan demonstr ating 5-cm tumour in body of pancreas.
Pain settled in hospital, but he developed insulin-dependent type II diabetes mellitus; he was discharged 13 days after admission. A further CT scan performed 1 month after discharge showed resolution of the pancreatitis, but the mass in the body of pancreas persisted. The radiological diagnosis was of a possible neuroendocrine tumour of the pancreas rather than a ductal adenocarcinoma in view of its enhancing nature (with intravenous contrast) and central necrosis. Ca19-9 assay and gastrointestinal hormone screen were normal.
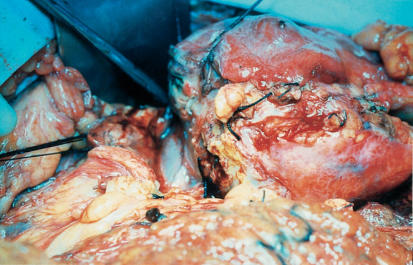
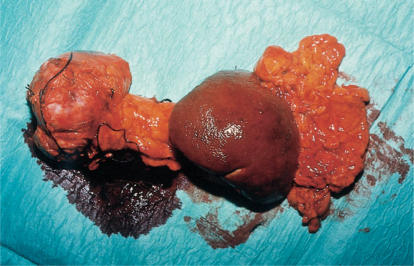
At laparotomy, a large tumour was found within the body of pancreas extending into the retroperitoneal space and the transverse mesocolon (Figure 2). There was no evidence of secondary spread. Distal pancreatectomy and splenectomy was performed (Figure 3). The patient's convalescence was unremarkable. Upon review 9 months postoperatively, he remains well although still diabetic.
Figure 2. .
Operative photograph showing tumour within body of pancreas.
Figure 3. .
Distal pancreatectomy and splenectomy specimen.
Pathological examination of the operative specimen revealed a moderately differentiated acinar cell carcinoma of the pancreas. There was some evidence of vascular invasion. Immunohistochemical staining for neuroendocrine cells was negative. There was no evidence of nodal spread. Elsewhere the pancreatic parenchyma demonstrated interstitial fibrosis.
Discussion
ACC is a rare pancreatic neoplasm representing 1% of exocrine pancreatic tumours and usually demonstrating a variable endocrine component 3. It is most common in Caucasian males in their seventh decade, the body of pancreas being the least common site (body 8%, head 56% and tail 36%) 3.
Macroscopically these tumours are solid, well circumscribed, fleshy lesions but they may occasionally display haemorrhage, necrosis or cystic characteristics 4,5,6. These circumscribed tumours therefore have a higher resectability rate (64%) than is the case with ductal adenocarcinoma (10–20%) 4,7,8.
The light microscopic appearance is one of acinar structures. Cells may demonstrate variable immunohistochemical positivity for enzymes including lipase and amylase. Up to 40% will demonstrate a minor endocrine component, and it has been suggested that this indicates a common embryological origin for both cell lines 3. Clinical presentation is varied, with jaundice being infrequent (12%) and complications of lipase release not uncommon. The latter include panniculitis, fat necrosis and polyarthralgia, and are probably more common with disseminated disease and associated with a poorer prognosis. Abdominal pain is present in around 32%, nausea and vomiting in 23% 4. However, acute pancreatitis remains unreported.
Half of all patients have evidence of metastatic disease on presentation, with a further 23% developing metastases to local lymph nodes, liver, lungs or spleen. Prognosis is considered better than that of ductal adenocarcinoma, with 1- and 3-year survival rates of 57% and 26%, respectively 4, whereas 1-year survival for ductal carcinoma may be <10%.
Although unreported in this particular type of tumour, pancreatitis secondary to other forms of pancreatic tumour is well known, albeit still rare. One study suggests that pancreatic carcinoma may present as acute pancreatitis in up to 13.8% of cases (24 of 174) 9. There are no recent case series, but it has been proposed that wherever pancreatic carcinoma is associated with pancreatitis, the diagnosis is often delayed. This may especially be the case when the tumour co-exists with gallstones, providing an alternative cause for pancreatitis 2. Possible causes of acute pancreatitis in our case may include release of amylase by the tumour itself or direct duct disruption. It is also possible that the subject's alcohol consumption played a part.
Of the various clinical parameters considered by Klimstra and co-workers 4, only age at presentation and the presence of symptoms of lipase secretion currently have a statistically significant negative impact on survival. It is possible that secretion of amylase and pancreatitis at presentation may be added to this list, although it is likely to be a long time before this association can be verified owing to the rarity of the tumour and this form of presentation. It is possible that with the advent of newer imaging modalities, more tumours of this type will be diagnosed at an earlier, resectable stage.
References
- 1.Klimstra DS, Rosai J, Heffhess CS. Mixed acinar-endocrine carcinomas of the pancreas. Am J Surg Pathol. 1994;18:765–78. doi: 10.1097/00000478-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Gambill EE. Pancreatitis associated with pancreatic carcinoma: a study of 26 cases. Mayo Clin Proc. 1971;46:174–7. [PubMed] [Google Scholar]
- 3.Virlos IT, Papazachariou IM, Wiliamson RCN. Acinar cell carcinoma of the pancreas with and without endocrine differentiation. HPB. 2002;4:87–90. doi: 10.1080/136518202760378452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klimstra DS, Heffness CS, Oertel JE, et al. Acinar cell carcinoma of the pancreas, a clinicopathologic study of 28 cases. Am J Surg Pathol. 1992;16:815–37. doi: 10.1097/00000478-199209000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Cantrell BB, Cubilla AL, Erlandson RA, et al. Acinar cell cystadenocarcinoma of the human pancreas. Cancer. 1981;47:410–16. doi: 10.1002/1097-0142(19810115)47:2<410::aid-cncr2820470232>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 6.Stamm BH, Burger H, Hollinger A. Acinar cell cystadenocarcinoma of the pancreas. Cancer. 1978;60:2542–7. doi: 10.1002/1097-0142(19871115)60:10<2542::aid-cncr2820601032>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Crinnion JN, Williamson RCN. Garden OJ. WB Saunders; London: 1997. Pancreatic neoplasia, Hepatobiliary and Pancreatic Surgery1st edn; pp. 321–51. [Google Scholar]
- 8.Sener SF, Fregmen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100, 313 patients diagnosed from 1985–1995, using the national cancer database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 9.Kohler H, Lankisch PC. Acute pancreatitis and hyperamylasaemia in pancreatic carcinoma. Pancreas. 1987;2:117–19. doi: 10.1097/00006676-198701000-00018. [DOI] [PubMed] [Google Scholar]