Abstract
Background
The aim of this retrospective study was to review all patients diagnosed with gallbladder cancer over a 10-year period to assess variables affecting survival.
Methods
Patients diagnosed with gallbladder cancer from January 1990 to December 1999 were identified from the Lothian Surgical Audit database and a case-note review was performed.
Results
The 44 patients who were studied (33 women, 11 men) had a mean age of 66 years (range 42–90 years). The diagnosis was established preoperatively in 25 patients (57%), intraoperatively in 5 patients (11%) and incidentally following pathological examination of cholecystectomy specimens in 14 patients (32%). None of the 25 patients diagnosed preoperatively underwent curative operations (median survival 4 months). All five patients diagnosed at the time of attempted cholecystectomy had advanced irresectable disease (median survival 1 month). The overall median survival in 14 patients with an incidental diagnosis of gallbladder cancer was 16 months; however, in eight of these patients who were considered to have had a potentially curative resection, the median survival was 38 months.
Discussion
The prognosis for patients diagnosed preoperatively or at the time of cholecystectomy is very poor. Patients with an incidental finding of gallbladder cancer have a significantly better prognosis and should be considered for further radical re-resection.
Keywords: gallbladder cancer, cholecystectomy, jaundice, hepatic resection, segment 3 cholangiojejunostomy
Introduction
Carcinoma of the gallbladder is an uncommon condition, but it is the most frequent tumour of the extrahepatic biliary tract, and the fourth commonest upper gastrointestinal malignancy. It carries a very poor prognosis 1,2 as patients tend to present late, only when contiguous structures and organs, such as the bile ducts or duodenum, are involved. At this stage the tumour is usually irresectable, and therefore the majority of patients undergo palliative treatment 3.
Some recent reports suggest an improved prognosis 4, particularly in a minority of patients with early tumours that are diagnosed incidentally on pathological examination of cholecystectomy specimens 5. Others have highlighted improved survival after radical surgery for advanced tumours 6. However, a recent multicentre review of 724 patients who underwent surgical treatment for gallbladder cancer failed to show improved survival rates compared with older reports 7.
The aim of this study was to review the investigation, diagnosis, treatment and survival of all patients with gallbladder carcinoma in a tertiary referral centre over a 10-year period.
Patients and methods
The case-notes of all patients with a diagnosis of gallbladder carcinoma between January 1990 and December 1999 were reviewed retrospectively. Patients were identified from the Lothian Surgical Audit database and were verified using records from the pathology, radiology and surgery departments. Information was obtained with regard to patient demographics, investigations, operative and non-operative treatment, pathological findings and survival. Patients in whom the diagnosis of gallbladder cancer was suspected before operation on abdominal ultrasonography underwent CT imaging of the abdomen and chest. Laparoscopic ultrasonography and mesenteric angiography were undertaken selectively to assess for evidence of widespread dissemination or vascular invasion. Patients presenting with jaundice from irresectable disease were considered for segment III cholangiojejunostomy if there was no evidence of direct tumour invasion into the left hemiliver or involvement of the supplying vessels with associated lobar atrophy. Endoscopic or percutaneous biliary stenting was undertaken in jaundiced patients who had advanced disease or were considered unsuitable for operation. The TNM classification and stage grouping criteria were used to stage the disease pathologically 8.
The cumulative survival rates were estimated by the Kaplan-Meier method. Assessment of the effect of discreet variables, such as patient age, sex, time of diagnosis, surgical procedure, tumour stage and tumour grade on survival was performed using the log-rank test. A p value of <0.05 was considered significant.
Results
Fifty-one patients were identified with a diagnosis of gallbladder cancer; however, seven sets of case-notes had been destroyed and therefore these patients were excluded from analysis. Of the 44 remaining patients, 33 were women (75%) and 11 were men (25%). The mean age at diagnosis was 66 years (range 42–90 years). Twenty-eight patients were referred from other hospitals, and the remaining patients were either referred directly to the hepatobiliary unit from their general practitioners (13 patients), or from other clinicians within the hospital (3 patients).
The commonest presenting complaints are shown in Table 1. The mean duration of symptoms was 14 weeks (range 1–150 weeks). Seventeen patients (39%) had a past medical history of gallstone disease or were noted to have radiological or pathological evidence of gallstones. No patient had a known family history of gallbladder cancer, or a prior history of another malignancy. Thirty-six patients (82%) had abnormal liver function tests at presentation, and the mean serum bilirubin concentration at the time of presentation was 124 mmol/L (range 3–460 mmol/L).
Table 1. Presenting symptoms of 44 patients with gallbladder cancer.
| Symptom | Number of patients |
|---|---|
| Abdominal pain | 36 |
| Vomiting | 13 |
| Weight loss | 13 |
| Jaundice | 13 |
| Pruritus | 10 |
| Anorexia | 8 |
| Abdominal distension | 1 |
Twenty-five patients (57%) were diagnosed with gallbladder cancer before operation on the basis of investigations (group 1). Five patients (11%) were diagnosed at the time of cholecystectomy (group 2), and in 14 patients(32%) the diagnosis was made on pathological examination of cholecystectomy specimens (group 3). Of the latter group, nine operations were performed laparoscopically, three were converted from an initial laparoscopic to an open procedure and two operations were performed entirely by the open technique. The various types of imaging modalities utilised in each of the three groups are shown in Table 2.
Table 2. Assessment of patients with gallbladder cancer.
| Investigation | Group 1 (n=25) | Group 2 (n=5) | Group 3 (n=14) |
|---|---|---|---|
| Abdominal ultrasound | 20 | 4 | 14 |
| CT | 21 | 4 | 10 |
| ERCP | 16 | 2 | 2 |
| PTC | 12 | 1 | 4 |
| Laparoscopic ultrasound | 12 | 0 | 2 |
| Mesenteric angiography | 6 | 0 | 3 |
Laparoscopic staging was performed in 14 patients. Of the 12 patients in group 1 who underwent laparoscopic staging, metastases were confirmed in three patients where radiological imaging had raised a suspicion of distant spread and six patients had metastases identified which were not seen by other imaging modalities. Laparoscopy was normal in three patients; however, disseminated disease was present at the time of laparotomy in two of these patients and the third patient did not undergo definitive surgical resection because of serious co-morbidity. Both patients in group 3 who underwent laparoscopic staging after diagnosis of their malignancy proceeded to laparotomy. One had a curative resection, the other was found to have lymph node metastases (porta hepatis and superior pancreatic) and had palliative treatment.
Surgical operation
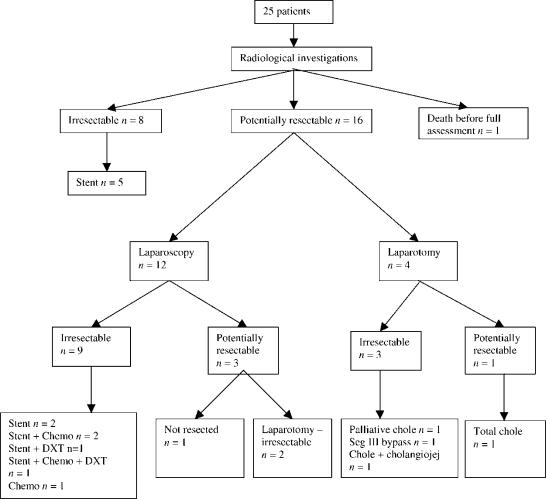
Of the 25 patients diagnosed preoperatively (group 1), 16 were considered to have potentially resectable disease and 8 were judged to have advanced disease on the basis of radiological findings. A further patient underwent percutaneous stenting and died from cholangitis, sepsis and multi-organ failure during the same admission, before a decision was made about resectability (Figure 1). Twelve patients underwent further assessment by laparoscopy and laparoscopic ultrasound and four patients underwent laparotomy without prior laparoscopic staging. Of the latter group, frozen section confirmed metastatic disease in three patients and therefore palliative procedures were undertaken (cholecystectomy, 1; cholecystectomy and cholangiojejunostomy, 1; segment III bypass, 1). Cholecystectomy was considered curative in one patient, but she represented 6 months later with metastatic disease.
Figure 1. .
Patients diagnosed preoperatively. Chemo, chemotherapy; Chole, cholecystectomy; cholangiojej, cholangiojejunostomy; Seg III, segment III; DXT, radiotherapy.
All five patients in whom the cancer was diagnosed intraoperatively were found to have advanced irresectable disease. In one case a cholecystectomy, and in another an open biopsy only had been performed in the referring hospital. Following transfer, radiological imaging confirmed the advanced nature of the disease and radical re-resection was not considered appropriate. The three patients treated primarily in our own unit underwent subtotal cholecystectomy (n=2), or total cholecystectomy and hepaticojejunostomy (n=1) as palliative procedures. Two of the five patients later required biliary stenting for recurrent jaundice, and one patient who developed gastric outlet obstruction due to disease progression 4 months after diagnosis required a subsequent gastrojejunostomy. Four patients from this group were therefore staged pathologically (stage IVa, 3; stage IVb, 1).
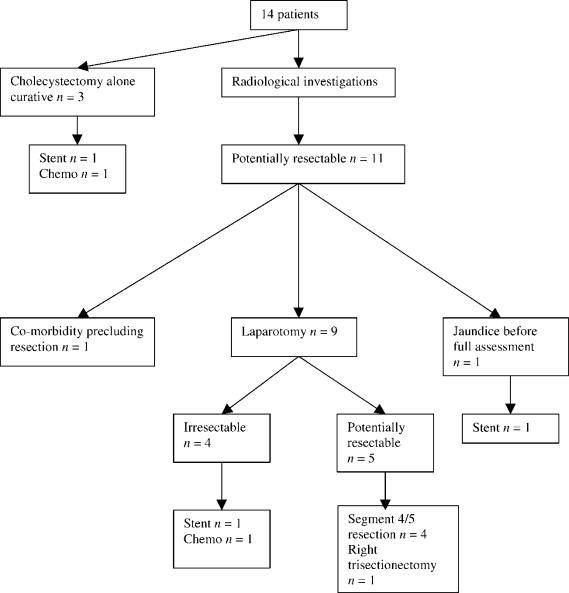
Of the 14 patients diagnosed on pathological examination of cholecystectomy specimens, cholecystectomy alone was considered to be potentially curative in three cases (one each with Tla, T1b and T2 tumours) (Figure 2). The patient with the Tla tumour was treated with adjuvant chemotherapy, as the gallbladder was perforated at the time of cholecystectomy. The patient with the T2 lesion did not undergo a radical re-resection because of the presence of granulomatous hepatitis. This patient developed jaundice due to tumour recurrence 2 years later and required palliative biliary stenting.
Figure 2. .
Patients diagnosed postoperatively. Chemo, chemotherapy.
Eleven patients were considered for radical re-resection; however, one patient was not suitable due to serious co-morbidity, and one patient developed jaundice due to disease progression before a decision was reached on further management. The remaining nine patients underwent further assessment (CT scan, 9; mesenteric angiography, 3; laparoscopy and laparoscopic ultrasound, 2; ERCP, 2; PTC, 1). Although CT scan revealed an abnormality in the region of the gallbladder bed in two patients and PTC demonstrated a high biliary stricture in a further patient, there were no absolute contraindications to further exploratory operation in any of the nine patients. Five patients proceeded to radical re-resection (four had T2 tumours and one had a T3 tumour), comprising radical bile duct excision combined with resection of segments 4/5 of the liver (n=4) or right trisectionectomy (n=1). Pathological examination of the resected specimens revealed no evidence of residual tumour in four cases, but one patient (with a T2 tumour) had an involved retroduodenal lymph node. This patient required a third laparotomy with revision of the biliary anastomosis 2 years later because of recurrent episodes of cholangitis secondary to an anastomotic stricture. A peritoneal metastasis was excised at the time of the third laparotomy, but the patient died 3 years after the initial diagnosis. Four patients who underwent a second exploratory laparotomy for possible radical re-resection were found to have irresectable disease (T2 tumour, 2; T3 tumour, 1; T4 tumour, 1). Of these patients, two had biliary stenting and two received chemotherapy.
Morbidity and mortality
There were no perioperative deaths in this series. Two of the 36 patients who underwent non-curative operations developed chest infections, but there were no other serious complications.
Of the five patients who underwent radical re-resection procedures, one patient required splenectomy for an inadvertent operative injury. Another was readmitted 2 weeks postoperatively with a major haematemesis from a hepatic artery pseudoaneurysm, which was treated successfully by embolisation at mesenteric angiography. Late complications occurred in two patients. One patient developed recurrent episodes of cholangitis due to an anastomotic stricture which was treated by stenting, and one patient developed an incisional hernia, which was repaired 1 year after operation. Four of the 20 patients who underwent biliary stenting developed recurrent jaundice due to stent blockage, and two of them had an episode of ascending cholangitis. All four patients had stent replacement.
Pathology
Definite histology was available in 39 cases (89%) and in the remaining five patients, radiological evidence and clinical course were consistent with gallbladder cancer. Thirty-seven tumours were adenocarcinomas, of which nine were classified as mucinous, one as papillary and one as tubular. One further tumour was classified as adenosquamous and one as a carcinosarcoma. Fourteen tumours were reported as poorly differentiated, 4 were moderately differentiated, none were well differentiated, and the remainder were unclassified.
Survival
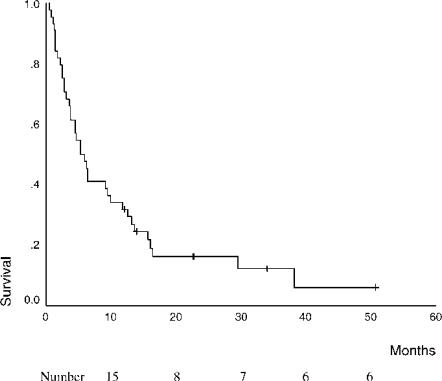
The overall median survival in this series of 44 patients was 5 months (Figure 3). Six patients remain alive at 12, 14, 23, 23, 34 and 51 months after initial diagnosis. Four of these patients underwent curative radical re-resection after an incidental diagnosis of gallbladder cancer was made following cholecystectomy, one patient had an early tumour for which cholecystectomy alone was considered to be curative, and one patient is still alive 34 months after diagnosis. The latter patient had a tumour that was considered resectable, but operation was not performed in view of serious co-morbidity. Patients with a normal serum bilirubin at diagnosis had a better outcome than patients who presented with jaundice (16 months vs 4 months, p = 0.0002).
Figure 3. .
Survival rates of all patients with gallbladder carcinoma.
T-stage alone or the stage grouping criteria were not predictive of patient survival using log-rank analysis. The median survival of Tl tumours (n=3) was 13 months, T2 tumours (n=9) 30 months, T3 tumours (n=3) 10 months and T4 tumours (n=6) 6 months. The median survival of patients with stage I disease (n=3) was 13 months, stage II disease (n=6) 30 months, stage III disease (n=2) 10 months, stage IVa disease (n=5) 5 months and stage IVb disease (n=14) 4 months. Of the three patients with stage I lesions, two had serious co-morbidity and died after 5 and 14 months, respectively. The remaining patient with stage I disease is still alive 51 months after diagnosis.
Patients with a poorly differentiated tumour had a median survival of 4 months, compared with 38 months in those with a moderately differentiated tumour (p = 0.015).
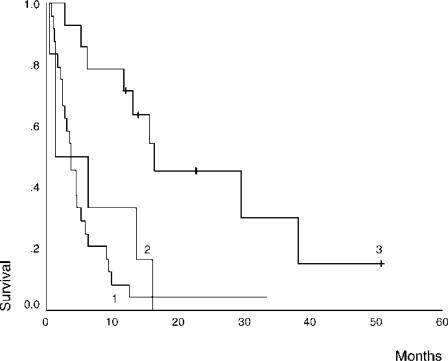
Patients diagnosed incidentally on pathological examination of cholecystectomy specimens had an improved survival compared with patients whose gallbladder cancer was diagnosed preoperatively or at the time of cholecystectomy (16 months vs 4 months vs 1 month, p = 0.0003) (Figure 4). Patients undergoing radical re-resection and those in whom a cholecystectomy alone was considered curative had a better outcome than those who did not undergo curative surgery (38 months vs 6 months, p = 0.001).
Figure 4. .
Comparison of survival rates according to timing of diagnosis, 1, on investigation (n=25); 2, at cholecystectomy (n=5); 3, incidental (n=14).
Discussion
The very poor prognosis in patients with gallbladder carcinoma is confirmed in this series, with an overall median survival of 5 months. Other series report similar overall cumulative survival rates, as most patients present with advanced disease 2,7. Possible management strategies should be evaluated in the light of the natural history of the disease, survival benefit, morbidity and mortality rates, and patient co-morbidity. Many studies have reported that T-stage or stage-grouping are of positive predictive value for survival 2,7, although the small numbers of patients with early disease has not allowed a significant correlation with survival to be demonstrated in this series.
Tl tumours are most commonly diagnosed incidentally on pathological examination of cholecystectomy specimens. Our review confirms that cholecystectomy alone is associated with excellent survival and agrees with the current consensus that no further treatment is required 7,9,10. If the tumour is recognised intraoperatively, then a cystic duct lymph node and periportal lymph nodes should be sampled to rule out stage II disease. However, if a Tl gallbladder cancer is recognised only incidentally on pathological examination, then no further surgery is indicated, as the incidence of lymph node involvement is almost non-existent in Tl disease 11. It is essential that the cystic duct margin is not involved with tumour. A positive margin dictates the need for re-resection. Shirai et al. reported recurrences in only 2 of 89 patients with Tl disease, but both patients had tumour involving the cystic duct margin and both went on to die of their disease 12.
The general consensus for patients with T2 gallbladder cancer is that an extended resection (involving hepatic resection and radical lymph node dissection in addition to cholecystectomy) should be undertaken, as previous studies have shown an improved survival compared with cholecystectomy alone 7,9. Lymph node metastases are common with T2 tumours; however, the cystic duct lymph node is rarely retrieved at laparoscopic cholecystectomy and therefore accurate staging of these patients is difficult. If a gallbladder carcinoma is diagnosed at laparoscopic cholecystectomy, the procedure should be converted to an open technique, to minimise the risk of dissemination, and a definitive procedure should be carried out if its is technically possible and if the surgeon has the necessary expertise 13. It is not our policy to undertake routine frozen section as this risks the dissemination of tumour cells; however, frozen-section histology of suspicious areas may be beneficial in selected patients. The cancer should ideally be recognised before disrupting the subserosal plane (which may be invaded by tumour) and an extended surgical resection undertaken. However, as most patients with T2 tumours will only be diagnosed postoperatively by pathological examination, further assessment and repeat exploratory laparotomy may be necessary.
Patients should be selected carefully for re-resection by assessment of their physiological status and by further staging investigations. The optimal restaging modalities are not clearly defined; however, in our own practice we rely primarily on CT scan with selective use of mesenteric angiography and laparoscopic ultrasonography.
The aim of hepatic resection is to achieve a negative resection margin 6,9,14, although the optimal extent of the resection is unclear. No difference in survival has been demonstrated between wedge resection and more extensive liver resection for T2 lesions 15. It is recommended that port sites should be excised if radical re-resection is undertaken for patients found incidentally to have gallbladder carcinoma after laparoscopic chole-cystectomy because of the high incidence of port site recurrence 16. In our experience, radical re-resection often merely allows more accurate staging, as in four of the five patients who underwent this procedure no residual cancer was identified. One patient had an involved lymph node but no evidence of tumour in the resected liver. Five-year survival figures of 60–85% have been reported in patients with T2 tumours after radical re-resection 6,9,17. De Aretxabala and colleagues reported an improved 5-year survival rate in 20 patients who underwent re-resection for incidental T2 tumours compared with 18 patients who did not undergo re-resection (70% vs 20%) 18.
Radical re-resection may be considered for selected patients with T3 or T4 tumours 13. The one patient in our series who had radical re-resection of a T3 tumour is alive without recurrence 12 months after diagnosis. Results in the literature for this subgroup of patients vary, although Tsukada and colleagues quote a 40% 5-year survival rate 6. Cubertafond and co-workers failed to show any improvement in survival when comparing radical surgery to cholecystectomy alone 7.
Lymph node status has been shown consistently to be an important prognostic factor 19, and variable results have been reported for aggressive lymphadenectomy 6,7. Some Japanese authors have attempted even more radical surgery including pancreatoduodenectomy 20 and para-aortic lymphadenectomy 21, but this is associated with a high perioperative mortality rate and there is much doubt as to whether there is a survival benefit. Resection of the common bile duct is commonly performed because lymphatic spread of the disease along the bile duct is an important mechanism of disease progression. However, this manoeuvre has not been shown to improve survival 22, and many authors would only advocate it if the tumour is close to or actually infiltrates the bile duct 13.
There were no postoperative deaths in this series. Mortality rates reported in the literature vary considerably. The large review of 724 patients by the French Surgical Association showed an overall mortality rate of 22% 7, whereas others have observed rates as low as 0.9% 6. Forty-eight operative procedures were undertaken in this series with an overall morbidity rate of 10%. This figure compares with a morbidity rate of 34% reported by Tsukada et al. after extended procedures 6, but is in keeping with an overall operative morbidity of 13% in series of patients undergoing a similar range of operative procedures reported by Donohue and colleagues 2.
As most patients with gallbladder cancer have incurable disease at the time of presentation, many will undergo palliative treatment 4. No one palliative treatment is suitable for all patients. Cholecystectomy or gallbladder drainage may be required to manage mucocele or acute cholecystitis resulting from obstruction of the cystic duct. Given the limited survival prospects, endoscopic or percutaneous drainage of the biliary tree may be appropriate, although selected patients may have improved palliation with a segment III cholangiojejunostomy. Gastrointestinal bypass may be necessary for gastric outlet obstruction 23,24.
In this review of our recent experience of gallbladder carcinoma it is evident that most tumours are irresectable at the time of presentation. Tumours generally present late with a short history of non-specific abdominal symptoms. The exceptions are tumours diagnosed incidentally on pathological examination of cholecystectomy specimens. The only prospect of cure is in patients with early tumours that have been removed entirely by cholecystectomy, or in selected patients with advanced tumours (T2, T3, T4) that are diagnosed incidentally and who go on to have radical re-resection. In this carefully selected group, mortality rates following radical re-resection are low and a significant survival advantage can be expected.
References
- 1.Oerth D, Herzog U, Tondelli P. Primary carcinoma of the gallbladder: operative experience during a 16 year period. EurJ Surg. 1993;159:415–20. [PubMed] [Google Scholar]
- 2.Donohue JH, Nagorney DM, Grant CS, et al. Carcinoma of the gallbladder. Does radical resection improve outcome? Arch Surg. 1990;125:237–41. doi: 10.1001/archsurg.1990.01410140115019. [DOI] [PubMed] [Google Scholar]
- 3.Piehler JM, Crichlow RW. Primary carcinoma of the gallbladder. Surg Gynecol Obstet. 1978;147:929. [PubMed] [Google Scholar]
- 4.Ouchi K, Suzuki M, Saijo S, Ito K, Matsuno S. Do recent advances in diagnosis and operative management improve the outcome of gallbladder carcinoma? Surgery. 1987;101:731–7. [PubMed] [Google Scholar]
- 5.Ouchi K, Sugawara T, Ono H, et al. Diagnostic capability and rational resectional surgery for early gallbladder cancer. Hepatogastroenterology. 1999;46:155–60. [PubMed] [Google Scholar]
- 6.Tsukada K, Hatakayama K, Kurosaki I, et al. Outcome of radical surgery for carcinoma of the gallbladder according to TNM stage. Surgery. 1996;120:816–20. doi: 10.1016/s0039-6060(96)80089-4. [DOI] [PubMed] [Google Scholar]
- 7.Cubertafond P, Gainant A, Cuchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg. 1994;219:275–80. doi: 10.1097/00000658-199403000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The UICC, TNMClassification of Malignant Tumours, 5th edn. New York: Wiley & Sons, 1997. [Google Scholar]
- 9.Shirai Y, Yoshida K, Tsukada K, Muto T. Inapparent carcinoma of the gallbladder. An appraisal of radical second operation after simple cholecystectomy. Ann Surg. 1992;215:326–31. doi: 10.1097/00000658-199204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouchi K, Owada Y, Matsuno S, Sato T. Prognostic factors in the surgical treatment of gallbladder carcinoma. Surgery. 1987;101:729–37. [PubMed] [Google Scholar]
- 11.Tsukada K, Kurosaki I, Uchida K, et al. Lymph node spread from carcinoma of the gallbladder. Cancer. 1997;80:661–7. [PubMed] [Google Scholar]
- 12.Shirai Y, Yoshida K, Tsukada K, Muto T, Watanabe H. Early carcinoma of the gallbladder. Eur J Surg. 1992;158:545–8. [PubMed] [Google Scholar]
- 13.Muratore A, Polastri R, Capussotti L. Radical surgery for gallbladder cancer: current options. Eur J Surg Oncol. 2000;26:438–43. doi: 10.1053/ejso.1999.0918. [DOI] [PubMed] [Google Scholar]
- 14.Chjiiwa K, Tanaka M. Carcinoma of the gallbladder: an appraisal of surgical resection. Surgery. 1994;115:751–6. [PubMed] [Google Scholar]
- 15.Yoshikawa T, Araida T, Azuma T, Takasaki K. Bisubsegmental liver resection for gallbladder cancer. Hepatogastroenterology. 1998;45:14–19. [PubMed] [Google Scholar]
- 16.Z'graggen K, Birrer S, Maurer CA, Wehrli H, Klaiber C, Baer HU. Incidence of port site recurrence after laparoscopic cholecystectomy for preoperatively unsuspected gallbladder carcinoma. Surgery. 1998;124:831–8. [PubMed] [Google Scholar]
- 17.Yamaguchi K, Chijiiwa K, Saiki K, et al. Retrospective analysis of 70 operations for gallbladder carcinoma. Br J Surg. 1997;84:200–4. [PubMed] [Google Scholar]
- 18.De Aretxabala XA, Roa IS, Burgos LA, et al. Curative resection in potentially resectable tumours of the gallbladder. EurJ Surg. 1997;163:419–26. [PubMed] [Google Scholar]
- 19.Morrow CE, Sutherland DER, Florack G, Eisenberg MM, Grage TB. Primary gallbladder carcinoma: significance of subserosal lesions and result of aggressive surgical treatment and adjuvant chemotherapy. Surgery. 1983;94:709–14. [PubMed] [Google Scholar]
- 20.Nakamura S, Nishiyama R, Yokoi Y, et al. Hepatopancreatoduodenectomy for advanced gallbladder carcinoma. Arch Surg. 1994;129:625–9. doi: 10.1001/archsurg.1994.01420300069010. [DOI] [PubMed] [Google Scholar]
- 21.Kondo S, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Uesaka K. Regional and para-aortic lymphadenectomy in radical surgery for advanced gallbladder carcinoma. Br J Surg. 2000;87:418–22. doi: 10.1046/j.1365-2168.2000.01384.x. [DOI] [PubMed] [Google Scholar]
- 22.Chijiva K, Tanaka M. Indications for and limitations of extended cholecystectomy in the treatment of carcinoma of the gallbladder. Eur J Surg. 1996;162:211–16. [PubMed] [Google Scholar]
- 23.Baxter I, Garden OJ. Surgical palliation of carcinoma of the gallbladder. Hepatogastroenterology. 1999;46:1572–7. [PubMed] [Google Scholar]
- 24.Kapoor VK, Pradeep R, Haribhakti SP, et al. Intrahepatic segment III cholangiojejunostomy in advanced carcinoma of the gallbladder. Br J Surg. 1996;83:1709–11. doi: 10.1002/bjs.1800831215. [DOI] [PubMed] [Google Scholar]