Abstract
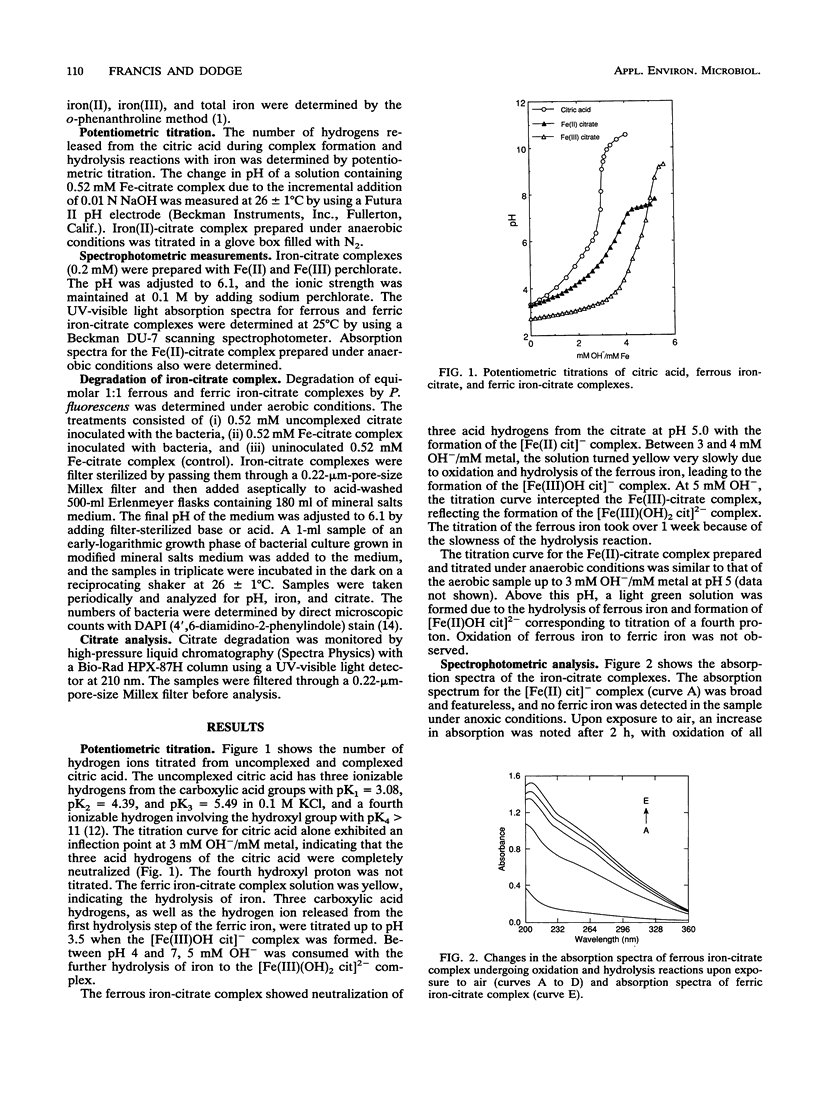
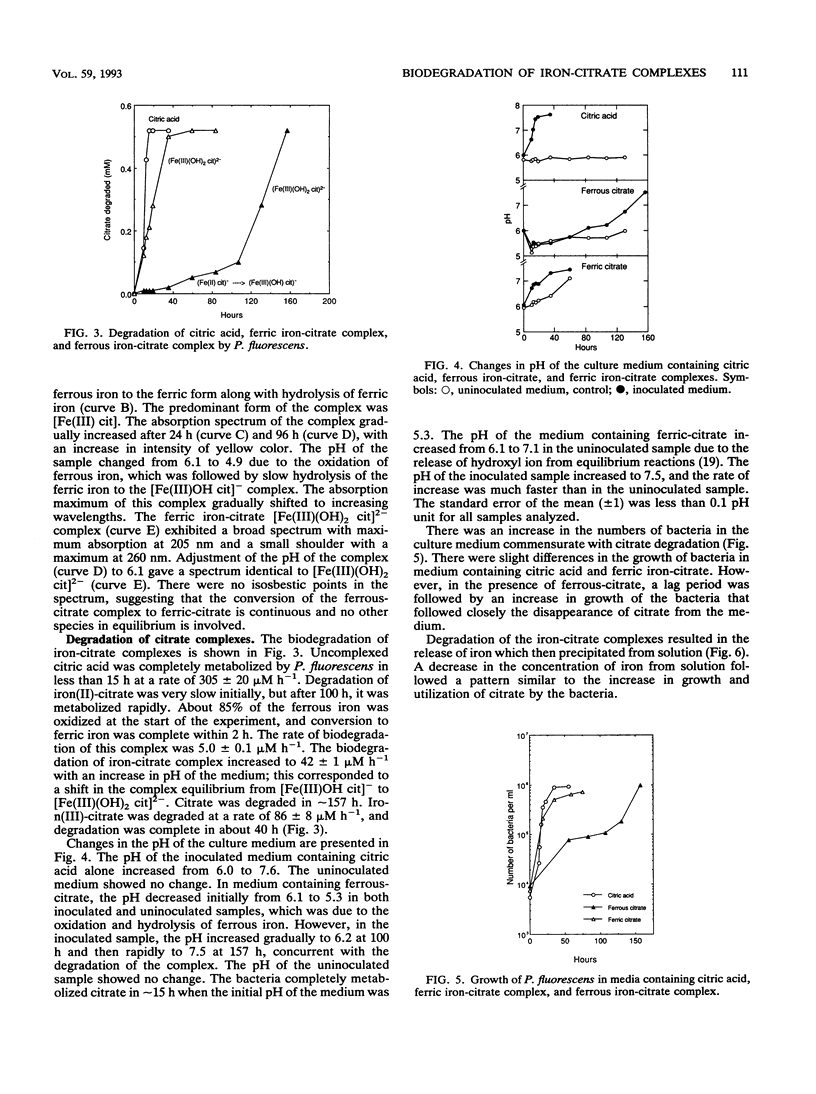
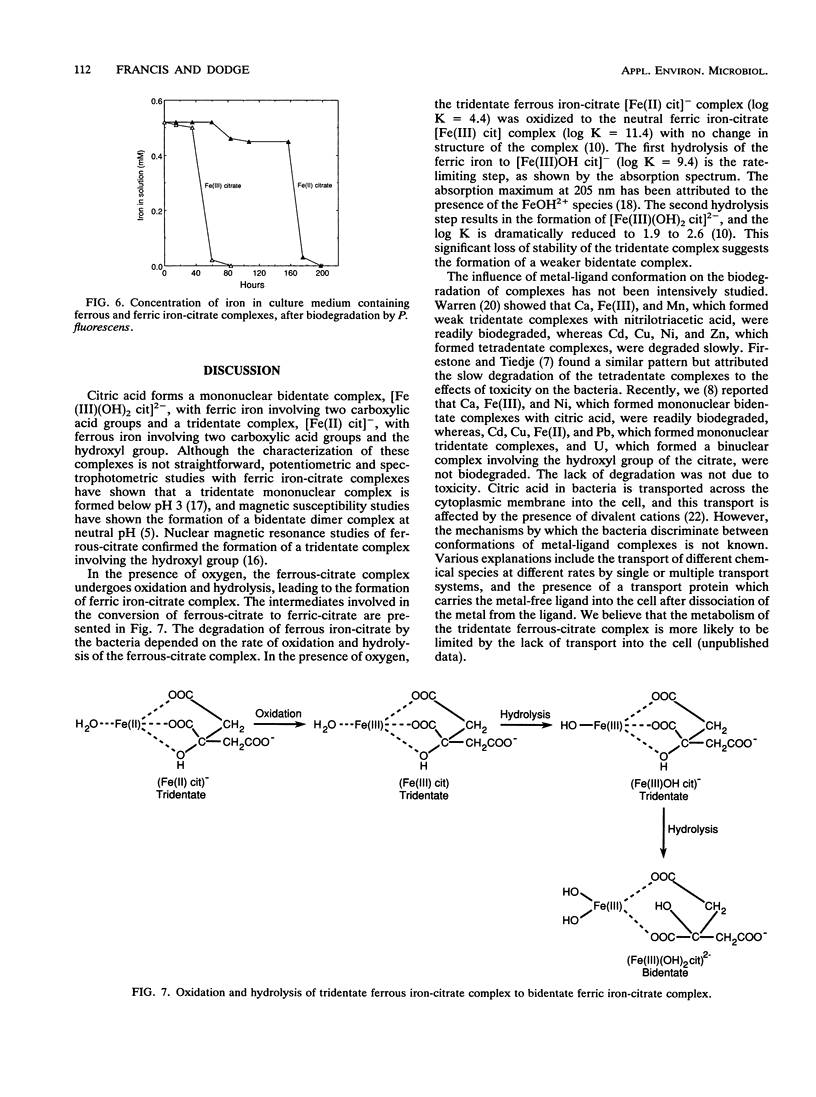
The biodegradation of iron-citrate complexes depends on the structure of the complex formed between the metal and citric acid. Ferric iron formed a bidentate complex with citric acid, [Fe(III) (OH)2 cit]2- involving two carboxylic acid groups, and was degraded at the rate of 86 μM h-1. In contrast, ferrous iron formed a tridentate complex with citric acid, [Fe(II) cit]-, involving two carboxylic acid groups and the hydroxyl group, and was resistant to biodegradation. However, oxidation and hydrolysis of the ferrous iron resulted in the formation of a tridentate ferric-citrate complex, [Fe(III)OH cit]-, which was further hydrolyzed to a bidentate complex, [Fe(III)(OH)2 cit]2-, that was readily degraded. The rate of degradation of the ferrous-citrate complex depended on the rate of its conversion to the more hydrolyzed form of the ferric-citrate complex. Bacteria accelerated the conversion much more than did chemical oxidation and hydrolysis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brynhildsen L., Rosswall T. Effects of cadmium, copper, magnesium, and zinc on the decomposition of citrate by a Klebsiella sp. Appl Environ Microbiol. 1989 Jun;55(6):1375–1379. doi: 10.1128/aem.55.6.1375-1379.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone M. K., Tiedje J. M. Biodegradation of metal-nitrilotriacetate complexes by a Pseudomonas species: mechanism of reaction. Appl Microbiol. 1975 Jun;29(6):758–764. doi: 10.1128/am.29.6.758-764.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot M. L., Meidl E. J., Plessner O. Citrate as a siderophore in Bradyrhizobium japonicum. J Bacteriol. 1990 Jun;172(6):3298–3303. doi: 10.1128/jb.172.6.3298-3303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen E. L., Alexander M. Effects of chemical speciation on the mineralization of organic compounds by microorganisms. Appl Environ Microbiol. 1985 Aug;50(2):342–349. doi: 10.1128/aem.50.2.342-349.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strouse J., Layten S. W., Strouse C. E. Structural studies of transition metal complex of triionized and tetraionized citrate. Models for the coordination of the citrate ion to transition metal ions in solution and at the active site of aconitase. J Am Chem Soc. 1977 Jan 19;99(2):562–572. doi: 10.1021/ja00444a041. [DOI] [PubMed] [Google Scholar]
- Willecke K., Gries E. M., Oehr P. Coupled transport of citrate and magnesium in Bacillus subtilis. J Biol Chem. 1973 Feb 10;248(3):807–814. [PubMed] [Google Scholar]


