Abstract
Background
Intraductal papillary mucinous tumours (IPMT) were described as a distinct entity in l982.The extent of surgical resection remains controversial.
Methods
Six patients with a diffuse dilatation of the main pancreatic duct were treated with total pancreatectomy for cure of IPMT.
Results
Histological examination showed one IPM adenoma, four IPM non-invasive carcinomas and one IPM invasive carcinoma. In all but one case multifocal extensive intraductal changes were found, affecting either most of the pancreas or the whole organ. All patients survived the operation and remain alive 5–56 months later. Post-pancreatectomy diabetes has been moderately well controlled.
Discussion
IPMTs represent a subgroup of pancreatic neoplasms with a favourable prognosis, and the resection should aim at removing all dysplastic foci. In cases with diffuse dilatation of the main pancreatic duct, widespread tumour involvement of the duct system can be expected, so total pancreatectomy should be the operation of choice.
Keywords: pancreatic intraductal papillary mucinous tumour, mucinous tumour, total pancreatectomy
Introduction
An increasing incidence of pancreatic cancer has made the disease the fifth leading cause of cancer death in the USA 1 and the sixth in the UK 2. The overall prognosis is extremely poor, only 10% of patients being alive 1 year after diagnosis and <3% at 5 years 2,3,4. Excluding ampullary carcinomas, only a minority of pancreatic cancers are suitable for operation, which offers the only hope of long-term survival.
With the recent description of intraductal papillary mucinous tumour (IPMT), a subgroup of pancreatic tumours – premalignant and malignant – has been identified for which aggressive surgical treatment is worthwhile and can be strongly recommended 5,6,7,8,9,10,11,12,13,14,15. A few hundred cases of IPMT have been described, but follow-up is sparse and short. Total pancreatectomy has been reported with follow-up in only 12 patients from seven centres 16,17,18,19,20,21,22. We therefore believe that it is important to present our experience of seven patients with IPMT, six of whom were operable and underwent a total pancreatectomy.
Patients and methods
During the last 5 years seven men with IPMT were diagnosed in this department. The median age was 68 years (range 39–75 years). All patients had a preoperative ultrasound scan (US) and endoscopic retrograde cholangiopancreatography (ERCP) with brush cytology. Three had a computed tomography (CT) scan and one a magnetic resonance (MR) scan. Fine-needle aspiration cytology (FNAC) was performed preoperatively in six patients. Preoperative biopsies were taken when needed. One 75-year-old man with biopsy-verified metastases in the liver was not operated. The other six patients recieved a total pancreatectomy. The clinical and demographic data are shown in Table 1. Patients were followed-up in their own county by specialists in internal medicine or endocrinology.
Table 1. Demographic data on seven male IPMT patients diagnosed between 1994 and 1998.
| Case | Age (yr) | Symptoms | US FNAC | CT | MR | ERCP/brush cytology |
|---|---|---|---|---|---|---|
| 1 | 46 | pain, loss of weight | cyst – | cyst, duct ectasia | – | communicating cysts/dysplasia, mucin, duct ectasia |
| 2 | 68 | loss of weight, steatorrhoea | multi-cystic | – | – | IPMT/CIS, duct ectasia |
| 3 | 54 | loss of weight, steatorrhoea | cystic mass in caput, normal cells | – | – | duct ectasia, normal cells, mucin, communicating cysts |
| 4 | 73 | loss of weight, pain, steatorrhoea | mass in head of pancreas, normal cells | mass in head of pancreas | – | duct ectasia, severe dysplasia, mucin |
| 5 | 65 | pain, diabetes, loss of weight, alcohol | mass in caput, dilated duct, normal cells | dilated duct | duct ectasia with tumour in duct | duct ectasia, dysplasia, mucin, tumour |
| 6 | 39 | pain, loss of weight, pancreatitis | dilated duct | – | – | duct ectasia, normal cells, suspicion for either tumour or mucin (filling defect). |
| 7 | 75 | loss of weight | liver metastases carcinoma | – | – | duct ectasia, dysplasia, mucin |
US = ultrasound; FNAC = fine-needle aspiration cytology; CT = computed tomography; MR = magnetic resonance imaging; ERCP = endoscopic retrograde cholangiopancreatography; IPMT = intraductal papillary mucinous tumour; CIS = carcinoma in situ; caput = head of pancreas.
Histology
The pancreatectomy specimens (Figure 1) were meticulously investigated by the pathologist. The histology of the tumours was described according to the WHO classification 23,24 as follows. (1) Intraductal papillary mucinous adenomas were papillary mucin-producing tumours with little or no cellular atypia. (2) Borderline papillary mucinous tumours were moderately dysplastic (hyperchromatic polarised nuclei with an occasional distinct nucleolus, nuclear crowding and stratification, variable mucin content). With adenomas the papillary projections had a fibrovascular stalk. (3) Intraductal papillary mucinous carcinoma, non-invasive, showed in-situ carcinoma of the papillary proliferations (nuclear pleomorphism, prominent nucleoli, mitotic figures of the epithelial cells with papillae without a fibrovascular stalk, cribriform pattern). (4) Intraductal papillary mucinous carcinoma, invasive. As for (3), but with clear signs of stromal invasion (Figure 2).
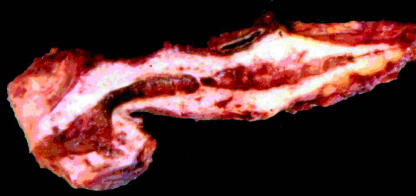
Figure 1. .
Pancreatectomy specimen from case no. 4, in which an intraductal papillary mucinous carcinoma was confined to the main duct and side branches of the head and neck of the pancreas.The main duct is distended and there is severe chronic obstructive pancreatitis of the whole organ.
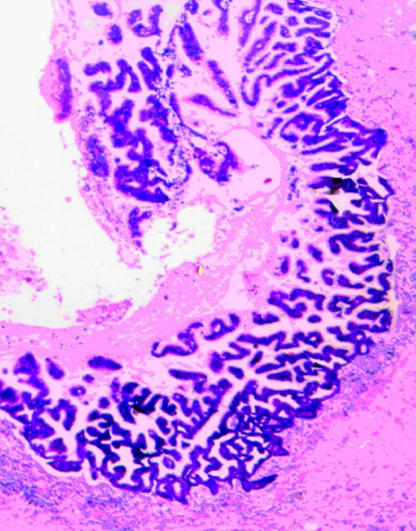
Figure 2. .
Part of dilated main duct in case no. 2 with severely dysplastic papillary epithelium representing an intraductal papillary mucinous carcinoma. Haematoxylin and eosin (×40).
Results
The results of the US, FNAC, CT, MR, ERCP and brush cytology are shown in Table 1. All seven patients had diffuse dilatation of the main pancreatic duct. Six patients underwent total pancreatectomy, while the seventh lived for 17 months without operation despite the presence of histologically proven liver metastases. Patient no. 3 was operated on suspicion of mucinous cystadenoma or cystadenocarcinoma in the pancreatic head. A Whipple's procedure was performed, and frozen section of the resection margin of the pancreas showed no tumour. This judgement was wrong: after further examination mucin-producing adenoma was detected at the resection margin. Ten days later the patient was reoperated for leakage from the pancreaticojejunostomy, and the rest of the pancreas was then removed.
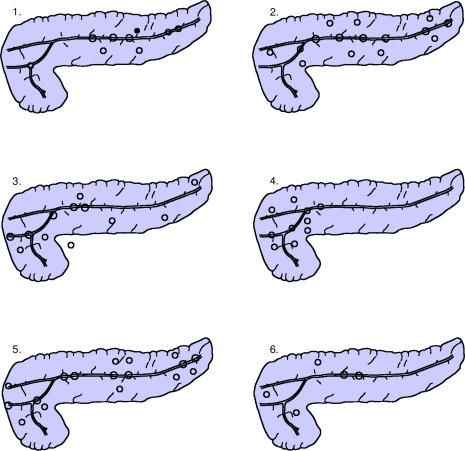
The widespread extension of papillary tumour in all six operated patients is illustrated in Figure 3. The morphology of these six tumours is shown in Table 2. All six operated patients are alive 5–56 months after total pancreatectomy, having regained their preoperative weight.
Figure 3. .
Schematic drawings of localisation of the papillary tumours in six patients. Open circles over the main duct represent in-situ main duct localisation. Open circles outside the main duct represent changes in side branches. In one patient (case no. 1) the closed circle in the body of the pancreas represents a focus of early invasion.
Table 2. Morphology of the six intraductal papillary pancreatic tumours.
| Patient | Type | Extent and secondary changes |
|---|---|---|
| 1 | IPMC (I) | Severe dysplastic changes practically confined to the body and tail with early invasion of the body |
| Main duct and side branches. Slight obstructive pancreatitis of the head and moderate/severe of the body and tail | ||
| 2 | IPMC (N) | Widespread severe dysplasia in main duct and side branches throughout the pancreas |
| Severe obstructive pancreatitis of the whole organ | ||
| 3 | IPMA | Slight dysplasia in the main duct and side branches of the head and in few scattered foci of the body and tail |
| Severe obstructive pancreatitis of the head, slight in body and tail | ||
| 4 | IPMC (susp.I) | Severe dysplasia with focus of suspected invasion |
| Changes confined to the main duct and side branches of head and neck | ||
| Severe obstructive pancreatitis of the whole organ | ||
| 5 | IPMC (N) | Widespread moderate-to-severe dysplasia in main duct and side branches of the entire pancreas |
| Moderate obstructive pancreatitis of the head – severe in body and tail | ||
| 6 | IPMC (N) | Focal dysplasia in side branches of the head and large focus in main duct of the body with moderate and severe dysplasia |
| Moderate obstructive pancreatitis of the head – severe in body and tail |
IPMA = intraductal papillary mucinous adenoma; IPMC = intraductal papillary mucinous carcinoma; N = noninvasive; I = invasive; susp.I = suspicious for invasion.
Only one patient needed daily strong analgesics. He had a 15-year history of Crohn's disease and was addicted to strong medication before the operation. Steatorrhoea was effectively treated in each patient by means of a low-fat diet and pancreatic enzyme substitution (6–12 Pancreon daily: median 9), and no patient had more than two stools per day.
Diabetes mellitus was moderately well regulated in all patients with Hgb Alc fractions between 0.062 and 0.099 (median 0.084; normal range: 0.044–0.064). None experienced diabetic ketoacidosis.
Discussion
In 1982 Ohhashi et al.25 described the clinical criteria associated with a specific group of neoplasms in the pancreas. The four criteria were as follows: an ectatic main pancreatic duct, mucin secretion, secretion of mucin through a patulous papilla and the presence of a mucin-producing papillary intraductal tumour (IPMT). IPMTs account for 0.5% of pancreatic tumours found at autopsy, 7.5% of clinically diagnosed tumours and 16.3% of tumours in resected cases 23. IPMTs were subclassified in 1992 by Furokawa et al.26 into four types according to their gross features: type 1, diffusely dilated main duct; type 2, focally dilated main duct; type 3, cystic sub-branches; type 4, dilated sub-branches. Type 1 seems to be the most common and all the present cases belong to this group.
Weight loss was seen in all our patients. This feature together with other symptoms of chronic pancreatitis, such as pain, steatorrhoea, and diabetes 27, should lead to a suspicion of IPMT 17,28. Recurrent or chronic pancreatitis without a known aetiological cause might lead the surgeon to suspect an intraductal obstruction 5, and the appropriate investigations should then be performed.
All seven patients in the present study were men, and the lesions involved the pancreatic head in every case. These characteristics separate IPMT from mucinous cystic neoplasms (MCN) 29, with which they otherwise have much in common. Both tumours originate from the duct epithelium and carry a relatively favourable prognosis, while MCN mainly affect middle-aged women, are mainly located in the body and tail of the pancreas, and do not usually communicate with the duct system.
US and CT will often suggest the diagnosis of IPMT 30,31, but in our experience they are very imprecise. All our patients were diagnosed by ERCP. The endoscopic findings of mucin leaking from the papilla, dilated pancreatic duct and filling defects due to mucin or papillomatous tumour all point to IPMT 32,33,34. ERCP with brush cytology and/or biopsy is the gold standard for evaluating patients suspected of IPMT.
The six operated patients received a total pancreatectomy due to the diffuse nature of the disease. Multifocal changes were subsequently found in the duct system in all but one patient (Figure 3). Indeed the reconstruction study of Furokawa and co-workers 26 showed that in the diffuse type of IPMT the tumour involved a wide ductal area, while Loftus and colleagues 19 demonstrated dysplastic epithelial changes throughout the entire duct system in the same subtype, which led them to advocate total pancreatectomy. In the other three types of IPMT, in which there are localised changes, a limited resection could be considered, although ductal changes can still extend into apparently normal segments either continuously or discontinuously 26. Thus at least a free resection margin is required plus careful follow-up.
In reviewing the treatment of 194 patients with IPMT in 17 centres worldwide, we found that about half of them were treated with Whipple's procedure and only 10% (including our own six patients) had a total pancreatectomy. There is no doubt that IPMT is a precancerous condition; at least 10% of cases are invasive at operation. The problem is that the course of this comparatively rare disease is uncertain. The risk of leaving IPMT in remaining pancreatic tissue is not well described, but Barbe et al.28 reviewed three patients retrospectively and six inoperable patients prospectively with serial ERCPs over a period of 6–50 months. Some progression of duct dilatation and mucin amount were observed in four patients, but no patient died of the disease during this time. Brat et al.35 presented two cases (one Whipple procedure and one distal resection) in which papillary hyperplasia in one and atypical hyperplasia at the resection margin in the other was followed by adenocarcinoma in the remnant pancreas after 9 and 10 years respectively.
It is important to note that these patients are not asymptomatic; most of them suffer variably from symptoms of chronic pancreatitis. Concerns about the radical nature of total pancreatectomy are based on the presumed difficulties in treating the resulting exocrine and endocrine insufficiency, but in the present series control was satisfactorily achieved.
In conclusion, IPMT represents a subgroup of pancreatic neoplasms with a very slow progression to manifest cancer. The diagnosis may be suspected in patients with non-alchoholic chronic pancreatitis. US, CT and MR imaging may lead to suspicion of IPMT, and ERCP with brush cytology very often provides the diagnosis. Histological heterogeneity makes exclusion of a malignant focus very difficult. The prognosis is very favourable. At operation the entire dysplastic epithelium should be removed. In our experience in which the final pathology demonstrated wide-spread and discontinuous lesions, total pancreatectomy was required and was tolerated in all six patients.
References
- 1.Carter DC. Churchill Livingstone; Edinburgh: 1987. Etiology and epidemiology of pancreatic and periampullary cancer, Trede M, Carter DC. “Surgery of the Pancreas; pp. 427–42. [Google Scholar]
- 2.Crinnion JN, Williamson RCN. WB Saunders; Philadelphia: 1997. Pancreatic neoplasia, Hepatobiliary and Pancreatic Surgery; pp. 321–51. [Google Scholar]
- 3.Hedberg M, Borgström A, Genell S, Janzon L. Survival fol lowing pancreatic carcinoma: a follow-up study of all cases recorded in Malmö, Sweden 1977–1991. Br J Surg. 1998;85:1641–4. doi: 10.1046/j.1365-2168.1998.00920.x. [DOI] [PubMed] [Google Scholar]
- 4.Sperti C, Pasquali C, Piccoli A, Pedrazolli S. Survival after resection for ductal adenocarcinoma of the pancreas. Br J Surg. 1996;83:625–31. doi: 10.1002/bjs.1800830512. [DOI] [PubMed] [Google Scholar]
- 5.Madura JA, Wiebke EA, Howard TJ, et al. Mucin-hypersecreting intraductal neoplasms of the pancreas: a precursor to cystic pancreatic maligscies. Surgery. 1997;122:786–93. doi: 10.1016/s0039-6060(97)90088-x. [DOI] [PubMed] [Google Scholar]
- 6.Grieshop NA, Wiebke EA, Kratzer SS, Madura JA. Cystic neoplasms of the pancreas. Am Surg. 1994;60:509–14. [PubMed] [Google Scholar]
- 7.Milchgrub S, Campuzano M, Casillas J, Albores-Saavedra J. Intraductal carcinoma of the pancreas. Cancer. 1992;69:651–6. doi: 10.1002/1097-0142(19920201)69:3<651::aid-cncr2820690309>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Matsui Y, Aoki Y, Ishikawa O, et al. Ductal carcinoma of the pancreas. Arch Surg. 1979;114:722–6. doi: 10.1001/archsurg.1979.01370300076013. [DOI] [PubMed] [Google Scholar]
- 9.Sugiyama M, Atomi Y, Kuroda A. Two types of mucin-producing cystic tumors of the pancreas: diagnosis and treatment. Surgery. 1997;122:617–25. doi: 10.1016/s0039-6060(97)90136-7. [DOI] [PubMed] [Google Scholar]
- 10.Rivera JA, Castillo CF, Pins M, et al. Pancreatic mucinous ductal ectasia and intraductal papillary neoplasms. Ann Surg. 1997;225:637–46. doi: 10.1097/00000658-199706000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Partensky C, Berger F, Ponchon T, Valette P. Pancréatectomie pour tumeur intracanalaire papillaire mucineuse du pancréas. Gastroenterol Clin Biol. 1996;20:938–45. [PubMed] [Google Scholar]
- 12.Azar C, Stadt JV, Rickaert F, et al. Intraductal papillary mucinous tumors of the pancreas. Clinical and therapeutic issues in 32 patients. Gut. 1996;39:457–64. doi: 10.1136/gut.39.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shyr Y, Su C, Tsay S, Lui W. Mucin-producing neoplasms of the pancreas. Ann Surg. 1996;223:141–6. doi: 10.1097/00000658-199602000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warshaw AL, Compton CC, Lewandrowski K, Cardenosa G, Mueller PR. Cystic tumors of the pancreas. Ann Surg. 1990;212:432–45. doi: 10.1097/00000658-199010000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada M, Kozuka S, Yamao K, Nakazawa S, Naitoh Y, Tsukamoto Y. Mucin-producing tumor of the pancreas. Cancer. 1991;68:159–68. doi: 10.1002/1097-0142(19910701)68:1<159::aid-cncr2820680129>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Castillo CF, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg. 1995;130:295–9. doi: 10.1001/archsurg.1995.01430030065013. [DOI] [PubMed] [Google Scholar]
- 17.Rickaert F, Cremer M, Devierre J, et al. Intraductal mucin-hypersecreting neoplasms of the pancreas. Gastroenterology. 1991;101:512–19. doi: 10.1016/0016-5085(91)90032-g. [DOI] [PubMed] [Google Scholar]
- 18.Obara T, Maguchi H, Saitoh Y, et al. Mucin-producing tumor of the pancreas: a unique clinical entity. Am J Gastroenterol. 1991;86:1619–25. [PubMed] [Google Scholar]
- 19.Loftus EV, Olivaraz-Packad BA, Batts KP, et al. Intraductal papillary-mucinous tumors of the pancreas: clinipathologic features, outcome and nomenclature. Gastroenterology. 1996;110:1909–18. doi: 10.1053/gast.1996.v110.pm8964418. [DOI] [PubMed] [Google Scholar]
- 20.Navarro F, Michel J, Bauret P, et al. Management of intraductal papillary mucinous tumors of the pancreas. Eur J Surg. 1999;165:43–8. doi: 10.1080/110241599750007496. [DOI] [PubMed] [Google Scholar]
- 21.Morohoshi T, Kanda M, Asanuma K, Klöppel G. Intraductal papillary neoplasms of the pancreas. Cancer. 1989;64:1329–35. doi: 10.1002/1097-0142(19890915)64:6<1329::aid-cncr2820640627>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 22.Adsay NV, Adair CF, Heffes CS, Klimstra DS. Intraductal oncocytic papillary neoplasms of the pancreas. Am J Surg Pathol. 1996;20:980–94. doi: 10.1097/00000478-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Pour PM, Konishi Y, Klöppel G, Longnecker DS. Springer Verlag; Berlin: 1994. Histological classification of exocrine pancreatic tumors, Atlas of Exocrine Pancreatic Tumors; pp. 43–66. [Google Scholar]
- 24.Klöppel G, Solcia E, Longnecker DS, Capella C, Sobin LH. Springer Verlag; Berlin: 1994. Histological Typing of Tumors of the Exocrine Pancreas2nd edn; pp. 11–21. [Google Scholar]
- 25.Ohhashi K, Murakami Y, Takekoshi T. Four cases of “mucin-producing” cancer of the pancreas on specific findings of the papilla Vater. Prog Dig Endosc. 1982;20:348–51. [Google Scholar]
- 26.Furokawa T, Takahashi T, Kobari M, Matsuno S. The mucus-hypersecreting tumor of the pancreas. Cancer. 1992;70:1505–13. doi: 10.1002/1097-0142(19920915)70:6<1505::aid-cncr2820700611>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi K, Ogawa Y, Chijiiwa K, Tanaka M. Mucin-hypersecreting tumors of the pancreas: assessing the grade preoperatively. Am J Surg. 1996;171:427–31. doi: 10.1016/S0002-9610(97)89624-9. [DOI] [PubMed] [Google Scholar]
- 28.Barbe L, Ponsot P, Vilgrain V, et al. Intraductal papillary mucinous tumors of the pancreas. Clinical and morphological aspects in 30 patients. Gastroenterol Clin Biol. 1997;21:278–86. [PubMed] [Google Scholar]
- 29.Lichtenstein DR, Carr-Locke DL. Mucin-secreting tumors of the pancreas. Gastrointest Endosc Clin North Am. 1995;5:237–58. [PubMed] [Google Scholar]
- 30.Kobayashi H, Itoh T, Itoh H, Konishi J. Duct ectasia due to mucin-producing cancers with intraductal extension: histopathologic correlation with radiologic imagings. Abdom Imaging. 1995;20:341–47. doi: 10.1007/BF00203368. [DOI] [PubMed] [Google Scholar]
- 31.Kyokane T, Furukawa H, Takayasu K, et al. CT diagnosis of intraductal papillary neoplasm of the pancreas in comparison with histopathologic findings. Int J Pancreatol. 1996;20:163–7. doi: 10.1007/BF02803764. [DOI] [PubMed] [Google Scholar]
- 32.Tenner S, Carr-Locke DL, Banks PA, et al. Intraductal mucin-hypersecreting neoplasm “mucinous ductal ectasia”: endo- scopic recognition and management. Am J Gastroenterol. 1996;91:2548–54. [PubMed] [Google Scholar]
- 33.Nickl NJ, Lawson JM, Cotton PB. Mucinous pancreatic tumors: ERCP findings. Gastrointest Endosc. 1991;37:133–8. doi: 10.1016/s0016-5107(91)70670-6. [DOI] [PubMed] [Google Scholar]
- 34.Procacci C, Graziani R, Bicego E, et al. Intraductal mucin-producing tumors of the pancreas: imaging findings. Radiology. 1996;198:249–57. doi: 10.1148/radiology.198.1.8539388. [DOI] [PubMed] [Google Scholar]
- 35.Brat DJ, Lillemoe KD, Yeo CJ, Warfield PB, Hruban RH. Progression of pancreatic intraductal neoplasias to infiltrating adenocarcinoma of the pancreas. Am J Surg Pathol. 1998;22:163–9. doi: 10.1097/00000478-199802000-00003. [DOI] [PubMed] [Google Scholar]