Abstract
Background
Despite improved diagnostic tools, it is often difficult to make a correct diagnosis of small hepatocellular carcinoma (HCC) in patients with obstructive jaundice.
Case outlines
Three cases of small HCC (<2 cm diameter) presenting as obstructive jaundice are reported. All tumours were initially diagnosed as hilar cholangiocarcinoma based on ultrasono-graphy, computed tomography, cholangiography and angiogra-phy. Because of insufficient hepatic function, none of the patients underwent hepatic resection. One patient died 8 months after first admission to our hospital, another died of disseminated intravascular coagulation I month after admission, and the third was treated with hepatic arterial infusion chemotherapy and survived >36 months.
Conclusion
It is important to consider HCC in the diagnosis of obstructive jaundice in patients who are predisposed to HCC because of liver cirrhosis and/or chronic viral hepatitis, and have elevated serum alpha-fetoprotein.
Keywords: obstructive jaundice, hepatocellular carcinoma
Introduction
Obstructive jaundice is uncommon as the initial presenting clinical feature in a patient with hepatocellular carcinoma (HCC). The bile duct can be physically obstructed by HCC in several ways: intraluminal tumour casts or tumour fragments, intraluminal blood clots from haemobilia, extensive tumour encasement of the intra-hepatic biliary system, or extrinsic compression of the hepatic and common bile ducts by the tumour or malignant lymph nodes 1. Lin and colleagues clinically classified such cases as icteric-type hepatomas 2. The identifying characteristics of this carcinoma still remain obscure, and these cases are often misdiagnosed as biliary carcinoma or choledocholithiasis. It is important to recognise patients with obstructive jaundice secondary to HCC because with appropriate treatment, good palliation with prolonged of survival and occasional curative resection are possible 3,4.
Tumour size is said to be large in most reported cases of HCC with obstructive jaundice. We report three cases of small HCC (<2 cm in diameter) that caused obstructive jaundice. HCC was diagnosed by post-mortem examination in two of these patients.
Case reports
Case no. 1
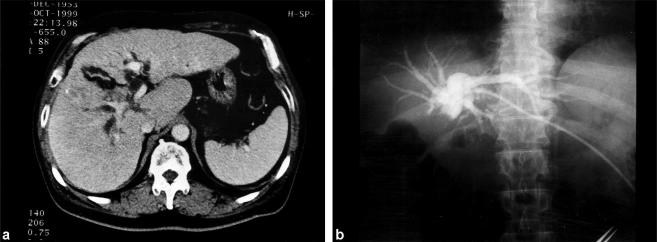
A 46-year-old man presented with right upper abdominal pain for 1 month followed by jaundice. He was positive for hepatitis B surface antigen, and alpha-fetoprotein (AFP) was 16.6 ng/ml. The peak serum bilirubin level was 16.0 SI units. Bile duct cancer at the hepatic hilus was diagnosed on the basis of ultrasonography (US) and abdominal CT (Figure 1a), and he was referred to this hospital, where angiography and percutaneous transhe-patic biliary drainage (PTBD) were performed (Figure 1b). Serum bilirubin fell around 5 SI units thereafter, but it never became within the normal range. Serum levels of transaminase were 100–150 U/L even after PTBD caused by chronic hepatitis. Indocyanine green retention rate at 15 min (ICG R 15) was only 35%. Liver function did not improve sufficiently with biliary decompression. The tumour was assessed as inoperable, so palliative treatment was attempted with a metal self-expandable stent following microwave coagulation therapy (MCT), which was performed through the PTBD route, to treat the obstructed portion of the bile duct. He subsequently experienced three further episodes of obstructive jaundice, and PTBD was performed another three times and MCT several times, with insertion of a metal self-expandable stent. He died of liver failure 8 months after the first admission. Autopsy revealed a 1-cm diameter HCC in segment IV. The right hepatic duct was completely obstructed by invasive tumour tissue. The metal stent was also filled with tumour tissue and necrotic debris. Histological studies revealed that the tumour was a moderately differentiated HCC immuno-negative for AFP.
Figure 1. .
Case no. 1. (a) CT scan shows an irregular surface of the liver, intrahepatic bile duct dilatation and a heterogeneous mass invading the right hepatic duct. (b) Cholangiography through a percutaneous transhepatic biliary drainage (PTBD) catheter reveals complete obstruction at the hilar area, suggesting a diagnosis of bile duct cancer at the hepatic hilus.
Case no. 2
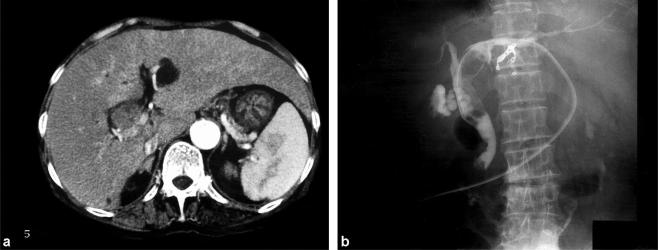
A 72-year-old woman presented with a complaint of general fatigue and was found to have obstructive jaundice on ultrasound (US). Hepatitis C antibody was positive. Serum AFP level was elevated to 4687 ng/ml and protein induced by vitamin K absence (PIVKA-II) was 1.2 AU/ml (normal range <0.1 AU/ml). Serum bilirubin level on admission was 8.6 SI units. Abdominal CT scans revealed dilatation of the intrahepatic bile duct (Figure 2a), which was thought to be due to cholangitis. PTBD was performed to relieve jaundice. Bile duct cancer at the hepatic hilus was diagnosed on the basis of the cholangiogram (Figure 2b). The patient developed haemobilia 1 month after PTBD and died of disseminated intravascular coagulation. Autopsy revealed an HCC that originated in the porta hepatis and expanded from the left and right hepatic ducts to the common bile duct along the lumen of the bile ducts. Histological studies revealed a poorly differentiated HCC.
Figure 2. .
Case no. 2. (a) CT scan shows dilatation of the left hepatic duct and an isodensity mass filling the bile duct. (b) Cholangiography through the PTBD catheter reveals an intraluminal filling defect in the left hepatic duct and common bile duct, which was diagnosed as bile duct cancer at the hepatic hilus.
Case no. 3
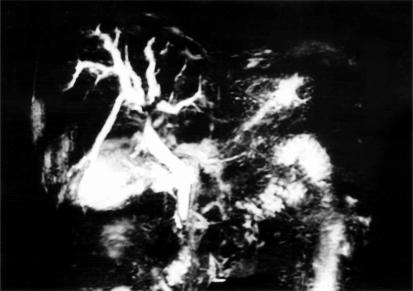
A 60-year-old man who was being followed up for diabetes mellitus and chronic hepatitis C began to suffer from general fatigue and jaundice. He was referred to this hospital with a diagnosis of bile duct cancer at the hepatic hilus based on US, CT, magnetic resonance cholangiography (MRCP) (Figure 3) and angiography. The tumour marker PIVKA-II level was elevated to 47 800 AU/ml, but AFP was within normal limits. Serum bilirubin on admission was 4–6 SI units. At laparotomy, because of severe liver cirrhosis, only a biopsy of the tumour was performed. The tumour was 2 cm in diameter and was situated in segment IV of the liver; the specimen revealed HCC. The patient underwent continuous hepatic arterial infusion chemotherapy with a daily dose of 500 mg 5-fluorouracil for 1 week accompanied by 8 mg of mitomycin C on the first day. This chemotherapy regimen was repeated on an outpatient basis once every 2 weeks for 12 months. The tumour reduced in size and the jaundice improved. He remained well 36 months after the diagnosis of HCC with no evidence of tumour enlargement.
Figure 3. .
Case no. 3. Magnetic resonance cholangiography (MRCP) showing a bile duct stricture at the hepatic hilus.
Discussion
Jaundice usually presents at a later stage of HCC and results from extensive hepatic parenchymal destruction by the tumour. The prognosis of patients in such circumstances is dismal. However, not all HCC patients who present with jaundice are terminally ill. It is important to differentiate between those with obstructive jaundice secondary to HCC and those with parenchymal liver destruction because of cirrhosis and tumour expansion.
A correct differential diagnosis between HCC, intra-hepatic bile duct cancer and choledocholithiasis is important in making treatment plans. The diagnosis of hepatic tumour with common bile duct invasion or compression is not difficult in patients with a tumour large enough to be detected by abdominal CT or US, but it is difficult to distinguish HCC from intrahepatic bile duct cancer 5. Percutaneous tumour biopsy of the liver has a danger of tumour cell implantation to the abdominal wall, so we generally avoid it. Biopsy of the tumour was only performed in case no. 3, because this patient underwent laparotomy. Sometimes tumour markers such as AFP can be useful in the differential diagnosis of these diseases; however, AFP levels have been reported to be elevated in some patients with intrahepatic bile duct cancer 6, while they are not always elevated in patients with HCC. Misdiagnosis of cholangiocarcinoma is often made if there is a small HCC tumour causing obstructive jaundice that is undetected by abdominal CT or US. In all three of our patients the initial diagnosis of bile duct cancer was based on abdominal CT, cholangiographic and angiographic findings, although in one patient serum AFP level was greatly elevated. The typical appearance of HCC has been reported on CT 7, magnetic resonance imaging (MRI) 8,9, cholangiography 1,10 and cholangioscopy 11, yet it can still be difficult to make a correct diagnosis. The treatment by MCT and a metal self-expandable stent was acceptable for inoperable bile duct cancer. When a patient has repeated episodes of obstructive jaundice in a short time, even after stenting the obstructed bile duct as in case no. 1, it is necessary to consider diseases other than bile duct cancer and other treatments including a local resection of the tumour. It is important to suspect HCC in any patient with risk factors such as liver cirrhosis, chronic viral hepatitis or elevated serum AFP.
‘Curative’ resection HCC improves the prognosis 3,4,10,12,13,14,15, but the patient's liver function needs careful evaluation, especially in cases of cirrhosis. Tada and colleagues recommended preoperative biliary drainage followed by transcatheter arterial embolisation for successful surgical treatment of icteric HCC 15. Satisfactory palliation is possible with appropriate treatment for patients with unresectable tumours 3,4,16. Thus in case no. 3, hepatic arterial infusion chemotherapy was effective. This patient has remained well 36 months after diagnosis of HCC with no evidence of extension of the tumour. Unnecessarily radical hepatic resection can be avoided if the proper diagnosis is made preoperatively or intra-operatively.
In conclusion, obstructive jaundice secondary to HCC is very rare, and it is difficult to make a correct diagnosis without histological examination. Nevertheless, HCC must be kept in mind as one of several differential diagnoses of obstructive jaundice, even if the tumour is very small as in the present small series, because long survival or good palliation can be expected with appropriate treatment.
References
- 1.Lau WY, Leow CK, Leung KL, Leung TW, Chan M, Yu SC. Cholangiographic features in the diagnosis and management of obstructive icteric type hepatocellular carcinoma. HPB Surg. 2000;11:299–306. doi: 10.1155/2000/79241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin TY, Chen KM, Chen YR, Lin WS, Wang TH, Sung JL. Icteric type hepatoma. Med Chir Dig. 1975;4:267–70. [PubMed] [Google Scholar]
- 3.Lau WY, Leung JW, Li AK. Management of hepatocellular carcinoma presenting as obstructive jaundice. Am J Surg. 1990;160:280–2. doi: 10.1016/s0002-9610(06)80023-1. [DOI] [PubMed] [Google Scholar]
- 4.Lua W, Leung K, Leung TWT, et al. A logical approach to hepatocellular carcinoma presenting with jaundice. Arm Surg. 1997;225:281–5. doi: 10.1097/00000658-199703000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang GT, Sheu JC, Lee HS, Lai MY, Wang TH, Chen DS. Icteric type hepatocellular carcinoma: revisited 20 years later. J Gastroenterol. 1998;33:53–6. doi: 10.1007/pl00009966. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi T, Mimura H, Kim H, Takakura N, Hamazaki K, Tsuge H. Diagnosis and extension pattern of cholan-giocellular carcinoma. Nippon Shoukakigeka Gakkai Zasshi (Jpn J Gastroenterol Surg) 1987;20:2572–8[in Japanese]. [Google Scholar]
- 7.Soyer P, Sibert A, Laissy JP. Intrahepatic bile duct dilatation secondary to hepatocellular carcinoma: CT features in 10 patients. Abdom Imaging. 1995;20:114–17. doi: 10.1007/BF00201516. [DOI] [PubMed] [Google Scholar]
- 8.Soyer P, Laissy JP, Bluemke DA, Sibert A, Menu Y. Bile duct involvement in hepatocellular carcinoma: MR demonstration. Abdom Imaging. 1995;20:118–21. doi: 10.1007/BF00201517. [DOI] [PubMed] [Google Scholar]
- 9.Tseng JH, Hung CF, Ng KK, Wan YL, Yeh TS, Chiu CT. Icteric-type hepatoma: magnetic resonance imaging and magnetic resonance cholangiographic features. Abdom Imaging. 2001;26:171–7. doi: 10.1007/s002610000136. [DOI] [PubMed] [Google Scholar]
- 10.Wu CS, Wu SS, Chen PC, et al. Cholangiography of icteric type hepatoma. Am J Gastroenterol. 1994;89:774–7. [PubMed] [Google Scholar]
- 11.Jan YY, Chen MF. Obstructive jaundice secondary to hepatocellular carcinoma rupture into the common bile duct: choledochoscopic findings. Hepatogastroenterology. 1999;46:157–61. [PubMed] [Google Scholar]
- 12.Jan YY, Chen MF, Chen TJ. Long term survival after obstruction of the common bile duct by ductal hepatocel lular carcinoma. Eur J Surg. 1995;161:771–4. [PubMed] [Google Scholar]
- 13.Shiomi M, Kamiya J, Nagino M, et al. Hepatocellular carcinoma with biliary tumor thrombi: aggressive operative approach after appropriate preoperative management. Surgery. 2001;129:692–8. doi: 10.1067/msy.2001.113889. [DOI] [PubMed] [Google Scholar]
- 14.Shimada M, Takenaka K, Hasegawa H, et al. Hepatic resection for icteric type hepatocellular carcinoma. Hepato-gastroenterology. 1997;44:1432–7. [PubMed] [Google Scholar]
- 15.Tada K, Kubota K, Sano K, et al. Surgery of icteric-type hepatoma after biliary drainage and transcatheter arterial embolization. Hepatogastroenterology. 1999;46:843–8. [PubMed] [Google Scholar]
- 16.Lauffer JM, Mai G, Berchtold D, Curd CG, Triller J, Baer HU. Multidisciplinary approach to palliation of obstructive jaundice caused by a central hepatocellular carcinoma. Dig Surg. 1999;16:531–6. doi: 10.1159/000018784. [DOI] [PubMed] [Google Scholar]