Abstract
Background
Throughout the history of liver transplantation many improvements have been made in the field of surgical technique. It is beyond the scope of this paper to review all aspects of surgical technique in liver transplantation; thus, in this review we focus on the description of our current technique in most cases, which is orthotopic liver transplantation with preservation of the inferior vena cava and temporary portocaval shunt. We advocate this technique because it has been demonstrated that it achieves better haemodynamic stability during the anhepatic phase, transfusion can be reduced and renal function is improved. The different options for vascular anastomoses are described, particularly the options for arterial anastomoses in case of finding a non-adequate recipient hepatic artery. Technical possibilities for patients with preoperative portal vein thrombosis and the procedure in case of domino or sequential liver transplantation are further explained.
Keywords: liver transplantation, surgical techniques
Introduction
Since the pioneering times of liver transplantation (LT) in the early 1960s, innumerable improvements have been made, not only in inmunosuppression 1 but also in surgical technique.
The technique of orthotopic liver transplantation (OLT) as first developed at the University of Colorado in Denver 2, and afterwards at the University of Pittsburgh 3,4 and at Cambridge 5,6, has been improved by some technical procedures mainly focused on achieving haemodynamic stability, adequate vascular and biliary anastomoses, and perfect haemostasis.
It is beyond the scope of this paper to review all aspects of LT; thus, we will try to review the main techniques used in OLT. In particular, we will describe our most frequently used technique, which is OLT with preservation of the inferior vena cava and temporary portocaval shunt; other technical options will also be named.
A brief review of some special situations (portal vein thrombosis and domino OLT and their technical possibilities is also provided.
Abdominal incision and exposure
Adequate exposure is crucial to allow the appropriate dissection needed for native liver hepatectomy. The most commonly used incisions are the bilateral subcostal incision with midline extension, named by Sir Roy Calne as the ‘Mercedes’ incision, and the inverted J incision, named the Makuuchi incision. With any of these incisions exposure is excellent. Currently, we advocate the use of Makuuchi's incision, except in cases of previous surgery or huge splenomegaly that can necessitate the part of the left-sided incision.
Native liver removal
The native liver removal begins with dissection of the hepatic hilum. The dissection is carried down to the hepatic artery, which is divided above its bifurcation. The most frequent site chosen for the subsequent anastomoses is the region of the common hepatic artery and gastroduodenal artery. Then the cystic duct is cannulated and division of the common duct above the cystic duct is carried out. It is important to take care to preserve the longitudinal vessels supplying the common bile duct.
The next step is the portal vein transection. Management of the portal vein will depend on the chosen OLT technique (veno-venous bypass or preservation of the inferior vena cava).
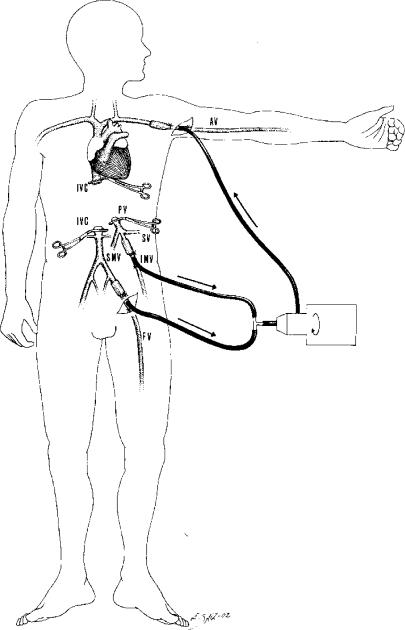
It had already been demonstrated by the initial experimental OLT in dogs that some type of bypass would be needed to tolerate the anhepatic phase 7. Even though cirrhotic patients could better tolerate portal clamping, it was shown by Pittsburgh studies in the early 1980s that OLT with veno-venous bypass, without anticoagulation 8, was performed with better haemodynamic stability and lower blood needs (Figure 1). Nevertheless, some complications have been described when bypass is used 9, and some haemodynamic alterations cannot be avoided 10. For this reason, in 1989 Tzakis et al. described the technique of OLT with preservation of the inferior vena cava, called the ‘piggy-back’ technique 11. It has been shown that with the use of this technique OLT can be performed with better haemodynamic stability, lower blood transfusion requirements and shorter operative time 12,13,14.
Figure 1. .
Portofemoroaxillar venous bypass. AV, axillary vein; IVC, inferior vena cava; PV, portal vein; SV, splenic vein; IMV, inferior mesenteric vein; SMV, superior mesenteric vein; FV, femoral vein.
Although the ‘piggy-back’ technique improves the haemodynamic stability during the anhepatic phase, the portal vein clamping induces portal hypertension and splanchnic congestion, which can induce renal dysfunction and make dissection more difficult. In 1993, Tzakis et al.15, and afterwards Belghiti et al. in 1995 16,17, described the use of temporary portocaval shunt in OLT. It has already been demonstrated that the use of temporary portocaval shunt associated with the preservation of the inferior vena cava can be performed safely in most patients, without the need for veno-venous bypass, and with haemodynamic and renal improvements (Table 1) 18,19. We could indeed confirm this fact with a prospective randomised study that demonstrated that the association of a temporary portocaval shunt with the preservation of the inferior vena cava technique achieved better haemodynamic stability during the anhepatic phase, and this was associated with lower blood transfusion requirements and better renal function, mainly in those patients with higher portal blood flow or higher portocaval gradient 20. It should be noted that temporary portocaval shunt is particularly useful in patients with fulminant hepatitis. These patients do not have hepatofugal circulation and tolerate poorly the fluid overload necessary to maintain haemodynamic status during the anhepatic phase 17.
Table 1. Temporary portocaval shunt during OLT with vena cava preservation.
| Parameter | PCS group | No PCS group | p value |
|---|---|---|---|
| All patients | |||
| CO decrease (%) | −9.6±23 | −19± 18 | 0.05 |
| SVR increase (%) | 22±43 | 41 ±49 | 0.07 |
| Urinary output (ml/kg/h) | 3.6 | 2.1 | 0.05 |
| OLT without transfusion (%) | 45 | 22 | 0.05 |
| Portal flow ≥ 1000 ml/min* | |||
| CO decrease (%) | −3.5±17 | −21±19 | 0.02 |
| SVR increase (%) | 27±19 | 27 ±39 | 0.9 |
| Urinary output (ml/kg/h) | 3.6 | 2.1 | 0.08 |
| OLT without transfusion (%) | 61 | 23 | 0.04 |
| P-C gradient ≥ 16 mmHg* | |||
| CO decrease (%) | −12±23 | −23±18 | 0.08 |
| SVR increase (%) | 11.3 ±34 | 50 ±50 | 0.07 |
| Urinary output (ml/kg/h) | 3.6 | 1.9 | 0.01 |
| OLT without transfusion (%) | 45 | II | 0.01 |
| Both characteristics* | |||
| CO decrease (%) | −8.8±28 | −27±17 | 0.1 |
| SVR increase (%) | 17.9±45 | 38 ±38 | 0.3 |
| Urinary output (ml/kg/h) | 5.5 | 2.1 | 0.03 |
| OLT without transfusion (%) | 67 | 15 | 0.04 |
PCS, temporary portocaval shunt. CO, cardiac output; SVR, systemic vascular resistance. CO decrease, SVR increase and urinary output refer to the anhepatic phase.
*At the beginning of OLT.
In view of these results, we advocate that this technique is performed in all cases.
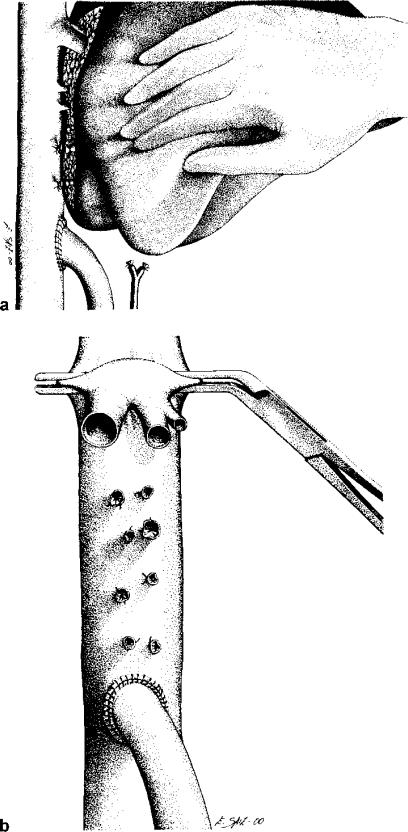
After arterial and hepatic duct section, the portal vein is dissected widely. Afterwards, the anterior face of inferior vena cava is exposed. After clamping and section of the portal vein, the vena cava is laterally clamped, and an end-to-side anastomosis with a running suture with 5/0 polypropylene (Prolene 5/0; Ethicon, Somerville, NJ, USA) is performed (Figure 2a, b). The hepatectomy can then be performed safely, with the preservation of the caval and portal flow. The early hepatic devascularization, and the absence of splanchnic congestion due to the portocaval shunt, facilitate the division of hepatic ligaments, ligation of small hepatic veins and further mobilisation of the liver.
Figure 2. .
(a) After end-to-side portocaval anastomoses, individual hepatic veins are ligated, exposing the major hepatic veins in a dry field without portal hypertension. (b) During the anhepatic phase, the three major hepatic veins are cross-clamped with a vascular clamp. Normal venous return to the heart is maintained because the lumen of the vena cava is only partially occluded and the portocaval anastomosis is still patent. (Reprinted with permission from Liver Transplantation).
Subsequently, the complete dissection of the anterior face of the lower caval vein is done by ligation of the small vessels that join the caudate lobe to the caval vein. To complete the exposure of the right hepatic vein, the hepatocaval or Makuuchi's ligament must be divided. Depending on the anatomic distribution and the size of the hepatic veins, we will decide to ligate and section the right hepatic vein, or if possible join it creating a common hole with the middle and left hepatic veins. In some cases the anatomic distribution of the recipient hepatic veins makes it difficult to join them. In such cases, it is preferable to create an orifice from the middle and left hepatic veins and extended caudally to the lower caval vein, thus performing a ‘face-à-face’ venacavaplasty 21,22.
It is essential to achieve enough long venous cuffs for the subsequent anastomoses. Once all hepatic veins have been exposed, they are clamped, while avoiding clamping the caval vein.
Vascular anastomoses
After the removal of the native liver, the liver graft can be implanted. Before initiating the vascular reconstruction, the surgical field must be completely ready, with perfect haemostasis, and adequate vascular cuffs prepared.
The liver allograft implantation begins with the suture of the donor upper vena cava to the cuff created with the three recipient hepatic veins (or the hepatic venous orifice created with the middle and left hepatic veins with an extension inferiorly onto the inferior caval vein), by a running 3/0 polypropylene suture (Prolene 3/0; Ethicon).
Afterwards, we can perform the portal or arterial anastomoses. If we begin with the portal one, then the portocaval anastomosis has to be taken down. If we begin with the arterial anastomoses, the portocaval shunt can be maintained until the arterial anastomoses are finished. The ‘piggy-back’ technique with temporary portocaval shunt allows one to choose the order of graft revascularization. We prefer to do the arterial anastomosis first and then the portal anastomosis and to perform simultaneous arterial and portal revascularisation of the graft. Although some studies have suggested some advantages with initial arterial revascularisation 23, further studies are needed to confirm these findings.
To take down the portocaval shunt, the portal side is clamped, and a vascular stapling device (TA 30; Autosuture, Elancourt, France) closes the caval defect. Before performing the portal anastomoses, the graft is washed with 1 L of lactate Ringer at 38 °C (although this is our preference, washing with blood or cold perfusion has also been described), with the patient positioned in the Trendelenburg position. The inferior opening of the donor vena cava is left open to allow the drainage of the effluent from the flush of the graft. After washing the graft, the caudal opening of the donor inferior vena cava is closed with a vascular stapling device (TA 30; Autosuture).
Then the portal anastomosis is performed, with a running 5/0 polypropylene suture (Prolene 5/0; Ethicon).
The arterial anastomosis is performed by a running or interrupted 6/0 polypropylene suture (Prolene 6/0; Ethicon) generally at the level of the common hepatic artery bifurcation, or at the cuff created with the gastroduodenal artery take-off. If the graft has some kind of arterial anomaly it should be solved at the bench surgery to create an appropriate vascular cuff. On the other hand, if the recipient hepatic artery is not adequate to perform a safe anastomosis, several alternatives have been evolved to solve this problem. In most cases, the arterialisation of the graft will be achieved using the aorta, through either direct anastomoses or the placement of an arterial conduit between the aorta and the donor hepatic artery 24. Although different grafts can be used, the preferable one is a graft of donor iliac artery. The location of choice for the graft is an end-to-side anastomosis to the supracoeliac aorta. Sometimes it is not possible to anastomose the graft at the supracoeliac aorta; in such cases, the origination of the arterial graft could be the infrarenal aorta 25. The graft then has to be passed through the transverse mesocolon, anterior to the pancreas, to reach the hepatic hilum.
One alternative to the graft, particularly suitable when splenomegaly is present, is to use the recipient's splenic artery for arterial reconstruction 26.
Biliary tract reconstruction
After performing the cholecystectomy, the biliary tract reconstruction is done. Our standard technique is the end-to-end choledocostomy with an interrupted suture using PDS 5/0 (Violet polydioxanone; Ethicon), without a T-tube stent.
In some cases of inadequacy of the biliary duct size, or depending on the underlying disease (primary sclerosing cholangitis), a Roux-en-Y hepatojejunostomy will be needed.
Like most centres, we classically used a choledocostomy stented with a T-tube. Nevertheless, since the report from Rouch et al. in 1990 demonstrating that most of the early biliary complications were T-tube-related 27 we decided to use a choledocostomy without a T-tube, whenever possible. With this approach we are confident with this type of anastomosis, with a rate of biliary tract complications similar to other series, and avoiding the T-tube-related complications 28. Thus, this has been our method of choice since 1991.
Haemostasis and closure
After finishing vascular and biliary anastomoses, perfect haemostasis must be achieved before closure. Efforts towards complete haemostasis will avoid re-explorations and postoperative renal and hepatic function disturbances.
Before the wound closure we usually perform a reperfusion biopsy at the left lateral sector. Then drainage is introduced; we place one to three drains depending on the case. The wound is then closed with a two-layer running suture.
Special situations and technical possibilities
Portal vein thrombosis
The presence of portal vein thrombosis was considered a contraindication to OUT. Nowadays, it is accepted that portal vein thrombosis does not preclude OLT, although it increases surgical complexity. Different operative techniques have been described to solve this problem; the use of any of these techniques will depend on the characteristics of the thrombosis (acute or chronic), the degree of thrombosis (partial or complete), and mainly its extension throughout the splenomesenteric tree 29. Although preoperative knowledge of portal vein disease greatly facilitates operative strategy, the status of the portal vein and the extent of thrombosis will definitively be determined after portal vein dissection.
A thrombectomy can be attempted in some cases (acute or partial thrombosis), but in case of complete chronic thrombosis thrombectomy would need some intimal dissection or eversion endarterectomy that may be related to higher postoperative portal thrombosis, and higher ischaemic times and transfusion requirements 29,30. Thus, in such cases of complete chronic thrombosis, we advocate the use of an extra-anatomic venous graft 31,32. As described, a venous graft from the donor iliac vein has to be placed from the infrapancreatic superior mesenteric vein to the donor portal vein. The graft is placed through a tunnel in the transverse mesocolon, anterior to the pancreas.
Other alternatives described for special cases include the use of some enlarged collateral vein or the coronary vein.
In the rare cases of thrombosis of the whole splenomesenteric tree, the arterialisation of the portal vein 33 or cavoportal hemitransposition 34 are the only technical options available.
Domino OLT
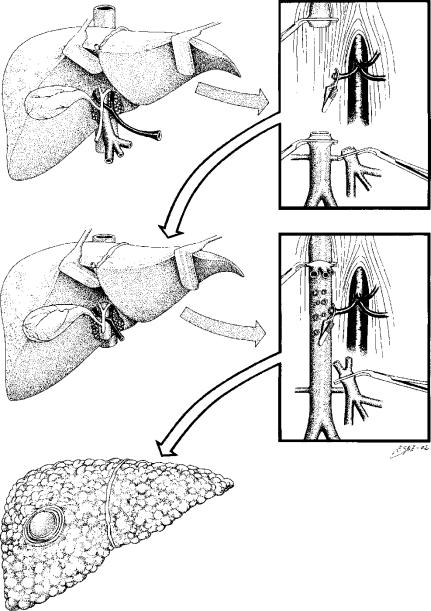
In case of domino OLT, the patient with familial amyloidotic polyneuropathy is at the same time donor and recipient 35,36. Hepatectomy in those patients begins with cholecystectomy and section of the hepatic duct. As the patient is the donor of the liver, resection of enough long retrohepatic caval vein is necessary (Figure 3); to achieve this, the suprarenal and diaphragmatic veins are ligated. Once enough caval vein has been dissected, a tolerance test is done, clamping both the caval and portal veins. In case of haemodynamic changes that would preclude performing the hepatectomy, a venous bypass will be required. In such cases, we use a portofemoroaxillar venous bypass 10 (Figure 1).
Figure 3. .
Domino orthotopic liver transplantation. Technique of hepatectomy of the domino donor. It is necessary to achieve long arterial and portal segments both for the graft and for the patient Resection of enough long retrohepatic caval vein is also necessary. The procedure of OLT in the domino liver recipient is done with the preservation of the inferior vena cava technique.
Next the hepatectomy is performed, without clamping the vascular flow until the last moment. Is necessary to achieve long arterial and portal segments both for the graft and for the patient. The common hepatic artery is clamped and divided just before the take-off of the gastroduodenal artery. The portal vein is clamped and divided just 1 cm below portal bifurcation. Finally, the caval vein is divided above and below the liver.
Once the hepatectomy has been performed, the liver has to be prepared to be the graft of another patient. Perfusion through the portal vein and the hepatic artery has to be done at the bench procedure. The biliary tract is also washed. We think it is not necessary to heparinise. The lower vena cava is closed with a running 3/0 polypropylene suture (Prolene 3/0; Ethicon). Arterial reconstruction is carried out if necessary.
The procedure of revascularisation in the patient with familial amyloidotic polyneuropathy is done as for the classical technique. The usual sequence begins with sewing the upper vena cava and the lower vena cava. The liver is then flushed through the portal vein, and the fluid is allowed to escape through the anterior part of the lower caval anastomoses. Whether the portal or arterial anastomoses are performed next, depends upon the tolerance. It should be noted that the arterial anastomosis has to be done at the common hepatic artery, because the cuff between the common hepatic artery and the gastroduodenal artery will be used in the recipient of the ‘domino’ liver.
References
- 1.Calne RY, Rolles K, White DJ, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreas and 2 livers. Lancet. 1979;2:1033–6. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 2.Starzl TE, Marchioro TL, Von Kaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–76. [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Groth CG, Brettschneider L, et al. Orthotopic homotransplantations of the human liver. Arm Surg. 1968;168:392–415. doi: 10.1097/00000658-196809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Iwatsuki S, Esquivel CO, et al. Refinements in the surgical technique of liver transplantation. Semin Liver Dis. 1985;5:349–56. doi: 10.1055/s-2008-1040632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calne RY, Williams R. Liver transplantation in man – I, Observations on technique and organization in five cases. BMJ. 1968;4:535–40. doi: 10.1136/bmj.4.5630.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calne RY. Surgical aspects of clinical liver transplantation in 14 patients. Br J Surg. 1969;56:729–36. doi: 10.1002/bjs.1800561009. [DOI] [PubMed] [Google Scholar]
- 7.Starzl TE, Kaupp HA, Brock DR, Lazarus RE, Johnson RV. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynecol Obstet. 1960;111:733–43. [PMC free article] [PubMed] [Google Scholar]
- 8.Griffith BP, Shaw BW, Hardesty RL, Iwatsuki S, Bahnson HT, Starzl TE. Veno-venous bypass without systemic anticoagulation for transplantation of the human liver. Surg Gynecol Obstet. 1985;160:271–82. [PMC free article] [PubMed] [Google Scholar]
- 9.Khoury GF, Mann ME, Porot MJ, Abdul-Rasool IH, Busuttil RW. Air embolism associated with venovenous bypass during orthotopic liver transplantation. Anesthesiology. 1987;67:848–51. doi: 10.1097/00000542-198711000-00048. [DOI] [PubMed] [Google Scholar]
- 10.Shaw BW, Martin DJ, Marquez JM, et al. Venous bypass in clinical liver transplantation. Ann Surg. 1984;200:524–34. doi: 10.1097/00000658-198410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzakis A, Todo S, Starzl TE. Orthotopic liver transplantation with preservation of the inferior vena Cava. Ann Surg. 1989;210:649–52. doi: 10.1097/00000658-198911000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salizzoni M, Andorno E, Bossuto E, et al. Piggyback techniques versus classical technique in orthotopic liver transplantation: a review of 75 cases. Transplant Proc. 1994;26:3552–3. [PubMed] [Google Scholar]
- 13.Figueras J, Sabaté A, Fabregat J, et al. Hemodynamics during the anhepatic phase in orthotopic liver transplantation with vena cava preservation: a comparative study. Transplant Proc. 1993;25:2588–9. [PubMed] [Google Scholar]
- 14.Jovine E, Mazziotti A, Grazzi GL, et al. Piggy-back versus conventional technique in liver transplantation: report of a randomized trial. Transpl Int. 1997;10:109–12. doi: 10.1007/pl00003824. [DOI] [PubMed] [Google Scholar]
- 15.Tzakis AG, Reyes J, Nour B, Marino IR, Todo S, Starzl TE. Temporary end to side portocaval shunt in orthotopic hepatic transplantation in humans. Surg Gynecol Obstet. 1993;176:181–3. [PMC free article] [PubMed] [Google Scholar]
- 16.Belghiti J, Noun R, Sauvanet A. Temporary portocaval anastomoses with preservation of caval flow during orthotopic liver transplantation. Am J Surg. 1995;169:277–9. doi: 10.1016/S0002-9610(99)80151-2. [DOI] [PubMed] [Google Scholar]
- 17.Belghiti J, Noun R, Sauvanet A, et al. Transplantation for fulminant and subfulminant hepatic failure with preservation of portal and caval flow. Br J Surg. 1995;82:986–9. doi: 10.1002/bjs.1800820741. [DOI] [PubMed] [Google Scholar]
- 18.Cherqui D, Lauzet J, Rotman N, et al. Orthotopic liver transplantation with preservation of the caval and portal flow. Transplantation. 1994;58:793–6. [PubMed] [Google Scholar]
- 19.Steib A, Saada A, Clever B, et al. Orthotopic liver transplantation with preservation of portocaval flow compared with venovenous bypass. Liver Transpl Surg. 1997;35:518–25. doi: 10.1002/lt.500030507. [DOI] [PubMed] [Google Scholar]
- 20.Figueras J, Lladó L, Ramos E, et al. Temporary portocaval shunt during liver transplantation with vena cava preservation. Results of a prospective randomized study. Liver Transpl. 2001;7:904–11. doi: 10.1053/jlts.2001.27870. [DOI] [PubMed] [Google Scholar]
- 21.Belghiti J, Panis Y, Sauvanet A, Gayet B, Fékété F. A new technique of side to side caval anastomoses during orthotopic hepatic transplantation without caval occlusion. Surg Gynecol Obstet. 1992;75:270–2. [PubMed] [Google Scholar]
- 22.Bismuth H, Castaing D, Shellock DJ. Liver transplantation by "face-à-face" venacavaplasty. Surgery. 1992;111:1515. [PubMed] [Google Scholar]
- 23.Ducerf C, Mechet I, Landry JL, et al. Hemodynamic profiles during piggyback liver grafts using arterial or portal revascularization. J Am Coll Surg. 2000;190:89–93. doi: 10.1016/s1072-7515(99)00227-6. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein RM, Secrest CL, Klintmalm GB, Husberg BS. Problematic vascular reconstruction in liver transplantation. Part I. Arterial. Surgery. 1990;107:544–8. [PubMed] [Google Scholar]
- 25.Shaked AA, Takiff H, Bussutil RW. The use of the supraceliac aorta for hepatic arterial revascularization in transplantation of the liver. Surg Gynecol Obstet. 1991;173:198–202. [PubMed] [Google Scholar]
- 26.Figueras J, Parés D, Aranda H, et al. Results of using the recipient's splenic artery for arterial reconstruction in liver transplantation in 23 patients. Transplantation. 1997;64:655–8. doi: 10.1097/00007890-199708270-00020. [DOI] [PubMed] [Google Scholar]
- 27.Rouch DA, Emond JC, Thistlethwaite JR, Mayes JI, Broelsch CE. Choledochocholedocostomy without a T tube or internal stent in transplantation of the liver. Surg Gynecol Obstet. 1990;170:239–4. [PubMed] [Google Scholar]
- 28.Torras J, Lladó L, Figueras J, et al. Biliary tract complications after liver transplantation: type, management, and outcome. Transplant Proc. 1999;31:2406. doi: 10.1016/s0041-1345(99)00404-2. [DOI] [PubMed] [Google Scholar]
- 29.Steiber AC, Zetti G, Todo S, et al. The spectrum of portal vein thrombosis in liver transplantation. Ann Surg. 1991;213:199–206. doi: 10.1097/00000658-199103000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cherqui D, Duvoux C, Rahmouni A, et al. Orthotopic liver transplantation in the presence of partial or total portal vein thrombosis: problems in diagnosis and management. World J Surg. 1993;17:669–74. doi: 10.1007/BF01659140. [DOI] [PubMed] [Google Scholar]
- 31.Sheil R, Thompson JF, Stephen MS. Mesoportal graft for thrombosed portal vein in liver transplantation. Clin Transplant. 1987;1:18–20. [Google Scholar]
- 32.Figueras J, Torras J, Rafecas A. Extra-anatomic venous graft for portal vein thrombosis in liver transplantation. Transpl Int. 1997;10:407–8. doi: 10.1111/j.1432-2277.1997.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 33.Ehrard J, Lange R, Giebler R, Rauen U, De Groot H, Eigler FW. Arterialization of the portal vein in orthotopic and auxiliary liver transplantation. Transplantation. 1995;60:877. [PubMed] [Google Scholar]
- 34.Tzakis A, Kirkegaard P, Pinna AD, et al. Liver transplantation with cavoportal hemitransposition in the presence of diffuse portal vein thrombosis. Transplantation. 1998;65:619–24. doi: 10.1097/00007890-199803150-00004. [DOI] [PubMed] [Google Scholar]
- 35.Azoulay S, Samuel D, Castaing D, et al. Domino liver transplants for metabolic disorders: experience with familial amyloidotic polyneuropathy. J Am Coll Surg. 1999;189:584–93. doi: 10.1016/s1072-7515(99)00208-2. [DOI] [PubMed] [Google Scholar]
- 36.Figueras J, Parés D, Munar-Ques M, et al. Experience with domino or sequential liver transplantation in patients with familial amyloidotic polyneuropathy. Transplant Proc. 2002;34:307–8. doi: 10.1016/s0041-1345(01)02776-2. [DOI] [PubMed] [Google Scholar]