Abstract
Background
The necessity of widening the indications for living donor liver transplantation (LDLT) has been emphasised. Clarification of the advantages and limitations of using a left liver graft for LDLT in adults is essential for donor safety.
Methods
Between June 1990 and November 2002, 185 patients underwent LDLT at Shinshu University Hospital, Japan. In 97 of these, the graft comprised the left liver with or without the left portion of the caudate lobe. The peri-hepatectomy profiles of the donors, significance of left liver grafts, postoperative courses of the donors and recipients, and survival of the recipients were investigated.
Results
All the donors recovered well and returned to a normal lifestyle. None required banked-blood transfusion or repeat surgery, and postoperative liver function tests had satisfactory results. The cold ischaemic time for the graft was 127±54 minutes. The graft volumes (GVs) ranged from 230 to 625 ml, and GV/standard liver volume (SV) ratios varied from 22% to 65%, at the time of transplantation. Although 85% of the liver grafts had GV/SV ratios <50%, no patient developed immediate postoperative liver failure. Patient survival rates were 89%, 84% and 84% at 1, 3 and 5 years, respectively.
Discussion
Although LDLT using a left liver graft imposes potential postoperative complications (a small liver is more vulnerable to injury, and recipients of small grafts are at higher risk of complications during recovery), such grafts have yielded acceptable results in adult LDLT, with minimal burden to the donors.
Keywords: living donor liver transplantation, left liver lobe, donor safety
Introduction
Living donor liver transplantation (LDLT) is now being carried out worldwide to alleviate shortages of cadaveric organs, and the necessity of widening its indications in adult patients has been emphasised 1,2,3,4. The most common way of meeting the need for LDLT in adults is to use the right liver from a living donor as the graft 1,2,4. However, before adopting this technique as standard, the potential advantages and limitations of using a left liver graft need to be investigated from the viewpoint of maximising donor safety.
In 1993, we reported the first successful LDLT using a left liver graft in an adult patient 5. This was performed against the backdrop of acceptable results of earlier LDLTs in adolescents 6 and investigation of the recipient's standard liver volume (SV) 7. Based on this early success, we have adopted LDLTs using left liver grafts for adult patients and have achieved fair results when the graft volume (GV) exceeds 30% of the SV. We have also devised a left-liver-plus-caudate-lobe graft, which avoids the limitations imposed by a low GV to some extent. The procedures involved in left hepatectomy pose less risk to the safety of the living donor than right liver removal. In this article, various issues regarding LDLT with a left liver graft are explored, including indications and results from our own experience at Shinshu University Hospital, Japan.
Materials and Methods
Between June 1990 and November 2002, 185 patients underwent LDLT at Shinshu University Hospital. LDLTs using the left liver, with or without the left portion of the caudate lobe 8,9, were performed in 97 of these patients (excluding LDLTs involving the left lateral segments or extended left lateral segments). These 97 LDLTs are the subject of this study. The recipients comprised 16 children (<18 years old; mean age 12.7±2.4 years, range 8–16 years) and 81 adults (>18 years old; mean age 43.2±13.9 years, range 18–69 years). The reasons for transplantation were primary biliary cirrhosis (21 patients), familial amyloid polyneuro-pathy (FAP; 19), liver cirrhosis due to viral hepatitis (14), fulminant hepatic failure (13), biliary atresia (9), adult-type citrullinaemia (7), primary sclerosing cholangitis (6), Allagiles syndrome (2), autoimmune hepatitis (2), Carolis disease (1), glycogen storage disease type la (1), late-onset hepatic failure (1) and Wilsons disease (1). The donors were the recipient's children (in 27 cases), mothers (20), fathers (17), siblings (16), husbands (15), cousin (1) or nephew (1). The ages of the donors ranged from 20 to 61 years (mean 40.6±11.7 years).
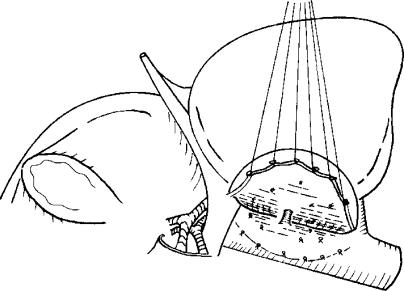
A lower limit of 30% of the recipient's SV was set as the GV necessary for LDLT. This criterion was based on experience gained during general hepatectomy, the observation of an uneventful postoperative course after LDLT using a left liver graft in adolescent recipients at our institution, and the reported acceleration of liver regeneration under immunosuppressant therapy 10. When the estimated GV did not exceed 30% of the recipient's SV if only the left liver was used, but did exceed 30% if the graft included the caudate lobe, we used the left liver plus the caudate lobe as the graft (Figure 1). The surgical procedures for both the donors and the recipients have been described elsewhere 3,8,9,11,12,13. To maximise the likelihood of success, efforts to shorten the graft ischaemic time were made in every case.
Figure 1. .
Left hepatic lobectomy including the left side of the caudate lobe. (Reprinted with permission from Société Internationale de Chirurgie 17).
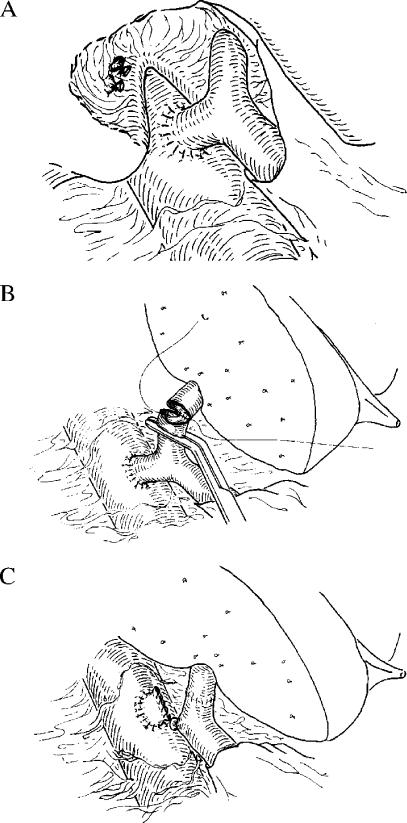
The indications for LDLT in 26 patients (27%) were non-cirrhotic liver diseases with a minimal portosystemic collateral circulation. Because the inferior vena cava is preserved in a manner similar to that used in piggyback liver transplantation during recipient hepatectomy for LDLT, prevention of portal congestion is only necessary during the anhepatic phase. We have previously described a technique in which a temporary shunt is inserted between the right portal vein and the inferior vena cava, thus completely avoiding portal congestion during surgery (Figure 2) 11. Briefly, the right portal vein is anastomosed end-to-side to the inferior vena cava, without interrupting blood flow through the left portal vein. Blood flow through this shunt is maintained until reperfusion of the graft is achieved after end-to-end anastomosis between the recipient's left portal vein and the left portal vein of the graft. As the volume of the left lobar graft is usually much smaller than the hepatic mass required by the recipient's metabolic demand in adult LDLT, avoidance of portal congestion is considered beneficial in minimising the functional burden on the graft.
Figure 2. .
(A) Temporary end-to-side shunt between the right branch of the portal vein and the inferior vena cava is completed before resection of the recipient's liver. (B) The recipient's left portal vein to graft left portal vein anastomosis is made in an end-to-end manner. Shunt flow is maintained during the anastomosis. (C) Immediately after graft reperfusion, the right portal vein is ligated and divided. The stump of the shunt on the inferior vena cava is closed with a running suture. (Reprinted with permission from the American College of Surgeons 11).
In LDLT for FAP, when the estimated GV does not exceed 30% of the recipient's SV even when the caudate lobe is included, temporary auxiliary partial orthotopic liver transplantation (APOLT) is the treatment of choice. In this technique, the auxiliary left lobar graft is orthotopically transplanted after resection of the recipient's left lobe, and the right portal vein is transected to induce compensatory hypertrophy of the graft. After confirming atrophy of the native liver and hypertrophy of the graft by computed tomography, the remnant native liver is removed around 2 months after APOLT. This technique guarantees an adequate safety margin for both the donor and the recipient in LDLT for FAP where the donor's left lobe is small.
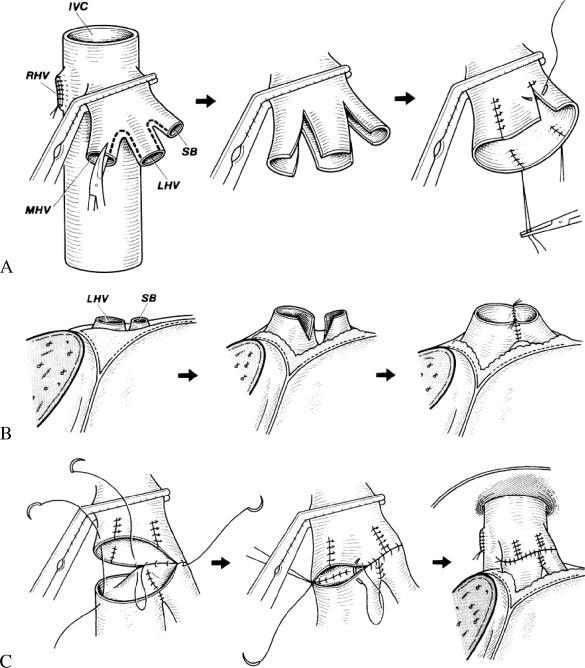
In our patients, hepatic artery and portal venous reconstruction were performed in an end-to-end manner. Because the diameters of these vessels are smaller than in whole liver transplantation, postoperative anticoagulant treatment was prescribed as described previously 12. The techniques used for Y-reconstruction of the hepatic venous outflow by end-to-end anastomosis after venoplasty have been reported in detail 13 (Figure 3). When the superficial branch of the left hepatic vein did not form a common orifice with the main left hepatic vein in the donor, venoplasty to construct such an orifice was undertaken to avoid partial congestion in grafts with a small volume.
Figure 3. .
End-to-end anastomosis techniques used for Y-reconstruction of outflow after living donor liver transplantation (LDLT). (A) Venopiasty of the middle and left hepatic veins in the recipient A common trunk was created by splitting and suturing. (B) Venopiasty of the left hepatic vein of the graft, involving dissecting and joining to ensure adequate length. (C) Y-shaped end-to-end anastomosis joining the common trunk of the middle left hepatic veins in the recipient and the left hepatic vein of the graft with continuous sutures. MHV, middle hepatic vein; LHV, left hepatic vein; SB, superficial branch of the LHV; RHV, right hepatic vein; IVC, inferior vena cava. (Reprinted with permission from the American College of Surgeons 13).
Initial postoperative immunosuppression consisted of tacrolimus and steroids; cyclosporin was substituted for tacrolimus if tacrolimus-related adverse effects occurred.
The perihepatectomy profiles of the donors, significance of left liver grafts, postoperative courses of the donors and recipients, and survival of the recipients were investigated. Data are expressed as mean±SD unless otherwise stated.
Results
Four (4%) of the donors experienced gastric volvulus (Table 1), but recovered without requiring surgery 14. All the LDLT donors recovered well and returned to a normal lifestyle. The perihepatectomy profiles of the donors are shown in Table 2. No donor required banked-blood transfusion or repeat surgery. Postoperative liver function tests in the donors had satisfactory results (Table 3).
Table 1. Postoperative complications in the left lobe donors (n = 97).
| Complication | Number | (%) |
|---|---|---|
| Gastric volvulus | 4 | (4) |
| Transient ulnar nerve palsy | 2 | (2) |
| Bile leakage | 2 | (2) |
| Peptic ulcer | 1 | (1) |
| Pleural effusion | 1 | (1) |
Table 2. Profiles of the donor hepatectomiesa.
| Graft | Transection timeb (min) | Amount of blood loss (ml) | Transfusion of autologous blood (ml) | Transfusion of autologous plasmac (ml) | Transfusion of heterologous blood |
|---|---|---|---|---|---|
| Left liver | 60±17 | 562±226 | 70±196 | 618±351 | 0 |
| Left liver + caudate lobe | 76±13 | 855±478 | 286±380 | 805±369 | 0 |
aValues are mean±SD; btime taken to transect the donor liver to obtain a graft; cautologous fresh frozen plasma.
Table 3. Postoperative liver function parameters in LDLT donors (n = 97)a.
| Parameter | Postoperative day |
||||
|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 14 | |
| AST | 234±118 | 134±60 | 76±39 | 64±35 | 44±29 |
| ALT | 245±137 | 205±109 | 144±69 | 129±64 | 85±61 |
| TB | 1.3±0.7 | 1.0±0.5 | 0.9±0.4 | 0.7±0.3 | 0.5±0.2 |
| PT | 61±12 | 76±12 | ND | 77±11 | 84±17 |
aValues are mean±SD. AST, aspartate aminotransferase (U/l); ALT, alanine aminotransferase (U/l); TB, total bilirubin (mg/dl); PT, prothrombin time (%); ND, no data.
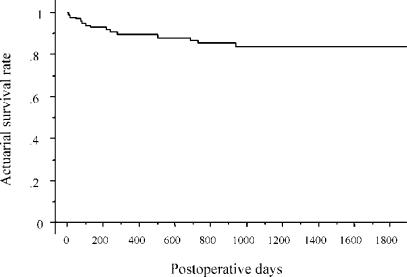
The cold ischaemic time for the graft was 127±54 minutes. The GVs ranged from 230 to 625 ml, and the GV/SV ratios from 22% to 65%, at the time of transplantation (Table 4) The postoperative liver function parameters are shown in Table 5. Although 85% of the liver grafts had GV/SV ratios of <50%, no patient developed immediate postoperative liver failure. One patient with FAP, who received the smallest liver graft among the cases excluding those of temporary APOLT (GV/SV ratio, 26%), showed a good recovery without significant liver dysfunction (peak serum total bilirubin level, 4.0 mg/dl). However, longstanding postoperative cholestasis was observed in three adult recipients who had received a transplant for end-stage liver cirrhosis, even after synthetic function had normalised. In patients who received a left-liver-plus-caudate-lobe graft, follow-up radiological estimation demonstrated adequate regeneration of the caudate lobe (Figure 4). Patient survival rates after LDLT using left liver grafts were 89%, 84% and 84% at 1, 3 and 5 years, respectively, as estimated by Kaplan-Meier analysis. The survival curves for the recipients are shown in Figure 5. The median follow-up period was 1015 days. Long-term liver function remains excellent. Currently, the follow-up values for total bilirubin and aspartate aminotransferase are 0.8±0.7 mg/dl and 38±28 IU/L, respectively.
Table 4. The preoperative estimated graft volume, actual graft volume and the actual graft volume/recipient's standard liver volume ratioa.
| Graft | Preoperative estimationb (ml) | Actual graft volume (ml) | GV/SV ratio (%) |
|---|---|---|---|
| Left liver | 431±91 | 426±76 | 41±10 |
| Left liver + caudate lobe | 387±69 | 421±66 | 34±10 |
aValues are mean±SD; bobtained by volumetry using computed tomography. GV, actual graft volume; SV, recipient's standard liver volume.
Table 5. Postoperative liver function parameters in the recipients (n = 97)a.
| Parameter | Postoperative day |
||||
|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 14 | |
| ALT | 262±I47 | 203±102 | 174±144 | 156±97 | 109±112 |
| TB | 6.0±4.5 | 3.9±2.9 | 4.0±3.0 | 3.8±3.2 | 3.5±4.5 |
aValues are mean±SD. ALT, alanine aminotransferase (U/l); TB, total bilirubin (mg/dl).
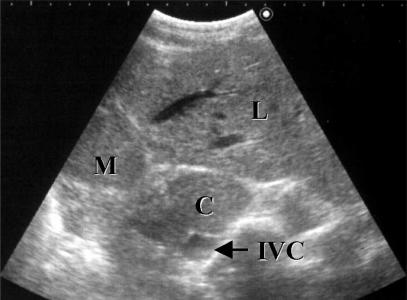
Figure 4. .
Ultrasonographic image of a left-liver-plus-caudate-lobe graft 6 months after LDLT. L, left lateral segment; M, medial segment; C, caudate lobe; IVC, inferior vena cava.
Figure 5. .
Survival curve for recipients of left liver grafts.
Discussion
LDLT has recently been recommended for adult patients 1,2,3,4,5,15. During our early experience, a paediatric patients who received a graft comprising 46% of the SV recovered uneventfully after transplantation. Accumulation of further knowledge concerning graft size has led us to expand the indications for LDLT to include adult patients 3,5,6,7,9,11,16. In 1993, a 53-year-old female patient patient with primary biliary cirrhosis was successfully treated by LDLT using the left lobe of her son's liver as a graft. Volumetric analysis demonstrated rapid enlargement of the graft from 402 to 1141 ml as early as 2 weeks after the operation 5. Although decisions regarding the indications for LDLT in adults should be made carefully, based on preoperative estimation of the GV/SV ratio 3,7,8, this procedure has become established. From the viewpoint of maximising donor safety, we have pursued adult LDLT techniques using the left liver, with or without the caudate lobe. In our series, this has resulted in no significant donor morbidity, no donor mortality, and fair results in the recipients (comparable to those obtained with right liver grafts 15). Efforts to avoid portal venous congestion and to shorten the graft preservation period might have contributed to these good results. In addition, the use of left-liver-plus-caudate-lobe grafts and temporary APOLT have allowed the indications to be widened to some extent.
When a donor undergoes left hepatectomy without removal of the caudate lobe, the left portion of the caudate lobe is left without a blood supply or bile drainage system, because of the anatomy of the liver. This results in atrophy of the left caudate lobe in the donor. On the other hand, when the graft includes the caudate lobe as well as the left liver, the caudate lobe is able to function in the recipient. To expand the indications for adult LDLT without additional risk to the living donor, we have developed a technique for harvesting the donor's left liver with the caudate lobe attached 9,17 (Figure 4). Regarding this technique, Takayama et al.18 described the routine reconstruction of the veins of the caudate lobe. Our policy is to reconstruct them only when a thick, short hepatic vein (>3 mm in diameter) is present between the caudate lobe and the inferior vena cava. We have obtained satisfactory results using this approach.
APOLT was initially introduced as a temporary or permanent support for patients with potentially reversible fulminant hepatic failure 19. Its indications have since been extended to include congenital metabolic disorders of the liver 20. A possible advantage of APOLT in metabolic disease is that the remnant native liver may work as a reservoir, which can be life-sustaining if the graft becomes severely dysfunctional. We chose to perform APOLT with ligation of the portal venous branch to the native liver 16, because it has several advantages: firstly, the remnant native liver can sustain the patient's life if the graft is destroyed by primary non-function, severe rejection or other potentially fatal events; secondly, this technique does not jeopardise portal flow in the graft; and thirdly, we can expect compensatory hypertrophy of the left lobar graft and atrophy of the native right lobe after portal branch ligation.
Four donors experienced temporary gastric volvulus in our series. We define gastric volvulus as rotation of the whole or part of the stomach by >180° to create a closed-loop obstruction. This description has also been used by others 21. We believe that this complication results from movement of the stomach into the cavity created by removal of the left liver, followed by adhesion between the stomach and the transection plane of the liver. Fortunately, all the donors with gastric volvulus recovered without any need for repeat surgery. The overall results were satisfactory.
Right lobar grafts have been successfully used to expand the donor pool for adult LDLT in some centres 1,2,4,22. Although this procedure has widened the availability of adult LDLT, there is no doubt that right hepatic lobectomy can be a serious burden for the donor. This point of view is supported by reports of hyperbilirubinaemia in some right lobe donors 23,24, and of donor deaths 25. Such complications have not been observed in left hepatectomized donors. Furthermore, based on our experience with small grafts, the number of patients for whom a right lobar graft is essential is limited. Considering previous reports of donor morbidity and mortality, careful selection of the donor procedure is necessary.
Conclusion
In LDLT, it is well known that although larger grafts are helpful for the recipients, they are harmful for the donors. This dilemma has led to limitations in the application of adult LDLT. Although LDLT using a left liver graft imposes potential postoperative complications, in that a small liver is more vulnerable to injury, and recipients of small grafts are at higher risk of complications during the recovery period, such grafts have yielded acceptable results in adult as well as adolescent LDLT, with minimal burden to the donors.
References
- 1.Marcos A, Ham JM, Fisher RA, et al. Surgical management of anatomical variations of the right lobe in living donor liver transplantation. Ann Surg. 2000;231:824–31. doi: 10.1097/00000658-200006000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trotter JF, Wachs M, Everson GT, Kam I. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346:1074–82. doi: 10.1056/NEJMra011629. [DOI] [PubMed] [Google Scholar]
- 3.Kawasaki S, Makuuchi M, Matsunami H, et al. Living related liver transplantation in adults. Ann Surg. 1998;227:269–74. doi: 10.1097/00000658-199802000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo C-M, Fan S-T, Liu C-L, et al. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg. 1997;226:261–70. doi: 10.1097/00000658-199709000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashikura Y, Makuuchi M, Kawasaki S, et al. Successful living-related partial liver transplantation to an adult patient. Lancet. 1994;343:1233–14. doi: 10.1016/s0140-6736(94)92450-3. [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki S, Makuuchi M, Ishizone S, et al. Liver regeneration in recipients and donors after transplantation. Lancet. 1992;339:580–1. doi: 10.1016/0140-6736(92)90867-3. [DOI] [PubMed] [Google Scholar]
- 7.Urata K, Kawasaki S, Matsunami H, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317–21. [PubMed] [Google Scholar]
- 8.Makuuchi M, Kawasaki S, Noguchi T, et al. Donor hepatectomy for living related partial liver transplantation. Surgery. 1993;113:395–402. [PubMed] [Google Scholar]
- 9.Miyagawa S, Hashikura Y, Miwa S, et al. Concomitant caudate lobe resection as an option for donor hepatectomy in adult living related liver transplantation. Transplantation. 1998;66:661–3. doi: 10.1097/00007890-199809150-00021. [DOI] [PubMed] [Google Scholar]
- 10.Francavilla A, Starzl TE, Barone M, et al. Studies on mechanisms of augmentation of liver regeneration by cyclosporine and FK 506. Hepatobgy. 1991;14:140–3. doi: 10.1002/hep.1840140123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawasaki S, Hashikura Y, Matsunami H, et al. Temporary shunt between right portal vein and vena cava in living related liver transplantation. J Am Coll Surg. 1996;183:74–6. [PubMed] [Google Scholar]
- 12.Hashikura Y, Kawasaki S, Okumura N, et al. Prevention of hepatic artery thrombosis in pediatric liver transplantation. Transplantation. 1995;60:1109–12. doi: 10.1097/00007890-199511270-00009. [DOI] [PubMed] [Google Scholar]
- 13.Matsunami H, Makuuchi M, Kawasaki S, et al. Venous reconstruction using three recipient hepatic veins in living related liver transplantation. Transplantation. 1995;59:917–19. [PubMed] [Google Scholar]
- 14.Chisuwa H, Hashikura Y, Mita A, et al. Living liver donation: preoperative assessment, anatomical considerations and long-term outcome. Transplantation (in press). [DOI] [PubMed] [Google Scholar]
- 15.Todo S, Furukawa H, Jin MB, Shimamura T. Living donor liver transplantation in adults: outcome in Japan. Liver Transpl. 2000;6(Suppl 2):S66–S72. doi: 10.1053/jlts.2000.19009. [DOI] [PubMed] [Google Scholar]
- 16.Ikegami T, Kawasaki S, Ohno Y, et al. Temporary auxiliary liver transplantation from a living donor to an adult recipient with familial amyloid polyneuropathy. Transplantation. 2002;73:628–30. doi: 10.1097/00007890-200202270-00027. [DOI] [PubMed] [Google Scholar]
- 17.Hashikura Y, Kawasaki S, Miyagawa S, et al. Recent advance in living donor liver transplantation. World J Surg. 2002;26:243–6. doi: 10.1007/s00268-001-0212-3. [DOI] [PubMed] [Google Scholar]
- 18.Takayama T, Makuuchi M, Kubota K, Sano K, Harihara Y, Kawarasaki H. Living-related transplantation of left liver plus caudate lobe. J Am Coll Surg. 2000;190:635–8. doi: 10.1016/s1072-7515(00)00255-6. [DOI] [PubMed] [Google Scholar]
- 19.Gubernatis G, Pichlmayr R, Kemnitz J, Gratz K. Auxiliary partial orthotopic liver transplantation (APOLT) for fulminant hepatic failure: first successful case report. World J Surg. 1991;15:660–5. doi: 10.1007/BF01789221. [DOI] [PubMed] [Google Scholar]
- 20.Kasahara M, Kiuchi T, Uryuhara K, et al. Treatment of ornithine transcarbamylase deficiency in girls by auxiliary liver transplantation: conceptual changes in a living-donor program. J Pediatr Surg. 1998;33:1753–6. doi: 10.1016/s0022-3468(98)90278-0. [DOI] [PubMed] [Google Scholar]
- 21.Wastelle C, Ellis H. Volvulus of the stomach. A review with a report of 8 cases. Br J Surg. 1971;58:557–62. doi: 10.1002/bjs.1800580802. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Tanaka K, Kiuchi T, et al. Anatomical variations and surgical strategies in right lobe living donor liver transplantation: lessons from 120 cases. Transplantation. 2002;73:1896–903. doi: 10.1097/00007890-200206270-00008. [DOI] [PubMed] [Google Scholar]
- 23.Strong RW. Whither living donor liver transplantation? Liver Transpl Surg. 1999;5:536–8. doi: 10.1002/lt.500050613. [DOI] [PubMed] [Google Scholar]
- 24.Surman OS. The ethics of partial-liver donation. N Engl J Med. 2002;346:1038. doi: 10.1056/NEJM200204043461402. [DOI] [PubMed] [Google Scholar]
- 25.Hashikura Y, Kawasaki S, Terada M, et al. Long-term results of living-related donor liver graft transplantation: a single-center analysis of 110 transplants. Transplantation. 2001;72:95–9. doi: 10.1097/00007890-200107150-00019. [DOI] [PubMed] [Google Scholar]