Abstract
Background
Strategies for the management of patients with necrotizing pancreatitis remain controversial. While consensus opinion supports operative necrosectomy for the treatment of infected pancreatic necrosis, the timing for surgical intervention is not completely resolved. Further, the indication for the surgical management of sterile pancreatic necrosis is also subject to debate.
Methods
The objective of this study was to evaluate outcome measures for the surgical management of necrotizing pancreatitis, independent of documented infection. A retrospective review was undertaken between 1994 and 2002 at a single county hospital.
Results
Twenty-one patients with CT-documented necrotizing pancreatitis underwent operative pancreatic necrosectomy with laparostomy within 21 days of initial diagnosis and had an average of three reoperations. Average length of stay (LOS) in the ICU was 36 days and in the hospital 67 days. Ten patients had documented infected necrosis based on initial intra-operative cultures, while I I had sterile necrosis. Overall, 95% (20/21) of the patients had a complication, with an average of three complications per patient. Common complications included ARDS (71%), sepsis (33%), renal failure (24%), and pneumonia (24%). The overall mortality rate was 14% (3/21), with a mean follow-up of 469 days.
Discussion
The surgical management of acute necrotizing pancreatitis, independent of documented infection, can be undertaken within 3 weeks of diagnosis with an acceptable morbidity and a low mortality rate. Creation of a laparostomy to enable ready, atraumatic debridement of the retroperitoneum is a safe alternative to standard repeat laparotomies and thus represents a useful adjunct to the surgical management of necrotizing pancreatitis.
Keywords: pancreas, necrosis, laparotomy, infection
Introduction
Acute necrotizing pancreatitis is a devastating disease. While only 10–15% of patients with acute edematous pancreatitis develop the necrotizing variant of the disease, mortality rates associated with necrosis range from 27% to 86% 1,2,3,4,5,6,7,8,9,10. Patients with necrotizing pancreatitis routinely require assisted ventilation, hemo-dynamic monitoring and extended stays in the ICU. Also, many affected patients require multiple operations for control of their disease. The specific indications and timing for surgical intervention are evolving and controversial. While consensus opinion supports operative necrosectomy for the treatment of infected pancreatic necrosis 11, the surgical management of sterile necrosis remains the subject of intense debate. Opponents of the operative treatment of necrotizing pancreatitis cite high mortality rates with operation 1,3. Moreover, many suggest that operation is often unnecessary in patients with sterile pancreatic necrosis 1,3,12. Proponents for the operative treatment of necrotizing pancreatitis cite decreased hospital stays and improved mortality rates 2. The timing of the initial operation is also unclear, although many authors advocate waiting at least 4–6 weeks before operative necrosectomy 13. We hypothesize that early surgical management of acute necrotizing pancreatitis, independent of documented infection, can be undertaken safely and with a low mortality rate. To address this hypothesis, we analysed 21 consecutive patients surgically treated for CT-documented necrotizing pancreatitis, independent of infection, at a county hospital.
Methods
Study design
A retrospective review from 1994 to 2002 was conducted at a single county hospital.
Patient characteristics
Twenty-one patients with pancreatic necrosis, documented by abdominal CT scan, were taken to the operating room for pancreatic debridement; their care was principally supervised by two attending surgeons.
Therapeutic interventions
All patients in this series received aggressive fluid resuscitation, with restitution of third space losses and correction of electrolyte abnormalities. Pulmonary artery catheters were used when indicated by previous cardio-pulmonary disease or hemodynamic instability. All patients were placed on bowel rest and placement of a nasogastric tube in those that were nauseous or vomiting. They received antibiotics and nutritional support (par-enteral and enteral) in accordance with the clinical practices of the two attending surgeons. The diagnosis of necrotizing pancreatitis was established using helical CT of the abdomen and pelvis with a rapid intravenous bolus of contrast material and was based on the imaging feature of partial or complete lack of perfusion of the pancreatic parenchyma. Indications for operative intervention were: 1) evidence of pancreatic infection or sepsis, 2) clinical instability, or 3) clinical intransigence, with or without documentation of infected pancreatic necrosis. Pre-operative pancreatic infection was determined via CT-guided fine-needle aspiration of the necrotic tissue followed by a gram stain that identified a bacterial or fungal pathogen. Sepsis was clinically defined as a systemic response to documented infection in addition to two or more of the following conditions: oral temperature >38.3°C or <36°C, heart rate >90 beats/min, respiratory rate >20 breaths/min or PaCO2<32 mmHg, white blood cell count >12×109/ L or >10% immature band forms 14. Clinical instability was determined based on: a) persistent or recurrent hypotension (systolic blood pressure <100 mmHg) requiring fluid resuscitation and/or vasopressors, b) worsening respiratory function (increasing oxygen or positive end-expiratory pressure requirement to maintain arterial pO2>100 mmHg over a period of >24h), or c) worsening systemic acidosis (progressive and resistant to fluid resuscitation for >24h). Clinical intransigence entailed patients who although clinically stable, failed repeated attempts over at least a 7-day period to either wean off vasopressors or mechanical ventilation or who failed to tolerate resumption of an oral diet due to increased abdominal pain and/or distension.
The surgical approach was uniform and consisted of an exploratory laparotomy through a midline incision, a blunt pancreatic necrosectomy and creation of a flank laparostomy. The pancreas was approached through the gastrocolic ligament and samples of the necrotic pancreas were obtained for aerobic and anaerobic culture. The extent of pancreatic debridement was guided by the gross appearance of the gland intra-operatively, in conjunction with the findings on CT scan. Following the initial necrosectomy, the operation was concluded with creation of a transverse flank laparostomy 15,16,17. Specifically, following adequate debridement of the devitalized pancreatic tissue, the surgeon placed his hand in the pancreatic bed and a plane was created along the retroperitoneum directed towards the nearest flank. Then, with one hand in this retroperitoneal space, a 10–12-cm transverse skin incision was made lateral to the mid-clavicular line, adequate in length to allow passage of the surgeon's hand. Wide Penrose drains of 5 cm diameter (approximately 20–25 in number) were then sutured together in bundles of 8–10, and inserted through the flank laparostomy incision into the previously created retroperitoneal space, so as to completely fill the pancreatic bed (Fig. 1). The midline incision was then closed in the standard fashion and allowed to heal (Fig. 2). The flank laparostomy incision was managed as a stoma using a large wound drainage bag. Subsequent pancreatic debridements were performed using blunt dissection and vigorous saline irrigation through the flank laparostomy without further violation of the peritoneal cavity. The Penrose drains were removed when all necrotic tissue had been debrided, and the flank laparostomy incisions were then allowed to close secondarily.
Figure 1. .
Creation of flank laparostomy for marsupialization of the retroperitoneum; 5-cm wide Penrose drains are then sutured together in bundles of 8–10 and inserted through the flank incision, completely filling the pancreatic bed.
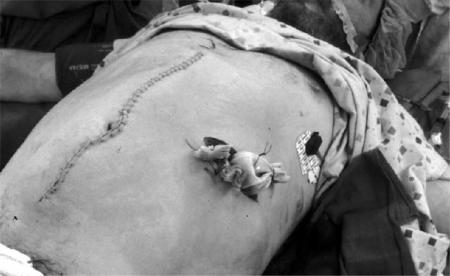
Figure 2. .
Closed abdomen after marsupialization of the retroperitoneum. Following operative debridement and creation of a left flank laparostomy, the midline incision is closed primarily. The Penrose drains are secured to the skin with non-absorbable sutures.
Main outcome measures
Primary outcome measures in this study were length of stay (LOS) in the ICU and in the hospital, and severity of illness as measured by several classification scores. A Ranson's score was calculated in the first 48 h 18, as was the more abbreviated Glasgow score, which is dependent on the prognostic factors age, serum lactate dehydrogenase (LDH), glucose, albumin, white blood cell (WBC), and partial pressure of oxygen 19. Acute Physiology and Chronic Health Evaluation (APACHE) II scores were calculated on admission, within 24 h of the initial surgical intervention, and at patient discharge 20.
Scoring of pancreas
CT examinations were obtained in all 21 patients for a total of 130 studies. All patients had an initial CT study at the time of the clinical diagnosis of severe acute pancreatitis and then a variable number of follow-up studies according to the clinical course of the patient. The number of follow-up studies ranged from 2 to 14 (mean = 6). All CT examinations of the abdomen and pelvis were performed with a 2% oral solution of sodium diatrizoate (Hypaque, Nycomed, Inc., New York, NY, USA) and 140 ml intravenous iohexol (Omnipaque 300, Nycomed, Inc.). A pancreatic protocol was used with a slice thickness of 5 mm through the area of the pancreas and of 7 mm through the remainder of the abdomen and pelvis. A radiologist with special expertise in gastrointestinal imaging (RFT) scored retrospectively the severity of the pancreatitis. Scoring was based on the Balthazar classification 21,22,23,24, which has independently been shown to be valuable in managing these patients by Bradley and colleagues 25. The radiologist was aware of the clinical diagnosis of pancreatitis, but was blinded to the clinical scoring, the clinical follow-up or eventual outcome. For the diagnosis of pancreatitis, presence or absence (= necrosis) of enhancement of the pancreas, appearance of pancreas and peripancreatic fat stranding were determined, and location, amount and number of fluid collections were noted (Fig. 3). Also, complications such as abscess formation (as evidenced by the presence of air within a fluid collection), hemorrhage, venous thrombosis, pseudocyst formation, bowel obstruction or bowel infarction were assessed. For the actual Balthazar scoring, only the morphologic appearance of the pancreas, pancreatic enhancement, peripancreatic fat stranding, and number of fluid collections were considered.
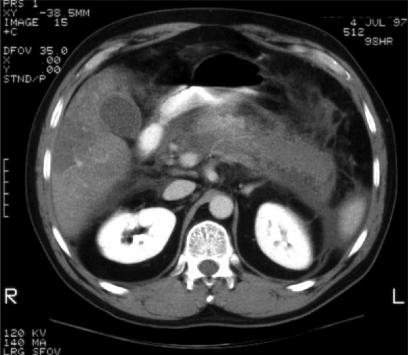
Figure 3. .
Representative helical CT image demonstrating diffuse pancreatic necrosis (lack of contrast enhancement of the gland) and peripancreatic edema (Balthazar score = 8).
Other outcome measures included nutritional requirements, nutritional status, time interval between diagnosis and first surgical debridement, number of surgical procedures, all complications (major and minor), and all-cause mortality.
Statistics
χ2 analysis was used to examine mortality as a function of pancreatic infection, p < 0.05 was assigned statistical significance.
Results
The patients in this series had a mean age of 42 years and were of diverse ethnic backgrounds. The etiology of necrotizing pancreatitis in our patient population was alcohol (9/21), gallstones (6/21), hypertriglyceridemia (4/21), or drug-induced (2/21). Severe necrotizing pancreatitis was confirmed in each case by several well-characterized injury severity scores (Table 1). Patients had an average (±SD) Ranson's score of 5.2 (±2.0), Glasgow score (also over the first 48 h) of 4–1 (±1.6), and an APACHE II score at the time of diagnosis of 8.9 (±5.6). The worst mean preoperative Balthazar CT score, based on the presence or absence of pancreatic edema, peri-pancreatic fluid collections, and partial lack of perfusion of the pancreas, was 7.4 (±1.9). The interval between diagnosis and operative intervention was 21 days (±10). Indications for operative intervention were clinical intransigence (43%), instability (33%), or infection (24%). Patients spent approximately 1 month in the ICU (mean 36 days), and over 2 months in the hospital (mean 67 days). Most patients (17/21 or 81%) were nutritionally depleted on presentation, as evidenced by a plasma albumin level of <3.2g/dl. Dietary supplementation included total parenteral nutrition (TPN) in 19/21 (90%) for an average of 26±20 days. However, over one-third of patients (9/21) successfully tolerated enteral feedings via jejunostomy tube within 7 days of their first operative debridement (mean 12 days, range 1–37 days).
Table 1. Physiologic characteristics of patients with necrotizing pancreatitis (n = 21).
| Severity of illness scores (mean±SD) | |
| Ranson (0–1 1) | 5.2±2.0 |
| Glasgow (0–6) | 4.1±1.6 |
| APACHE II (0–67) | |
| Admission | 8.9±5.6 |
| Preoperative | 8.3±5.4 |
| Discharge | 2.9±2.6 |
| Balthazar CT (0–10) | 7.4±1.9 |
| Number of necrosectomies | 3 (range 1–7) |
| Time from admission to initial operation | 21 days (range 1–91 days) |
| Number of antibiotics used | 7 (range 2–14) |
| Duration of antibiotic administration | 45 days (range 8–83 days) |
| Duration of TPN administration | 26 days (range 0–69) |
| Length of stay (hospital, mean±SD) | 67 days (±39) |
| Length of stay (ICU, mean±SD) | 36 days (±25) |
Ninety-five percent (20/21) of all patients had a complication, with an average of three complications per patient. Complications ranged from those that were relatively benign, such as urinary tract infection, to those that were potentially life-threatening, such as adult respiratory distress syndrome (ARDS) in 15 patients and systemic sepsis in 7 patients (Table 2). The most common gastrointestinal complication was fistula, which occurred in five of our patients. One patient developed both small and large bowel fistulas, which required percutaneous drainage. Two patients developed colonic fistulas that were also drained percutaneously. The remaining two patients had pancreaticocutaneous fistulas, which were of low output (<100 ml of output per day) and closed spontaneously. Renal failure occurred in five of our patients.
Table 2. Complications after necrosectomy in 21 patients with necrotizing pancreatitis.
| Complications | Number |
|---|---|
| ARDS | 15 |
| Sepsis | 7 |
| Renal failure | 5 |
| Pneumonia | 5 |
| Gastrointestinal complications | |
| Infected pancreatic necrosis | 10 |
| Intra-abdominal abscess | 9 |
| Gl hemorrhage | 3 |
| Intestinal fistula | 3 |
| Pancreatic fistula | 2 |
| Infarcted bowel | 1 |
| Small bowel obstruction | 1 |
| Abdominal compartment syndrome | 1 |
| Other complications | |
| Ventral hernias | 2 |
| Wound dehiscence | 1 |
| Urinary tract infection | 1 |
| Pulmonary embolism | 1 |
Ten patients had documented infected pancreatic necrosis, based on intraoperative cultures from the first necrosectomy. Table 3 details the organisms obtained from the initial operative debridement. While S. aureus was the single most common organism isolated (4/10), gram-negative coliforms were collectively more prevalent. Half the patients with infected pancreatic necrosis yielded multiple isolates (range 2–5). Interestingly, whereas 10 of the patients developed diabetes mellitus as a result of their pancreatic inflammation and necrosis, only one patient manifested clinical exocrine deficiency requiring pancreatic enzyme supplementation. Importantly, the overall 30-day mortality rate in the series was 10% (2/21 patients). The all-cause mortality was 14.2% (3/21) with a mean follow-up of 469 days. There was no difference in mortality rate as a function of pancreatic infection (χ2=0.48). Table 4 compiles the results of several clinical series, thereby placing our results within the context of the published literature on the topic.
Table 3. Microbiology results from the initial pancreatic necrosectomies (n = 10).
| Isolates | Number |
|---|---|
| Staphyiococcus aureus | 4 |
| Escherichia coli | 3 |
| Streptococcus viridans | 2 |
| Proteus spp. | 2 |
| Enterococcus sp. | 1 |
| Morganella sp. | 1 |
| Klebsiella pneumoniae | 1 |
| Candida glabrata | 1 |
| Gram-negative rods | 8 |
Table 4. Comparative results of 7 series: patients with necrotizing pancreatitis.
| Authors | Year | n | Ranson's (mean) | Infected (%) | LOS (days) | Mortality (%) |
|---|---|---|---|---|---|---|
| Altmeier & Alexander | 1963 | 32 | – | 72 | – | 44 |
| Beger et al. [26] | 1988 | 74 | 4.5 | 43 | 8 | |
| Stanten & Frey [8] | 1990 | 50 | 3.6 | 90 | 59 | 14 |
| Fugger et al. [16] | 1991 | 102 | – | 15 | – | 35 |
| Bradley et al. [25] | 1993 | 71 | 5.2 | 100 | 42 | 14 |
| Mier et al. [9] | 1997 | 36 | 3.9 | 61 | – | 47 |
| Fernandez-del-Castillo et al. [2] | 1998 | 64 | – | 56 | 41 | 6 |
| Total | – | 429 | 4.3 | 63 | 47 | 24 |
| Harris et al. [present study] | 2004 | 21 | 5.2 | 48 | 67 | 14 |
Discussion
Necrotizing pancreatitis is frequently lethal. The indications and timing for its surgical management are controversial issues. We hypothesized that the surgical management of acute necrotizing pancreatitis could be undertaken safely and with a low mortality rate independent of documented infection but rather dictated by clinical criteria. A total of 21 patients with CT-documented pancreatic necrosis went to the operating room within 3 weeks of their presentation and underwent an average of three operative necrosectomies, using a flank laparostomy for repeated access to the necrotic retroperitoneum. As in other series, our patients were ill, with an average Ranson's score of 5, which carries a predicted mortality of 40% 18. APACHE II scores (8.9 ±5.6) were also indicative of severe pancreatic disease. A prospective examination of APACHE II scores and outcome by Wilson and associates reported an average score of 6.3 for uncomplicated cases of acute pancreatitis, 9.4 for complicated cases, and 14.2 for fatal cases 20. The highest preoperative Balthazar CT score of 7.4 ±1.9 in our patient population indicated severe necrotizing pancreatitis 23. As expected, our patients with necrotizing pancreatitis had lengthy ICU and hospital stays. Nevertheless, the overall mortality rate was only 14% despite the severity of disease, as reflected by the various scores.
The patients developed numerous complications, as has been generally reported with this condition, with an average of three per patient. Approximately two-thirds of patients developed ARDS. Pancreaticocutaneous fistula occurred in only two of our patients and closed spontaneously, as did the intestinal fistulas.
The low mortality rate of 14% is particularly important when considered within the context of the current literature. As seen in Table 4, several recent series evaluating the operative treatment of necrotizing pancreatitis have reported high mortality rates. For example, Beger and associates 26 reported a mortality rate for patients with necrotizing pancreatitis of 37%, and in the study by Mier and co-workers, almost half of the patients with necrotizing pancreatitis died during the study period (47%) 9. In fact, despite advances in critical care and in our understanding of the pathophysiology of acute pancreatitis, only three groups in the last 12 years have reported mortality rates <10% 2,26,27. Based largely on these data, one of the principal arguments against surgical therapy for sterile necrotizing pancreatitis has been that it contributes to prohibitively high mortality rates 1,3. Our mortality rate of 14% confounds this argument as there was no difference in mortality rate as a function of pancreatic infection. While limited by the retrospective nature of these data, this series is noteworthy as it supports the concept that necrotizing pancreatitis can be safely managed using clinical criteria as indicators for surgical intervention.
Why our patients experienced such a low mortality rate compared with several previously published reports is subject to discussion. Among the various potential contributory factors is the fact that a standardized, relatively atraumatic surgical approach was employed. Clinically unstable patients with CT-verified necrotizing pancreatitis were taken to the operating room, with or without documented infection. For many patients the initial operative necrosectomy occurred within a few days of their presentation to the hospital, although the average length of time prior to operation was 21 days. This is a significantly shorter interval than that reported and recommended by many authors 2,13,26. Patients also experienced an earlier return of bowel function and were thus able to start enteral feeding soon after the initial operation. As a result there may have been decreased bacterial translocation and a decreased incidence of central venous line infection that is frequently associated with prolonged total parenteral nutrition. Nine patients tolerated enteral feeding within 7 days of the initial operation, and bowel function typically returned before the pancreatic necrosis had resolved completely. Since retroperitoneal inflammation is thought to be responsible for the ileus observed in these patients, the relatively rapid return of bowel function suggests that operative debridement facilitated resolution of the inflammatory process.
Our operative technique involved blunt necrosectomy, without formal pancreatic resection, as performed in selected earlier studies 7. Repeat debridements through the flank laparostomy were undertaken until there was complete evacuation of the necrotic soft tissue. The large-bore, rigid drains that were prevalent in earlier studies 3,28 were avoided, potentially accounting for the low incidence of enteric fistulae and gastrointestinal bleeding observed in our study. In addition, the contribution from improved imaging technologies cannot be overstated. Contrast-enhanced helical CT imaging of the pancreas has greatly advanced our ability to diagnose pancreatic necrosis. In the present study, this superior diagnostic capability with helical CT led to improved patient selection and the avoidance of operation in patients without pancreatic necrosis. Enhanced imaging also guided surgical therapy by precisely localizing the areas of necrosis that required debridement.
Flank laparostomy with effective marsupialization of the retroperitoneum is not an entirely new procedure. It was described by Davidson and Bradley 20 years ago for the treatment of pancreatic abscess and has been detailed sporadically in the literature since that time 15. Derived from the Latin root marsupium, marsupialization is simply the creation of a pouch. The pouch exteriorizes a compartment, aiding in both access and drainage. The technique is advantageous for necrotizing pancreatitis, in which there is an evolving inflammatory process and subsequent necrosis. Marsupialization using flank laparostomy incisions provides ready access to the pancreatic bed, and obviates the need for repetitive violation of the peritoneal cavity.
References
- 1.Bradley EL, 3rd, Allen K.A prospective longitudinal study of observation versus surgical intervention in the management of necrotizing pancreatitis. Am J Surg 1991; 161:19–24; discussion 24–5. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-del Castillo C, Rattner DW, Makary MA, Mostafavi A, McGrath D, Warshaw AL. Debridement and closed packing for the treatment of necrotizingpancreatitis. Ann Surg. 1998;228:676–84. doi: 10.1097/00000658-199811000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley EL., 3rd A fifteen year experience with open drainage for infected pancreatic necrosis. Surg Gynecol Obstet. 1993;177:215–22. [PubMed] [Google Scholar]
- 4.Uomo G, Visconti M, Manes G, Calise F, Laccetti M, Rabitti PG. Nonsurgical treatment of acute necrotizing pancreatitis. Pancreas. 1996;12:142–8. doi: 10.1097/00006676-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Teerenhovi O, Nordback I, Isolauri J. Influence of pancreatic resection on systemic complications in acute necrotizing pancreatitis. Br J Surg. 1988;75:793–5. doi: 10.1002/bjs.1800750823. [DOI] [PubMed] [Google Scholar]
- 6.Smadja C, Bismuth H. Pancreatic debridement in acute necrotizing pancreatitis: an obsolete procedure? Br J Surg. 1986;73:408–10. doi: 10.1002/bjs.1800730532. [DOI] [PubMed] [Google Scholar]
- 7.Schroder T, Sainio V, Kivisaari L, Puolakkainen P, Kivilaakso E, Lempinen M. Pancreatic resection versus peritoneal lavage in acute necrotizing pancreatitis. A prospective randomized trial. Ann Surg. 1991;214:663–6. doi: 10.1097/00000658-199112000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanten R, Frey CF. Comprehensive management of acute necrotizing pancreatitis and pancreatic abscess. Arch Surg 1990; 125:1269–74; discussion 1274–5. [DOI] [PubMed] [Google Scholar]
- 9.Mier J, Leon EL, Castillo A, Robledo F, Blanco R. Early versus late necrosectomy in severe necrotizing pancreatitis. Am J Surg. 1997;173:71–5. doi: 10.1016/S0002-9610(96)00425-4. [DOI] [PubMed] [Google Scholar]
- 10.Fugger R, Gotzinger P, Sautner T, et al. Necrosectomy and laparostomy – a combined therapeutic concept in acute necrotising pancreatitis. Eur J Surg. 1995;161:103–7. [PubMed] [Google Scholar]
- 11.Hartwig W, Werner J, Uhl W, Buchler MW. Management of infection in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:423–8. doi: 10.1007/s005340200052. [DOI] [PubMed] [Google Scholar]
- 12.Aultman DF, Bilton BD, Zibari GB, McMillan RW, McDonald JC. Nonoperative therapy for acute necrotizing pancreatitis. Am Surg 1997;63:1114–7; discussion 1117–18. [PubMed] [Google Scholar]
- 13.Uhl W, Warshaw A, Imrie C, et al. IAP Guidelines for the Surgical Management of Acute Pancreatitis. Pancreatology. 2002;2:565–73. doi: 10.1159/000071269. [DOI] [PubMed] [Google Scholar]
- 14.Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112:460–7. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson ED, Bradley EL., 3rd “Marsupialization” in the treatment of pancreatic abscess. Surgery. 1981;89:252–6. [PubMed] [Google Scholar]
- 16.Fugger R, Schulz F, Rogy M, Herbst F, Mirza D, Fritsch A. Open approach in pancreatic and infected pancreaticnecrosis: laparostomies and preplanned revisions. World J Surg 1991;15:516–20; discussion 520–1. [DOI] [PubMed] [Google Scholar]
- 17.Margulies AG, Akin HE. Marsupialization of the pancreas for infected pancreatic necrosis. Am Surg. 1997;63:261–5. [PubMed] [Google Scholar]
- 18.Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Localio SA. Objective early identification of severe acute pancreatitis. Am J Gastroenterol. 1974;61:443–51. [PubMed] [Google Scholar]
- 19.Williams M, Simms HH. Prognostic usefulness of scoring systems in critically ill patients with severe acute pancreatitis. Crit Care Med. 1999;27:901–7. doi: 10.1097/00003246-199905000-00023. [DOI] [PubMed] [Google Scholar]
- 20.Wilson C, Heath DI, Imrie CW. Prediction of outcome in acute pancreatitis: a comparative study of APACHE II, clinical assessment and multiple factor scoring systems. Br J Surg. 1990;77:1260–4. doi: 10.1002/bjs.1800771120. [DOI] [PubMed] [Google Scholar]
- 21.Balthazar EJ, Ranson JH, Naidich DP, Megibow AJ, Caccavale R, Cooper MM. Acute pancreatitis: prognostic value of CT. Radiobgy. 1985;156:767–72. doi: 10.1148/radiology.156.3.4023241. [DOI] [PubMed] [Google Scholar]
- 22.Balthazar EJ. Prognostic value of CT in acute pancreatitis: is the early CT examination indicated? Radiology. 1987;162:876–8. doi: 10.1148/radiology.162.3.3809511. [DOI] [PubMed] [Google Scholar]
- 23.Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiobgy. 1990;174:331–6. doi: 10.1148/radiology.174.2.2296641. [DOI] [PubMed] [Google Scholar]
- 24.Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology. 2002;223:603–13. doi: 10.1148/radiol.2233010680. [DOI] [PubMed] [Google Scholar]
- 25.Bradley EL, 3rd, Murphy F, Ferguson C. Prediction of pancreatic necrosis by dynamic pancreatography. Ann Surg 1989;210:495–503; discussion 503–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beger HG, Buchler M, Bittner R, Block S, Nevalainen T, Roscher R. Necrosectomy and postoperative local lavage in necrotizing pancreatitis. Br J Surg. 1988;75:207–12. doi: 10.1002/bjs.1800750306. [DOI] [PubMed] [Google Scholar]
- 27.Baril NB, Rails PW, Wren SM, et al. Does an infected peripancreatic fluid collection or abscess mandate operation? Ann Surg. 2000;231:361–7. doi: 10.1097/00000658-200003000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vauthey JN, Lerut J. An “open-closed” technique for the treatment of necrotizing pancreatitis. Am J Surg. 1993;165:277–81. doi: 10.1016/s0002-9610(05)80526-4. [DOI] [PubMed] [Google Scholar]