Abstract
Background
Selective internal radiation therapy (SIRT) is a new and developing modality for treating non-resectable liver tumours. Evidence is emerging that it is very efficacious in patients with hepatocellular cancer and colorectal liver metastases.
Discussion
SIRT generally involves a single delivery of 90yttrium micro-spheres into the hepatic artery. Preferential uptake is achieved into liver tumours, because of their predominant hepatic arterial blood supply. Average tumour doses of radiation in excess of 200 Gy are achieved. The treatment is generally well tolerated and has been documented by a number of groups internationally to achieve response rates of around 90% in patients with extensive colorectal cancer (CRC) liver metastases. Since the product obtained FDA approval in the USA in 2002, it is being more widely employed and investigated. Unlike other ablative therapies being applied to non-resectable liver tumours, SIRT is indicated even in patients with an extensive burden of liver tumour. Indications, dosing schedules and expected outcomes will become better defined as more groups take up the treatment.
Keywords: selective internal radiation therapy, SIRT, liver tumours, radiation
Introduction
The options and possibilities for the management of colorectal liver metastases have changed immensely over the last 25 years. Operative techniques and the knowledge to support liver resection 1 have developed alongside a number of ablative methods for the management of non-resectable liver metastases including cryotherapy 2, radiofrequency ablation 3 and laser electrocoagulation 4. Regional chemotherapy has been extensively used and promoted by many centres 5,6. At the same time a number of modalities that received attention in the 1970s and 1980s, including devascularising techniques, have faded from usage 7.
Systemic chemotherapy has undoubtedly improved with the development of the newer chemotherapy agents of capecitabine, irinotecan and oxaliplatin, and the emergence of chronomodulation 8, but response rates and overall survival gains remain rather disappointing. For this reason efforts to seek further modalities for treatment remain as important as ever. Selective internal radiation therapy (SIRT) is one such modality, which is emerging as being worthy of use and investigation in the management of non-resectable colorectal liver metastases 9,10,11. The technique entails the delivery of 90yttrium microspheres into the hepatic artery to obtain a degree of selective uptake into hepatic tumours, by virtue of the predominant hepatic arterial supply to tumours as opposed to the predominant portal venous supply to normal liver parenchyma 12. In this way large and lethal doses of radiation can be delivered to tumours while ensuring that the dose received by the non-tumorous areas of liver is tolerated without the development of serious or even fatal radiation hepatitis.
Background
External beam radiation has not found an appreciable place in the management of liver tumours because the liver is poorly tolerant of radiation therapy. Whole-liver doses in excess of 30 Gy may cause fatal radiation hepatitis, which is characterised by the development of portal hypertension, ascites, progressive liver failure and death as a result of a veno-occlusive-type lesion 13,14,15.
The delivery of currently recommended doses of 90yttrium microspheres leads to average calculated doses of radiation in excess of 150 Gy being delivered to liver tumours, while average doses received by unaffected liver parenchyma are of the order of 20–25 Gy 16,17. Problems of clinically detected radiation hepatitis are rare.
90Yttrium is a high-energy, pure β-emitter with a half-life of 64 hours and maximum tissue penetration of 11 mm, which makes it very suitable for treatment of liver tumours. It is relatively straightforward to use, with few issues relating to radioprotection for the patient, family or attending staff. The microspheres are approximately 35 µm diameter, which means that they become permanently trapped at the arteriolar end of the capillary bed. The number of microspheres administered is such that the procedure has little or no devascularising component. Two different types of 90yttrium microspheres are commercially available at the present time. The first, SIR-spheres® (Sirtex Medical Ltd, Sydney, Australia), were fully approved by the FDA in the USA for use in colorectal cancer (CRC) liver metastases in March 2002 and have more recently been similarly approved for use throughout Europe. They are resin microspheres, and a typical dose involves administration of 20–40 million microspheres. The second, Therasphere® (MDS Nordion, Toronto, Canada), is not fully FDA-approved but has a humanitarian device exemption (HDE) for the treatment of hepatocellular carcinoma (HCC). They are glass microspheres and a typical dose involves administration of 5–8 million microspheres. Thus the Therasphere® has both higher specific gravity and specific activity than the SIR-spheres®, which confer some theoretical if not actual disadvantages relative to SIR-spheres®. At this stage, there are more published reports for the SIR-spheres® than for Therasphere and larger numbers of patients have been treated with both HCC and CRC liver metastases using SIR-spheres®.
Technique of administration
The microspheres may be delivered into the hepatic artery via either a surgically implanted port or a percutaneous transfemoral hepatic artery catheter. Both methods are appropriate, and the choice will generally depend on what other treatment is to be offered (regional chemotherapy, systemic chemotherapy or nil). In either case it is imperative that the hepatic circulation is isolated from that of other foregut structures such as the duodenum, stomach and pancreas. This requirement means that vessels such as the gastroduodenal artery, the right gastric artery and pancreaticoduodenal branches should be ligated or embolised before treatment is administered. Failure to observe this precaution puts the patient at risk of development of serious radiation damage to the foregut with the possibility of fatal perforation. Alternatively, the microspheres can be delivered through a catheter placed beyond the take-off of these vessels, which may imply administration to only one side of the liver at a time. This is emerging as the usual method of administration in the USA at the present time, but the situation may change as experience is accrued.
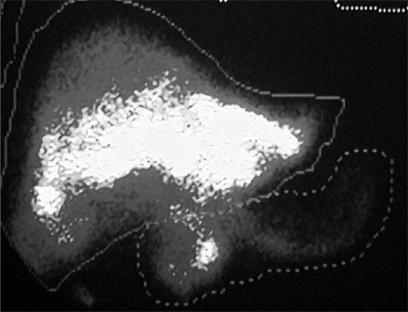
The safety of the technique dictates that any arteriovenous shunting through the liver or the tumour is assessed before SIRT is performed. This assessment involves scanning of the chest and abdomen with a gamma camera after administration of 99mTc-macro-aggregated albumin (MAA) into the hepatic artery 18. Because the MAA aggregates are of comparable size to the microspheres, their distribution after hepatic arterial delivery will be similar to that of administered micro-spheres. In the event that the shunt exceeds 13%, the SIRT should either not be administered, or a dose reduction should be made. Failure to observe this safety aspect may lead to the patient developing serious or even fatal radiation pneumonitis. Such a complication has never been documented provided that this caveat is heeded, but we are aware of at least one instance where fatal radiation pneumonitis occurred when this caveat was ignored. In our experience it is most unusual to encounter a shunt of >2% in patients with CRC liver metastases, although a shunt exceeding this level is seen in 10–20% of those with HCC being considered for SIRT. The MAA scan also provides valuable confirmation of the area of the liver to be treated, plus reassurance that extrahepatic foregut structures are not being accessed by the delivery system (see Figure 1).
Figure 1. .
Macroaggregated albumin scan showing some isotope distribution to the duodenum and pancreas after hepatic arterial injection. This finding was due to a pancreaticoduodenal artery that had not been ligated at the time of port placement. Selective internal radiation therapy was not administered.
SIRT is typically performed under light sedation with intravenous narcotic analgesia. Administration itself is straightforward and takes about 15 minutes, during which time the microspheres are gently flushed into the hepatic artery in a total volume of approximately 50 ml sterile water. Following the procedure patients are returned to their room and may stay in hospital for 24–48 hours. No special radiation safety precautions are necessary because the radiation is β in nature and is contained within the body. Decay takes place over the next 3 weeks, without hazard to family or friends.
Patient tolerance
The treatment is generally very well tolerated, although approximately one-third of patients will experience severe pain and nausea towards the end of the procedure. These symptoms are managed with intravenous narcotic analgesia and anti-emetics and usually subside within 24 hours. Most patients will experience marked lethargy and anorexia after the procedure for 3–6 weeks. No other specific problems are usually encountered. The adverse events that we have encountered in performing 100 procedures are detailed in Table 1. Three deaths have occurred in a total of 165 treatments (1.8%). The first was the result of severe radiation gastritis, which led to perforation, peritonitis and death 30 days after SIRT given for metastatic melanoma via a transfemoral catheter. The second was the result of progressive radiation hepatitis. In this instance jaundice developed 7 weeks after successful and uneventful administration of 2 GBq SIR-spheres® for multiple CRC liver metastases. The third death occurred from acute hepatic necrosis 5 days after repeat SIRT in a patient with HCC who had an excellent response to the initial treatment 9 months earlier; autopsy failed to provide an explanation for the hepatic necrosis. We have repeated SIRT in 15 other patients without clinical evidence of hepatic failure. Peptic ulceration after SIRT may result from inadvertent delivery of microspheres to the stomach or duodenum, but may also occur as a result of metabolic stress in patients with advanced malignancy.
Table 1. Adverse events in 100 consecutive patients treated with SIRT for liver metastases from from colorectal cancer.
| Adverse event | Number of patients |
|---|---|
| Acute pain and nausea | 28 |
| Peptic ulceration | 8 |
| – major bleeding | 2 |
| – requiring operation | 1 |
| Radiation hepatitis (fatal) | 1 |
| Radiation pneumonitis | 0 |
| Lethargy/anorexia for 3–6 weeks | 100 |
| Treatment-related death | 1* |
*From radiation hepatitis.
Clinical results
At the present time the principal clinical reports of experience with SIRT in patients with CRC liver metastases have come from Perth, Australia where the SIR-spheres® were developed, and from Wellington, New Zealand. In 1992 Gray and colleagues reported their early experience with the technique, which showed that response rates to SIRT were high 19. In 2000 they reported in greater detail the results in 71 CRC patients who received SIRT and subsequent hepatic artery chemotherapy (HAC) with floxuridine (FUDR) 20. A response rate of 85% and median survival time from treatment of 13.5 months was reported. In 2001 our own group reported the results of our first 50 patients with CRC liver metastases treated with SIRT and subsequent HAC with 5-fluorouracil (5FU). The response rate in our hands was 92% and median survival from treatment was 14.5 months 9. In our own report 14% of patients had very extensive (>50% liver replacement) disease.
In 2001 Gray and co-workers reported the results of a small (n=74), prospective, randomised trial comparing SIRT followed by HAC with HAC alone. The response rate in the former group of 36 patients was better (72% vs 47%), time to progression was longer (15.9 months vs 9.7 months) and survival at 2 years was improved (39% vs 29%) 10. Another small (n=21) prospective, randomised trial comparing systemic chemotherapy alone with SIRT followed by systemic chemotherapy was reported by Gray and associates in 2002. The chemotherapy regimen consisted of 5FU plus leucovorin. Those treated with SIRT plus chemotherapy fared markedly better than those who received the chemotherapy alone, both in terms of response rate (91% vs 0%) and in time to progression (15.6 months vs 4.7 months) 21. Further clinical trials comparing systemic chemotherapy with oxaliplatin and irinotecan with and without SIRT are planned. Our own experience of CRC liver metastases treated with SIRT plus HAC now comprises 120 patients, and the results are in line with our earlier published experience, with response rates of approximately 90%.
A number of reports from other centres have appeared in the literature in recent years detailing small experiences (generally less than 20 patients) with the use of Theraspheres® in treating CRC liver metastases, often mixed with a variety of other tumour types 22,23. Fewer conclusions can be drawn from these reports, but they do generally concur with the high response rates achieved and reported for SIR-spheres®.
While complete destruction of metastases can occur with SIRT, the chance of this occurring in all of multiple lesions is slim. For this reason it seems sensible that some additional effort is made to control regrowth in the liver with either regional or systemic chemotherapy. We have experience of a number of patients whose bilateral disease was sufficiently modified to allow subsequent liver resection and/or ablation.
Repeat treatment
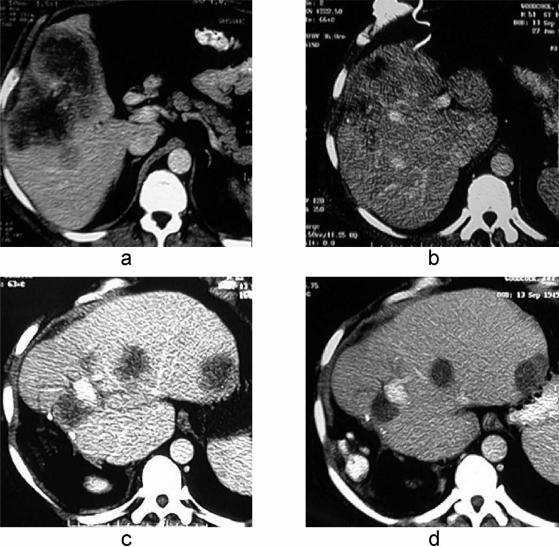
Re-treatment of liver metastases with SIRT has been undertaken both in Perth and in Wellington on occasions, with no penalty. We have re-treated a total of 16 patients (10%) and have come to expect a similar high rate of response as is seen after the first treatment. We would only re-treat if the first treatment had achieved a beneficial response, and have done so after intervals of between 9 and 18 months. Figure 2 shows the CT scans of one such patient, showing a good response after both SIRT treatments. At this stage there are no publications specifically addressing this issue, although the safety and value of repeat treatment for HCC was discussed by Lau and co-workers at the Chinese University in Hong Kong. In their report of 71 patients with HCC treated by SIRT with SIR-spheres®, 15 patients received up to five treatments 17.
Figure 2. .
(a) CT scans showing colorectal liver metastases before selective internal radiation therapy (SIRT) (a) and 18 months later (b) demonstrating an excellent response. An extended right hepatectomy was subsequently performed with cryodestruction of two residual sites in segments 2 and 3. Recurrent disease developed in the liver remnant within 12 months (c) and was retreated with repeat SIRT, a good response being recorded 9 months later (d).
Evidence of radiation hepatitis
While serious radiation hepatitis is very seldom seen after SIRT with the doses currently recommended, there is reason to believe that some degree of radiation hepatitis does occur more commonly than this fact suggests. We have seen evidence of mild portal hypertension develop in some individuals after SIRT, as noted at the time of any subsequent abdominal operation. This complication has generally not been of clinical relevance and has not precluded further operative procedures such as hepatic resection or cryotherapy. While liver function tests after SIRT generally show a temporary and minor deterioration before improving, they may at times demonstrate worsening levels of alkaline phosphatase, gamma-glutamyl transferase and transaminases over a number of months, in the absence of progressive disease. Gradual improvement may then follow. These changes presumably reflect a degree of radiation damage to the liver. Moroz and collegues have reported increased splenic volumes (by an average of 48%) and diminished normal liver parenchyma volumes (by an average of 17%) 12 months after SIRT 24. These findings are likely to reflect the development of mild portal hypertension from subclinical radiation hepatitis. Further work needs to be undertaken to define more thoroughly the extent of liver damage after SIRT, as this is likely to be relevant not only to the tolerance of repeat treatments, but also to the tolerance of subsequently administered cytotoxic drugs that are metabolised by the liver.
Assessment of response
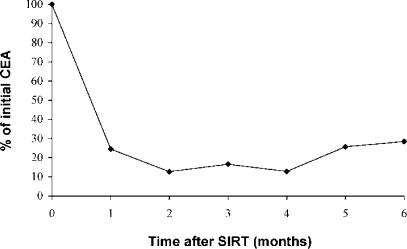
It has become apparent that the conventional method of assessing tumour response to treatment (as recommended by the WHO) using changes in the sum of the products of perpendicular diameters of index lesions on serial CT scans is rather unreliable following SIRT. As with other ablative-type therapies, size changes may occur very slowly or not at all, even in the presence of complete tumour destruction. In a study of 54 patients with CRC liver metastases we have reported 9% complete disappearance of all index lesions, but over an interval of up to 18 months. In a further 76% of patients, all index lesions reduced in size by >10%, but over an interval of up to 21 months. In another 9% of patients, the index lesions neither increased, nor decreased by more than 10%. Only in 6% of patients did the index lesions show progression on the first scan performed 3 months after SIRT 25. We consider that the changes observed in serum carcinoembryonic antigen (CEA) 1–2 months after SIRT give the best and most immediate indication of the response to treatment (see Figure 3). By this criterion, around 90% of patients with CRC liver metastases can be expected to show a response to SIRT. Positron emission tomography (PET) scan evidence is becoming available to support the view that size of lesions assessed by CT scanning after SIRT is an unreliable indicator of response and that tumour marker data are more reliable in this respect 26.
Figure 3. .
Graph showing median reduction in serum carcinoembryonic antigen (CEA) after selective internal radiation therapy in 100 patients treated for CRC liver metastases.
Predicting a response to SIRT
CRC metastases have traditionally been considered relatively avascular lesions, and one might wonder whether the degree of hepatic arterial vascularity could be a determinant of response after SIRT. It is possible to determine the ratio of tumour-to-normal tissue uptake (TNR) on the MAA scan and relate this to tumour response. While it has been suggested in the past that TNR is predictive of a response to HAC 27,28, this relationship appears not to be the case after SIRT 29. This finding suggests that the doses currently being employed may well be somewhat in excess of what is required. At this stage it is not possible to predict which patients with CRC liver metastases are unlikely to respond well to the treatment. Plausible prognostic features such as tumour size, grade and CEA level, which might be considered candidates for predicting response, do not predict a successful response.
It is apparent from clinical reports that one of the principal determinants of survival after SIRT in patients with CRC is the subsequent development of clinically relevant extrahepatic disease 9,10. In our own reported experience of 100 patients treated with SIRT plus HAC, we noted median survival times of 8.3 months for those who developed extrahepatic disease within 6 months of SIRT compared with 12.6 months for those who did not (p < 0.001) 11. It is possible that pre-SIRT PET scanning might identify those patients who will develop extrahepatic disease soon after SIRT and thereby be useful in selecting those who will gain most benefit from a regional approach to their liver metastases. Confirmation of this possibility is awaited.
Summary
SIRT is an exciting new addition to our armamentarium for the regional management of colorectal and other liver tumours. At this stage there are almost no data comparing this treatment with other modalities, particularly systemic chemotherapy, so such information must be awaited. However, the response rates to SIRT are sufficiently high to make it likely that the treatment will have some useful impact on the management and survival of patients with this disease. It also seems relatively inappropriate to use the treatment as a standalone modality for most patients. Rather, it should be considered in conjunction with either regional chemotherapy (in those with disease confined to the liver) or systemic chemotherapy. Considerably more information concerning its place and value in treatment will become available as more centres make use of SIRT.
References
- 1.Blumgart L.Liver resection – liver and biliary tumours, Surgery of the Liver and Biliary Tract. In: , Vol 2Edinburgh: Churchill Livingstone, 1988;1251–80. [Google Scholar]
- 2.Seifert JK, Junginger T, Morris DL. A collective review of the world literature on hepatic cryotherapy. J R Coll Surg Edinb. 1998;43:141–54. [PubMed] [Google Scholar]
- 3.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies. Ann Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heisterkamp J, van Hillegersberg R, Ijzermans JN. Interstitial laser coagulation for hepatic tumours. Br J Surg. 1999;86:293–304. doi: 10.1046/j.1365-2168.1999.01059.x. [DOI] [PubMed] [Google Scholar]
- 5.Meta-Analysis Group in Cancer. Reappraisal of hepaticarterial infusion in the treatment of nonresectable liver metastases from colorectal cancer. J Natl Cancer Inst 1996; 88:252–8. [DOI] [PubMed] [Google Scholar]
- 6.Kemeny NE, Ron IG. Hepatic arterial chemotherapy in metastatic colorectal patients. Semin Oncol. 1999;26:524–35. [PubMed] [Google Scholar]
- 7.Ohlsson B, Lindell G, Lundstedt C, et al. Dearterialization of colorectal liver cancer: institutional experience. Dig Surg. 1999;16:229–35. doi: 10.1159/000018713. [DOI] [PubMed] [Google Scholar]
- 8.Levi F, Giacchetti S, Adam R, et al. Chronomodulation of chemotherapy against metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Eur J Cancer. 1995;31A:1264–70. doi: 10.1016/0959-8049(95)00242-b. [DOI] [PubMed] [Google Scholar]
- 9.Stubbs RS, Cannan RJ, Mitchell AW. Selective internal radiation therapy with 90yttrium microspheres for extensive colorectal liver metastases. J Gastrointest Surg. 2001;5:294–302. doi: 10.1016/s1091-255x(01)80051-2. [DOI] [PubMed] [Google Scholar]
- 10.Gray B, van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres® plus chemotherapy versus chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12:1711–20. doi: 10.1023/a:1013569329846. [DOI] [PubMed] [Google Scholar]
- 11.Stubbs RS, Wickremesekera SK, Boppudi S, Mitchell AW. Selective internal radiation therapy (SIRT) for the treatment of advanced colorectal liver metastases. J Gastrointest Surg. 2003;7:262–3. [Google Scholar]
- 12.Taylor I, Bennett R, Sherriff S. The blood supply of colorectal liver metastases. Br J Cancer. 1979;39:749–56. doi: 10.1038/bjc.1978.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawrence TS, Robertson JM, Anscher MS, et al. Hepatictoxicity from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237–48. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 14.Ingold JA, Reed GB, Kaplan HS, Bagshaw MA. Radiation hepatitis. Am J Roentgenol Radium Ther Nucl Med. 1965;93:200–8. [PubMed] [Google Scholar]
- 15.Concannon JP, Edelmann A, Frich JC, Jr, Kunkel G. Localized “radiation hepatitis” as demonstrated by scintillation scanning. Radiology. 1967;89:136–9. doi: 10.1148/89.1.136. [DOI] [PubMed] [Google Scholar]
- 16.Ho S, Lau WY, Leung TW, et al. Partition model for estimating radiation doses from yttrium-90 microspheres in treating hepatic tumours. Eur J Nucl Med. 1996;23:947–52. doi: 10.1007/BF01084369. [DOI] [PubMed] [Google Scholar]
- 17.Lau WY, Ho S, Leung TW, et al. Selective internal radiation therapy for nonresectable hepatocellular carcinoma with intraarterial infusion of 90Yttrium microspheres. Int J Radiat Oncol Biol Phys. 1998;40:583–92. doi: 10.1016/s0360-3016(97)00818-3. [DOI] [PubMed] [Google Scholar]
- 18.Leung TW, Lau WY, Ho SK, et al. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90Yttrium-microspheres for inoperable hepatic tumours. Int J Radiat Oncol Biol Phys. 1995;33:919–24. doi: 10.1016/0360-3016(95)00039-3. [DOI] [PubMed] [Google Scholar]
- 19.Gray BN, Anderson JE, Burton MA, et al. Regression of liver metastases following treatment with Yttrium-90 microspheres. Aust N Z J Surg. 1992;62:105–10. doi: 10.1111/j.1445-2197.1992.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 20.Gray B, van Hazel G, Buck M, et al. Treatment of colorectal liver metastases with SIR-spheres plus chemotherapy. GI Cancer. 2000;3:249–57. [Google Scholar]
- 21.Gray B, van Hazel G, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone on advanced colorectal hepatic metastases. Presented to the American Society of Clinical Oncology (ASCO)2002; Abstract no. 59. [Google Scholar]
- 22.Anderson JH, Goldberg JA, Bessent RG, et al. Glassyttrium-90 microspheres for patients with colorectal liver metastases. Radiother Oncol. 1992;25:137–9. doi: 10.1016/0167-8140(92)90020-u. [DOI] [PubMed] [Google Scholar]
- 23.Andrews JC, Walker SC, Ackermann RJ, et al. Hepatic radioembolization with Yttrium-90 containing glass microspheres: preliminary results and clinical follow-up. J Nucl Med. 1994;35:1637–44. [PubMed] [Google Scholar]
- 24.Moroz P, Anderson JE, Van Hazel G, Gray BN. Effect of selective internal radiation therapy and hepatic arterial chemotherapy on normal liver volume and spleen volume. J Surg Oncol. 2001;78:248–52. doi: 10.1002/jso.1162. [DOI] [PubMed] [Google Scholar]
- 25.Stubbs RS, Wickremesekera SK, Boppudi S, Nowitz M. Assessment of response to selective internal radiation therapy (SIRT) in patients with colorectal liver metastases. J HPB Surg. 2002;9(Suppl 1):76. [Google Scholar]
- 26.Wong CY, Salem R, Raman S, et al. Evaluating 90Y-glass microsphere treatment response of unresectable colorectal liver metastases by [18F]FDG PET: a comparison with CT or MRI. Eur J Nucl Med. 2002;29:815–20. doi: 10.1007/s00259-002-0787-4. [DOI] [PubMed] [Google Scholar]
- 27.Daly JM, Butler J, Kemeny N, et al. Predicting tumour response in patients with colorectal hepatic metastases. Ann Surg. 1985;202:384–93. doi: 10.1097/00000658-198509000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kemeny N, Niedzwiecki D, Shurgot B, Oderman P. Prognostic variables in patients with hepatic metastases from colorectal cancer. Importance of medical assessment of liver involvement. Cancer. 1989;63:742–7. doi: 10.1002/1097-0142(19890215)63:4<742::aid-cncr2820630423>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 29.Stubbs RS, Dhabuwala A, Lamerton P, Cannan R. Relationship between 99mTc-labelled macroaggregated albumin (MAA) uptake by colorectal liver metastases and response to selective internal radiation therapy (SIRT) J HPB Surg. 2002;9(Suppl 1):76. [Google Scholar]