Abstract
Background
Besides its haematopoietic effect, erythropoietin (EPO) has multiple protective effects, i.e. antiapoptotic, antioxidant and angiogenic properties. The neuroprotective effects of EPO against ischaemia have all been demonstrated in cell culture and animal models. The aim of the study was to evaluate the effect of erythropoietin on ischaemia-reperfusion injury (I/R injury) of the liver.
Methods
Forty-eight adult male Sprague-Dawley rats weighing 250–300 g were divided into three groups: group I, hepatic ischaemia-reperfusion (Hepatic I/R); group II, hepatic ischaemia-reperfusion + EPO (Hepatic I/R+ EPO); group III, sham. Hepatic ischaemia was created by placing a microvascular clamp on the hepatic pedicle for 45 minutes. EPO was given to group II at a dose of 1000 U/kg 120 minutes before the onset of the ischaemia. Blood samples and liver tissues were obtained after 45 minutes of reperfusion from half of the rats in each group. The remaining rats were killed after a 24-hour observation period and blood and tissue samples were obtained. Blood alanine aminotransferase, tumour necrosis factor-α (TNF-α), interleukin-2 (IL-2) and liver tissue malondialdehyde (MDA) levels were determined. Liver tissue histopathology was also evaluated by light microscopy.
Results
In rats with hepatic ischaemia, serum levels of ALT, TNF-α, IL-2 and liver tissue levels of MDA were reduced by the administration of erythropoietin and the histopathological score was also less severe.
Conclusion
This study demonstrates that pre-ischaemic administration of EPO has protective effects on hepatic I/R injury.
Keywords: hepatic I/R, EPO, TNF-α, IL-2, MDA
Introduction
Ischaemia/reperfusion (I/R) -induced injury is one of the major perioperative complications in liver surgery. A lengthy period of ischaemia is required for a number of liver procedures, especially in the setting of hepatic transplantation 1. The liver is exposed to a further and more pronounced injury after restoring the blood supply (i.e. reperfusion), thus aggravating the damage already caused by ischaemia. The increased insult following reperfusion is the result of different interacting mechanisms and mediators acting especially on sinusoidal endothelial cells of the liver 2. Endothelial cell swelling, vasoconstriction, leucocyte entrapment and platelet aggregation within the sinusoids result in microcirculation failure during the early stages of reperfusion. These mechanisms actually prolong the hypoxia that has already begun during the period of ischaemia. The aggravated hypoxia after reperfusion in turn is followed by the activation of Kuppfer cells and neutrophils, which produce inflammatory cytokines and reactive oxygen species, thereby further aggravating the hepatic injury 3. Elevation of the inflammatory cytokine, tumour necrosis factor-alpha (TNF-α) has previously been observed during I/R injury of the liver 4. There have been several reports of reduction in tissue injury by means of drugs, mechanical or heat preconditioning following the I/R insult 4,5,6.
Erythropoietin (EPO) is a hypoxia-inducible haemopoietic growth factor that is mainly expressed in the kidney. It also has multiple protective effects, including antiapoptotic 7, antioxidant 8,9 and angiogenic properties 10. Furthermore EPO has a neuroprotective effect against ischaemia in cell culture and animal models 12,13. It therefore seemed possible that the administration of EPO before ischaemia might protect the liver from I/R injury, and the present study was planned to test this hypothesis.
Materials and Methods
The experiments were performed in the Surgical Research Centre at Osmangazi University and were approved by the University Ethics Committee. Adult male Sprague-Dawley rats weighing 250–300 g and housed four or five to a cage were given standard rat chow with free access to water ad libitum. Rats were fasted for 12 hours before the experiment. Under anaesthesia with intraperitoneal ketamine (Ketalar, Parke-Davis), a 3–4-cm long midline laparotomy was performed and rats were then randomly divided into three groups, as follows. Group I, hepatic ischaemia-reperfusion (Hepatic I/R); group II, hepatic ischaemia-reperfusion + EPO (Hepatic I/R + EPO); group III, sham.
Hepatic ischaemia was created by placing a micro-vascular clamp on the hepatic pedicle for 45 min. EPO (recombinant human erythropoietin, Beta-Neore-cormon, Roche) was prepared to a final concentration of 400 U/ml saline and was given as a single dose of 1000 U/kg by subcutaneous injection of the rats in group II 120 minutes before the beginning of ischaemia. The rats in the sham group were subjected only to the anaesthesia protocol and no further surgical manipulation was performed.
Blood and liver tissue samples were obtained after 45 min of reperfusion from half of the rats in all three groups. The remaining rats were followed for 24 h and similar samples were also obtained under anesthesia before they were killed.
Liver tissue samples were divided into two parts. One part was frozen in liquid nitrogen and stored at −20 °C for determination of lipid peroxidation, while the other was stored in 10% buffered formaldehyde for light microscopy.
Biochemical Analysis
Blood samples were immediately centrifuged at 5000 rpm for 5 minutes. Alanine aminotransferase (ALT) levels in serum were determined with a Hitachi 911 automatic analyser (Boehringer Mannheim, Germany).
TNF-α and interleukin-2 Assays
Serum samples were stored at –70 °C until the assay was performed. Plasma concentrations of TNF-α and inter-leukin-2 (IL-2) were measured by ELISA using the commercially available kits for rat ELISA TNF-α and IL-2 (R&D system, Quantikin M Murine, MN, USA).
Measurement of Lipid Peroxidation
Liver tissue rinsed with ice-cold saline was frozen at −70°C until assay. The tissues were homogenised in 0.1M phosphate-buffer (pH 7.4) with an Ultra Turrax homogeniser (IKA T18 basic, Wilmington, NC, USA). Homogenates were centrifuged at 5000 rpm at 4 °C for 10 min; the supernates were removed and used for MDA analysis. The levels of malondialdehyde (MDA) were measured as described previously 14.
Light Microscopy
Light microscopy studies were evaluated by a pathologist who was 'blinded' to the experiment. Paraffin sections were stained with haemotoxylin-eosin (H&E). Four sections were evaluated for 10 random fields at 20× magnification for congestion and hepatocellular changes (fatty change, intracellular oedema: none = 0, zone III = 1, zone II–III = 2, zone I–II–III panacinar = 3) and necrosis (none = 0, single cell or focal (zone I) = 1, submassive necrosis (bridging necrosis) = 2, bridging necrosis + massive necrosis + infarction = 3). These parameters were combined into a table, and a semi-quantitative scale was used as originally adapted by Hauet and associates for renal studies 15. Total histopathological score was obtained by summation of all the parameters for each sample.
Statistical Analysis
Data are expressed as mean±SEM. Values of p < 0.05 were considered statistically significant. Biochemical results and histopathological findings were assessed by Kruskal-Wallis non-parametric test between the groups. Mann-Whitney U test was used to compare the data between the paired groups.
Results
Biochemical Parameters
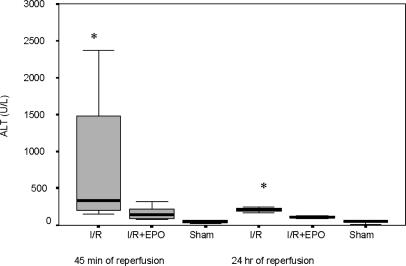
The mean ALT value was 805.3±255.6 U/L in the Hepatic I/R group, and EPO treatment decreased this level to 166.4±26.5 U/L at 45 min of reperfusion (p = 0.01) (Figure 1). The ALT values ranged between 20 and 71 U/L in rats in the sham-operated group. Furthermore, ALT levels decreased in both groups, while these levels were closer to normal in the Hepatic I/R + EPO group at 24 h of reperfusion.
Figure 1. .
ALT values (U/L) at 45 min and 24 h of reperfusion after liver ischaemia. Values are increased at 45 min in both groups exposed to hepatic ischaemia, but the increment is lower in the Hepatic I/R + EPO group (p < 0.05 for Hepatic I/R vs Hepatic I/R + EPO groups). At 24 h ALT levels were decreased in both groups, but more so in the Hepatic I/R + EPO group. *p < 0.05 for Hepatic I/R + EPO groups vs Hepatic I/R and sham groups.
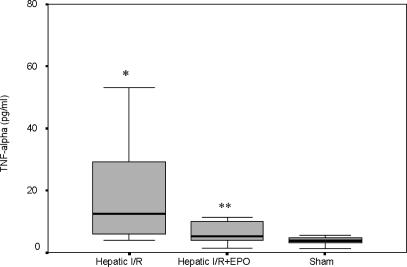
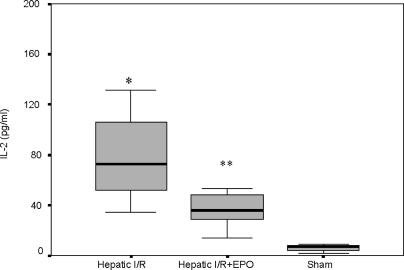
The TNF-α levels were also significantly higher in the Hepatic I/R group (22.06±7.01 pg/ml) vs the Hepatic I/R + EPO group (6.18±1.05 pg/ml) (p = 0.034) (Figure 2). EPO pre-treatment caused an approximately threefold decrease in IL-2 levels according to the results obtained with the Hepatic I/R group (39.76±6.52 pg/ml vs 93.52±23.86 pg/ml, p = 0.013) (Figure 3).
Figure 2. .
TNF-α values (pglml) at 45 min of reperfusion after liver ischaemia. Values are increased at 45 min of reperfusion in both groups exposed to hepatic ischaemia, but the increment in Hepatic I/R + EPO group was less statistically (*p < 0.05 for Hepatic I/R vs Hepatic I/R + EPO and sham groups, **p < 0.05 for Hepatic II R + EPO vs sham group).
Figure 3. .
IL-2 values (pglml) at 45 min of reperfusion after liver ischaemia. Values are increased at 45 min of reperfusion in both groups exposed to hepatic ischaemia. However, EPO pre-treatment caused approximately threefold decrease in IL-2 levels according to the Hepatic I/R group. *p < 0.05 for Hepatic I/R vs Hepatic I/R + EPO and sham groups, **p < 0.05 for Hepatic I/R + EPO vs sham group.
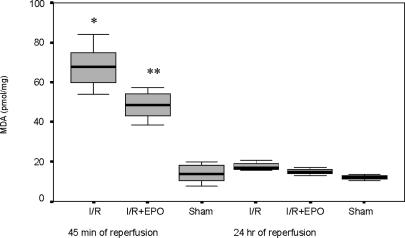
The liver tissue MDA levels were 67.71±3.58 pmol/mg protein in the Hepatic I/R group but decreased to 48.35±2.39 pmol/mg protein (p = 0.03) with EPO premedication (Figure 4).
Figure 4. .
The liver tissue MDA values at 45 min of reperfusion after liver ischaemia. Values are increased at 45 min of reperfusion in both groups exposed to hepatic ischaemia. EPO pre-treatment decreased the MDA levels significantly as compared with the Hepatic I/R group (*p < 0.05 for Hepatic I/R vs Hepatic I/R + EPO and sham groups, **p < 0.05 for Hepatic I/R + EPO vs sham group at 45 min of reperfusion).
Histopathological Parameters
Histopathological evaluation revealed that congestion, necrosis (p < 0.001) and hepatocellular changes (p < 0.05) were more severe in the Hepatic I/R and Hepatic I/R + EPO groups than in the sham-operated group at the either 45 min or 24 h. Total scores were also higher in ischaemic groups than in the sham group (Table 1). However, congestion (p < 0.05), necrosis, hepatocellular changes and total histopathological score (p < 0.001) were less in the Hepatic I/R + EPO group than in the Hepatic I/R group after 45 min of reperfusion. Histopathological improvement was more prominent with EPO retreatment in the Hepatic I/R + EPO group than in the Hepatic I/R group at 24 h (P<0.01 for congestion and hepatocellular changes, p < 0.001 for necrosis and total sum of score).
Table 1. Total histopathological scores of the groups15.
| 45 min | 24 h | |||||
|---|---|---|---|---|---|---|
| Sham | Hepatic I/R | Hepatic I/R + EPO | Sham | Hepatic I/R | Hepatic I/R + EPO | |
| Congestion | 0.5±0.18 | 2.75±0.16 | 2.12±0.22 | 0.37±0.18 | 2.62±0.18 | 1.75±0.25 |
| Hepatocellular changes | 0.5±0.18 | 1.5±0.18 | 1.12±0.22 | 0.37±0.18 | 1.62±0.18 | 0.87±0.12 |
| Necrosis | 0.00±0.00 | 2.62±0.26 | 0.75±0.25 | 0.00±0.00 | 2.87±0.29 | 0.62±0.26 |
| Total score | 1.00±0.39 | 6.87±0.51 | 4.00±0.61 | 0.75±0.34 | 7.12±0.41 | 3.25±0.44 |
Congestion, hepatocellular changes and necrosis were less in the hepatic I/R + EPO group than in the Hepatic I/R group.
Discussion
Although EPO has a demonstrable protective effect on neuronal ischaemia 12, according to our knowledge its role in liver I/R has not been investigated previously. The present study demonstrates that EPO protects liver tissue from I/R injury, as reflected by a lower histopathological score as well as attenuated ALT and TNF-α levels and decreased lipid peroxidation.
The cytokines TNF-α and IL-2 are important mediators of I/R injury. These cytokines play several critical roles in this injury including upregulation of the expression of major histocompatibility complex surface antigens and adhesion molecules. They also stimulate other proinflammatory cytokines and act as chemoattractants for neutrophil activation and migration, which eventually result in increased lipid peroxidation 3. Additionally TNF-α is correlated with I/R injury of the liver 4. The present study has shown that EPO premedication decreases the elevated levels of TNF-α and IL-2 levels observed after liver I/R. Lipid peroxidation was also ameliorated after EPO premedication, as detected by decreased MDA level. Thus EPO might prevent I/R injury in liver surgery, especially in traumatic injuries or transplantation, which require a substantial period of ischaemia. The receptor-associated tyrosine kinase, janus-kinase 2, is the main intracellular signal for the effects of EPO on haemopoiesis and neuroprotection. As EPO is the main stimulator of this enzyme-signalling pathway, its protective effect on liver I/R might be mediated through this mechanism, as in haemopoiesis and neuroprotection 13,16. However, Yamamoto and co-workers have previously shown that genistein suppresses the cellular injury following hepatic ischaemia/reperfusion 4, and genistein is a tyrosine kinase inhibitor. It may be that EPO acts upon the liver via mechanisms other than the tyrosine kinase pathway, i.e. that the protective effects of EPO on liver and nerves operate via different mechanisms.
References
- 1.Serracino-lnglott F, Habib NA, Mathie RT. Hepatic ischaemia-reperfusion injury. Am J Surg. 2001;181:160–6. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 2.McKeown CM, Edwards V, Phillips MJ, Harvey PR, Petrunka CN, Strasberg SM. Sinusoidal lining cell damage: the critical injury in cold preservation of liver allografts in the rat. Transplantation. 1988;46:178–91. [PubMed] [Google Scholar]
- 3.Colletti LM, Kunkel SL, Walz A, et al. The role of cytokine networks in the local liver injury following hepaticischaemia-reperfusion in the rat. Hepatology. 1996;23:506–14. doi: 10.1002/hep.510230315. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto S, Shimizu K, Oonishi I, et al. Genistein suppresses cellular injury following hepatic ischaemia/reperfusion. Transplant Proc. 1996;28:1111–15. [PubMed] [Google Scholar]
- 5.Anthuber M, Farkas S, Rihl M, et al. Conditioning of liver grafts by donor bolus pretreatment with epoprostenol. Transplantation. 1996;62:13–17. doi: 10.1097/00007890-199607150-00003. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson B, Friman S, Wallin M, Gustafsson B, Delbro D. The liver protective effect of ischemic preconditioning may be mediated by adenosine. Transpl Int. 2000;13(Suppl 1):S558–S561. doi: 10.1007/s001470050402. [DOI] [PubMed] [Google Scholar]
- 7.Sakanaka M, Wen TC, Matsuda S, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Nad Acad Sci USA. 1998;9:635–40. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribatti D, Presta M, Vacca A, et al. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood. 1999;93:2627–36. [PubMed] [Google Scholar]
- 9.Yamaji R, Okada T, Moriya M, et al. Brain capillary endothelial cells express two forms of erythropoietin receptor mRNA. Eur J Biochem. 1996;239:494–500. doi: 10.1111/j.1432-1033.1996.0494u.x. [DOI] [PubMed] [Google Scholar]
- 10.Chattopadhyay A, Choudhury TD, Bandyopadhyay D, Datta AG. Protective effect of erythropoietin on the oxidative damage of erythrocyte membrane by hydroxyl radical. Biochem Pharmacol. 2000;59:419–25. doi: 10.1016/s0006-2952(99)00277-4. [DOI] [PubMed] [Google Scholar]
- 11.Ates E, Gene E, Erkasap N, et al. Renal protection by brief liver ischaemia in rats. Transplantation. 2002;74:1247–51. doi: 10.1097/00007890-200211150-00009. [DOI] [PubMed] [Google Scholar]
- 12.Siren AL, Ehrenreich H. Erythropoietin – a novel concept for neuroprotection. Eur Arch Psychiatry Clin Neurosci. 2001;251:179–84. doi: 10.1007/s004060170038. [DOI] [PubMed] [Google Scholar]
- 13.Grasso G. Neuroprotective effect of recombinant human erythropoietin in experimental subarachnoid hemorrhage. J Neurosurg. 2001;45:7–14. [PubMed] [Google Scholar]
- 14.Okhawa H, Ohishi N, Yagi K. Assay for lipid peroxidase in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 15.Hauet T, Mothes D, Goujon JM, et al. Trimetazidine prevents renal injury in the isolated perfused pig kidney exposed to prolonged cold ischaemia. Transplantation. 1997;64:1082–6. doi: 10.1097/00007890-199710150-00025. [DOI] [PubMed] [Google Scholar]
- 16.Klingmüller U. The role of tyrosine phosphorylation in proliferation and maturation of erythroid progenitor cells – signals emanating from the erythropoietin receptor. Eur J Biochem. 1997;249:637–47. doi: 10.1111/j.1432-1033.1997.t01-1-00637.x. [DOI] [PubMed] [Google Scholar]