Abstract
Background
The laparoscopic approach for liver resections is still limited and controversial. Nevertheless the advantages connected with a mini-invasive approach are significant, especially in cirrhotic patients. In recent years the progress of laparoscopic procedures and the development of new and dedicated technologies have made endoscopic hepatic surgery feasible and safe. The aim of this study was to report the results of our experience in laparoscopic liver surgery for hepatocellular carcinoma (HCC) in cirrhotic patients.
Methods
From 2000 to 2003, 16 patients (10 male, 6 female; age 48–69 years; mean age 60.1 years) with HCC and associated severe but well compensated liver cirrhosis underwent laparoscopic hepatic resections at our department. Mean tumour size was 2.9 cm (range 1–3.9). Seven of these lesions were in the left liver and nine in the right lobe. Laparoscopy was performed under CO2 pneumoperitoneum. The liver was always examined using laparoscopic ultrasound (US) to confirm the extension of the lesions and their relationships to the vasculature. The Pringle manoeuvre was not used. The transection of liver parenchyma was obtained by the use of a harmonic scalpel. The specimens were placed in a plastic bag and removed without contact to the abdominal wall.
Results
There was one conversion to laparotomy for inadequate exposure. In the remaining 15 patients we performed 13 non-anatomical resections, I segmentectomy and I anatomical left lobectomy. The mean operative time was 152 min (range 80–180). Mean blood loss was 280 ml and none of the patients required blood transfusions. In two patients the resection margin was <1 cm but the capsule was not infiltrated at histology. One patient died on the third postoperative day from a severe respiratory distress syndrome. Major morbidities occurred in two patients who developed moderate postoperative ascites, which resolved successfully with conservative treatment. The mean postoperative hospital stay was 8.8 days. Mean follow-up time has been 18 months, and to date no recurrences at the site of resection or port-site metastases have been observed.
Discussion
Limited laparoscopic liver resections in cirrhotic patients are technically feasible with a low complication rate when careful selection criteria are followed (hepatic involvement limited and located in the left or anterior right segments, tumour size smaller than 5 cm, Child-Pugh class A). This approach could be considered the best option for the treatment of small esophitic or subcapsular HCC on well compensated cirrhosis and a useful option when it is necessary to perform a left lateral anatomical resection or non-anatomical resection in well selected patients. In fact the mini-invasive approach can minimise the postoperative morbidity rate, which is still too high in this group of patients. It must be performed in highly specialised units by surgeons assisted by all requested technologies and with extensive experience in hepatobiliary and advanced laparoscopic surgery.
Keywords: laparoscopy, liver resection, HCC, cirrhosis, Pringle manoeuvre
Introduction
About 15 years have passed since the first laparoscopic cholecystectomy performed by P. Mouret in 1987. In this period the laparoscopic approach has been applied more and more frequently to the full spectrum of abdominal surgery, becoming the gold standard in the surgical treatment of many pathologies such as biliary lithiasis and gastro-oesophageal reflux. Furthermore, it is routinely used in other procedures such as splenectomy, surrenalectomy, appendicectomy, groin hernia and colectomy 1.
For a few solid organs, especially liver and pancreas, laparoscopic surgery has developed more slowly, despite the fact that in recent years many reports of limited series of laparoscopic liver resections have been published 2,3,4,5,6,7,8,9,10. Although limited in numbers and lacking long-term results, these series have shown the feasibility and the safety of laparoscopic liver resections and further suggested that laparoscopy will improve the postoperative course and reduce hospital stay when compared with the traditional open approach 11,12,13.
There are many reasons for the slow diffusion of the laparoscopic hepatic surgery, such as the presumed technical difficulties, the complicated management of the bleeding, the lack of dedicated tools and the fear of gas embolism 14.
Nevertheless, the advantages connected with a mini-invasive approach are important and significant, especially in cirrhotic patients 4,12,15. The development of new and innovative laparoscopic instruments associated with growing experience in laparoscopy convinced us to try a laparoscopic approach in all the patients suffering with benign or malignant liver diseases in accordance with our inclusion criteria.
In this report we analyse the results of our experience in laparoscopic liver surgery for hepatocellular carcinoma (HCC) in cirrhotic patients.
Materials and Methods
Between May 2000 and July 2003, 37 laparoscopic procedures for benign and malignant hepatic diseases were performed at the Department of General and Hepato-Pancreato-Biliary Surgery, S.M. Loreto Nuovo Hospital, Naples, Italy, of which 16 (43.22%) were for HCC in cirrhotic patients. All the patients were evaluated preoperatively according to a specified protocol that included blood examinations (aspartate transaminase, alanine transaminase, serum albumin, total and direct bilirubin, Pt, α-fetoprotein), abdominal ultrasound and angio-CT scan. We also performed an EGDS to evaluate oesophageal varices and spirometry to check the pulmonary function. In some cases we performed an angio-MRI or, more recently, a contrast-enhanced harmonic sonography. Evaluation of hepatic function was done with the use of the Child-Pugh classification of liver dysfunction 16.
A laparoscopic approach was proposed only for the 16 patients with HCC who fulfilled the following criteria: esophitic or subcapsular tumours localised to the left (II-III) or anterior right (IVb-V-VI) segments, maximum size 4–5 cm, well compensated chronic liver disease (Child-Pugh class A) (Table 1). The characteristics of the 16 patients are summarised in Table 2. The mean age of the patients was 60.1 years (range 48–69); 10 patients were male and 6 were female.
Table 1. Indications for laparoscopic approach in patients with HCC.
| • Esophitic or subcapsular tumours |
| • Left segments (II-III) |
| • Right anterior segments (IVb-V-VI) |
| • Ø Max 4–5 cm |
| • Well compensated chronic liver disease (Child-Pugh class A) |
Table 2. Characteristics of patients.
| Patient no. | Age | Sex | Size (cm) | Site | Diagnosis | Cirrhosis | Surgical technique |
|---|---|---|---|---|---|---|---|
| 1 | 61 | M | 3.5 | VI | HCC | Yes | Non-anatomical resection |
| 2 | 58 | M | 3 | IV | HCC | Yes | Non-anatomical resection |
| 3* | 62 | F | 3 | IV-V | HCC | Yes | Non-anatomical resection |
| 4** | 56 | M | 3.6 | VII | HCC | Yes | Non-anatomical resection |
| 5 | 51 | F | 2.3 | III | HCC | Yes | Non-anatomical resection |
| 6 | 60 | M | 3.9 | VI | HCC | Yes | Segmentectomy |
| 7 | 65 | M | 3.4 | V-VI | HCC | Yes | Non-anatomical resection |
| 8 | 57 | F | 3.2 | II | HCC | Yes | Non-anatomical resection |
| 9 | 66 | M | 2.6 | II | HCC | Yes | Non-anatomical resection |
| 10 | 63 | F | 2.3 | VI | HCC | Yes | Non-anatomical resection |
| 11 | 66 | F | 1 | VI | HCC | Yes | Non-anatomical resection |
| 12 | 69 | F | 2.9 | III | HCC | Yes | Non-anatomical resection |
| 13 | 67 | M | 3.2 | V-VI | HCC | Yes | Non-anatomical resection |
| 14 | 54 | M | 2.2 | IV | HCC | Yes | Non-anatomical resection |
| 15 | 59 | M | 3.6 | II-III | HCC | Yes | Anatomical left lobectomy |
| 16 | 48 | F | 2.7 | V | HCC | Yes | Non-anatomical resection |
*P.O. exitus; **converted.
All the patients presented hepatocellular carcinoma (HCC) (two of them were completely esophitic) related to hepatitis C virus (HCV) infection, which can lead to chronic hepatitis or cirrhosis, depending on the degree of chronic liver damage. In our group all the patients had histologically confirmed cirrhosis (Child-Pugh class A) classified according to Ishak score for fibrosis 17.
The average size of the lesions was 2.9 cm (range 1–3.9); nine were localised in segments V-VI of the right liver, two in segment IV and five in the left lobe (segments II-III). The level of α-fetoprotein was >200 ng/ml in 10 patients. In five patients EGDS provided evidence of initial oesophageal varices (grade Fl). None of the patients had had previous abdominal operations and we never performed an associated procedure. According to the ASA (American Society of Anesthesiologists) classification there were 14 ASA 1 and 2 ASA II but in one patient this classification was probably wrong for an understimated respiratory syndrome (ASA III).
The clinico-pathologic characteristics and the types of resection of the 16 patients are shown in Table 3.
Table 3. Cinicopathologic and surgical data.
| Clinical data | |
| No. of patients | 16 |
| Sex (M/F) | 10/6 |
| Age (years) | 60.1 (range 48–69) |
| ASA status (I/II) | 14/2 |
| HCV (positive/negative) | 16/0 |
| Serum AFP level (<200 ng/ml/ > 200 ng/ml) | 6/10 |
| Child-Pugh score (A/B/C) | 16/0/0 |
| Pathologic data | |
| Ishak score for fibrosis | |
| F 5 | 2 |
| F 6 | 14 |
| HCC (yes/no) | 16/0 |
| Tumour size (cm) | |
| mean | 2.9 |
| range | 1–3.9 |
| Tumour location | |
| Segments II-III | 5 |
| Segment IV | 2 |
| Segments V-VI | 9 |
| Oesophageal varices (F1) | 5 |
| Procedures | |
| Non-anatomical resection | 13 + 1 converted |
| Segmentectomy | 1 |
| Anatomical left lobectomy | 1 |
Operative technique
All the operations were done under general anaesthesia. The patients were placed in the ‘French’ position with the primary surgeon positioned between the legs with one assistant on each side. For lesions localised in segments II-III-IV-V we used a Lloyd – Davis position, whereas in the case of lesions in right lateral segments we preferred a moderate left-lateral decubitus. With an open technique (in order to preserve the umbilical vein) continuous CO2 pneumoperitoneum was induced at a pressure <12 mmHg to prevent the risk of gas embolism. Usually we used a four-trocars (rarely five-trocars) configuration. A 12-mm port at the umbilicus housed a 30° video-camera. The other three trocars were positioned according to the location of the liver lesion (usually along a semicircular line with the concavity facing the right subcostal margin) and they also carried a 5–12-mm port to allow an easier change of the instruments, to give the opportunity of using the harmonic scalpel with both the right and left hand and to facilitate the introduction of endo-staplers.
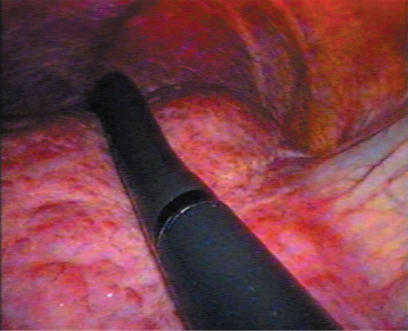
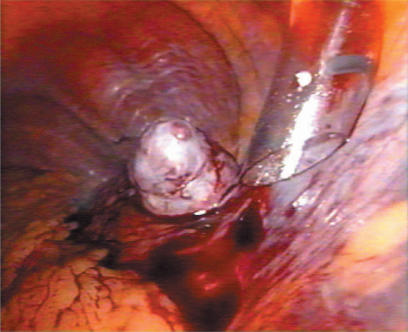
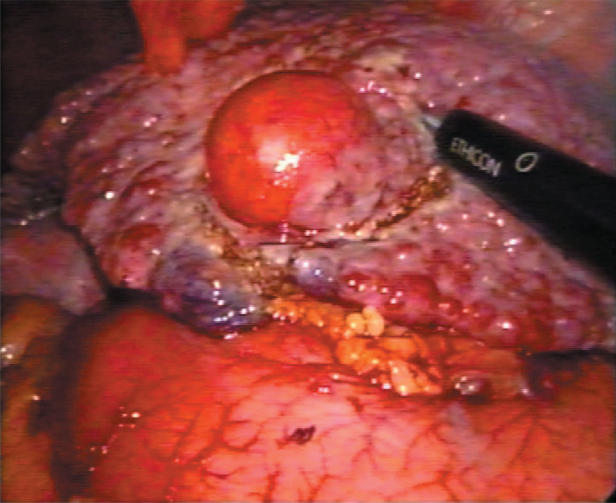
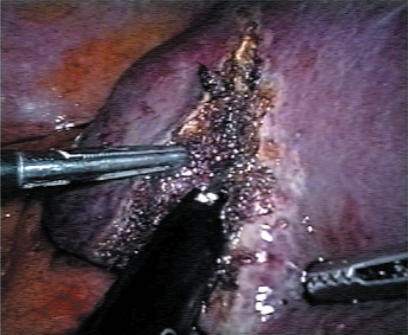
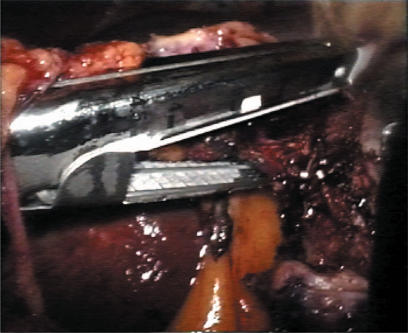
A standard diagnostic and staging laparoscopy was performed and the liver was examined using laparoscopic ultrasonography (US) (Aloka, Tokyo, Japan) (Figures 1 and 2) to confirm the extension of the tumours and their relationships to the vasculature 18. Neither mobilisation of the liver nor round ligament transection was performed. In this way is possible to preserve the umbilical vein, an important way of collateral blood flow in cirrhotic patients. In some cases the falciform ligament was incised to allow a more comfortable management of the laparoscope and of the instruments. Once resection had been decided, the hepato-gastric ligament was opened and the porta hepatis was surrounded by a tape and passed through a 16 F rubber drain for use as a tourniquet to enable performance of a Pringle's manoeuvre, if necessary. This was only preventive and when difficult, for the presence of large collateral veins, we did not perform it. The area to be resected was marked by electrocautery. The hepatic resection was performed with a no-touch technique and the harmonic scalpel was used for the parenchymal transection (Ultracision; Ethicon Endosurgery, Cincinnati, USA) (Figures 3 and 4). Intraparenchymal control of the major vessels was achieved with clips while biliary and vascular radicle division was obtained with clips or stapling devices. In case of anatomical left lobectomy, the left hepatic vein was transected with a linear endo-cutter (vascular cartridge type) (Figure 5). The resected specimen was placed in a plastic bag and externalised through the enlarged periumbilical incision or a mini-laparotomy in the suprapubic region 15. The Argon Beam coagulator (Conmed System 7500, Conmed Corporation, Utica, NY, USA) was applied on the raw surface of the liver to control blood oozing from the stump (Figure 6) and a fibrin glue (Tissucol; Baxter, Vienna, Austria) was spread on the parenchymal transection line to ensure haemostasis and biliostasis (Figures 7 and 8). In all cases a drain was inserted next to the site of resection.
Figure 1. .
Laparoscopic intraoperative ultrasonography.
Figure 2. .
HCC IV segment.
Figure 3. .
Laparoscopic resection for HCC (III segment) using harmonic scalpel.
Figure 4. .
Laparoscopic resection for HCC (VI segment) using harmonic scalpel.
Figure 5. .
Resection of left hepatic vein by linear endo-stapler.
Figure 6. .
Use of argon – beamer on the hepatic stump.
Figure 7. .
Application of fibrin glue.
Figure 8. .
End of the procedure.
Results
The laparoscopic procedure was completed in 15 patients. One patient, with an HCC preoperatively localised in segment VI but intraoperatively found posterior in the segment VII, was converted to laparotomy because of inadequate exposure that could lead to an unsafe procedure. This patient was excluded from the analysis of surgical results because laparoscopic resection had not started.
In the remaining 15 patients we performed 13 non-anatomical resections, 1 segmentectomy and 1 left lateral lobectomy (segmentectomy II-III) according to the Couinaud classification 19. The mean operative time was 152 min (range 80–180) (Table 4). No intraoperative complications occurred in the entire study cohort. The Pringle manoeuvre was prepared in nine patients (56.2%) but it was never needed. The blood loss was more than acceptable (mean 280 ml, range 100–550) and no intraoperative or perioperative blood transfusions were required.
Table 4. Results.
| Parameter | Value |
|---|---|
| Mean operative time (min) | 152 (range 80–180) |
| Pringle manoeuvre | 0 |
| Mean blood loss (ml) | 280 (range 100–550) |
| Transfusions | 0 |
| Conversion rate | 1/16 |
| Hospital mortality | 1/16 |
| Mean hospital stay (days) | 8.8 (range 5–15) |
| <sp = 1/2 > | |
| Complications | |
| Ascites | 2(13.3%) |
| Liver failure | 0 |
| Haemorrhage | 0 |
| <sp = 1/2 > | |
| Surgical margins | |
| < l cm | 2 (capsule not infiltrated) |
| > l cm | 13 |
One patient died in the hospital on the third postoperative day from severe respiratory distress syndrome (ARDS), probably due to a wrong preoperative evaluation of the respiratory function (ASA III instead of ASA II). In retrospect she may not have been an appropriate candidate for surgery, laparoscopic or open. On the other hand this patient was affected by a small subcapsular HCC on cirrhosis located in segment V (next to the gallbladder fundus) that was unsuitable for treatment with percutaneous ablation therapy.
Oral intake of fluid started on the second postoperative day. Patients were usually given a low sodium diet.
Major morbidities occurred in two patients (13.3%) who developed moderate postoperative ascites, which resolved successfully with conservative treatment. The mean postoperative hospital stay was 8.8 days (range 5–15), significantly longer in the two patients with postoperative ascites.
Surgical margins were examined in all the histological reports. In 13 patients the resection margin was >1 cm while in 2 cases it was < 1 cm; in these cases the tumour was capsulated and the capsule was not infiltrated at histology.
All the patients were followed with a standard protocol of surveillance 20 that included a multi-slice CT scan followed by liver function test, serum α-fetoprotein level and ultrasonography at 3 months after resection; serum α-fetoprotein level and abdominal US were repeated every 3 months, and multi-slice CT scans every 6 months. The median follow-up period was 18 months (range 9–46). To date no recurrences in the site of resection and no port site metastases have been observed.
Discussion
During the last two decades progress in preoperative patient assessment, refinement of the indications for resection, improved surgical technique, and the development of new surgical devices have greatly enhanced the safety of open hepatectomy in normal and even in cirrhotic liver 6,21,22.
In contrast, laparoscopic liver surgery is still a developing field, and scientific evidence of its benefits over those of traditional open technique has not been well shown 6,15,23. Even though more than 10 years have already elapsed since the first laparoscopic hepatic resection by Gagner 24 the spread of this technique is still limited and controversial 14. In fact a recent review of the literature reveals only 186 laparoscopic procedures performed for liver surgery, half of them were for malignant diseases. The reported mortality and morbidity rates were 0.54% and 16%, respectively, with a global conversion rate of 7% 25.
Many factors have slowed down the diffusion of this technique, such as the technical difficulties connected with the laparoscopic approach, the difficult management of the bleeding, the lack of dedicated tools and the fear of gas embolism 14. Initial laparoscopic procedures on the liver included staging of tumours and treatment of non-parasitic cysts by unroofing 26,27,28. More recently an increasing number of publications have reported on the laparoscopic treatment of benign liver tumours 1,7,10,26,29,30, but a mini-invasive approach to liver malignancies remains controversial. The number decreases if we consider HCC developed in a previous condition of liver cirrhosis, until recently considered a contraindication 7,31,32
However, we believe that the advantages connected with this approach are important and significant, especially in cirrhotic liver, and that this technique is feasible and safe in carefully selected patients, whatever the nature of the lesion. So, since May 2000, we began to treat patients with HCC on cirrhosis laparoscopically.
The most important factors in the selection of candidates for laparoscopic resections are the location of the lesion and its size 5,6,10,11,13,20. According to other authors 15,31, small (Ø Max 4–5 cm), superficial or peripheral lesions essentially located in the left lateral segment of the liver (segment II-III) or in the anterior segments of the right liver (IVb-V-VI) constitute a good indication for the laparoscopic approach. A case-control study, published in 2003 by Lesurtel et al.12, showed that laparoscopic left lateral lobectomy was as safe as open resection, with lower blood loss and less specific complications of hepatic surgery, but was associated with a longer surgical and clamping time. In our series mean tumour size was 2.9 cm and most of the neoplasms were in the right liver (in segment V-VI in nine patients), in segment IV in two patients (one esophitic) and five were in the left lobe (one esophitic in segment III). These locations allow a good exposure of the whole operation field and safe vascular control.
In only one case we were compelled to convert to laparotomy for a lesion located posteriorly in segment VII (but preoperatively wrongly staged in segment VI); a similar conversion rate (10–15%) is reported in other studies published recently 14,31,33.
Large tumours (deeply sited or located in superior and posterior segments) and lesions close to the portal bifurcation or to the suprahepatic junction should not be considered for laparoscopic resection 25,31,34, especially in cirrhotic patients. In fact an inadequate exposure of the liver is the most frequent reason for conversion to laparotomy after uncontrolled haemorrhage (15.3% and 23%, respectively, in the review by Biertho et al.) 25. All 16 patients in our group had a well compensated liver cirrhosis (Child-Pugh class A,) HCV-related, especially the patient submitted to anatomic left lobectomy (Ishak score for fibrosis 5). As in open surgery we never perform laparoscopic resection in patients with severe liver insufficiency 10.
The other data for our series (operating time, blood loss, transfusion rate, mortality, morbidity and hospital stay) are comparable with other reports 13,15,31 (Table 5).
Table 5. Reported series of laparoscopic liver resections for malignant tumours.
| Parameter | Kaneko [6] | Huscher[4] | Shimada [41] | Gigot [31] | Laurent [15] | Lesurtel [12] | Morino [13] | Belli (current series) |
|---|---|---|---|---|---|---|---|---|
| Year | 1996 | 1997 | 2001 | 2002 | 2003 | 2003 | 2003 | 2003 |
| Patients | 11 | 29 | 17 | 37 | 13 | 18 | 30 | 16 |
| Benign/malignant | 4/7 | 4/25 | 0/17 | 0/37 | 0/13 | 11/7 | 16/14 | 0/16 |
| HCC | 4 | 15 | 17 | 10 | 13 | 5 | 3 | 16 |
| Operative time (min) | 197 | 189 | 325 | NA | 267 | 202 | 148 | 152 |
| Blood loss (ml) | NA | 380 | 400 | NA | 620 | 236 | 320 | 280 |
| Transfusion rate | NA | 24.1% | 5.9% | 16% | 7.6% | 0% | 13% | 0% |
| PTC | NA | 58.6% | NA | 16% | 100% | 62% | 13% | 0% |
| Conversion rate | 9% | 3.4% | 0% | 13.5% | 15.3% | 11% | 0% | 6.2% |
| Complications | NA | 43% | 5.9% | 22% | 15.3% | 11% | 6.6% | 13.3% |
| Mortality | NA | 1/29 | 0 | 0 | 0 | 0 | 0 | 1/16 |
| Hospital stay (days) | NA | 10 | 12 | 7 | 15.3 | 8 | 6.4 | 8.8 |
| Surgical margin <1 cm | NA | NA | 41% | 30% | 15.3% | 6% | 43% | 13.3% |
PTC, portal triad clamping; NA, data not available.
Haemostasis certainly represents the major problem connected with hepatic surgery, either open or laparoscopic. In fact blood loss and perioperative transfusions are the most important prognostic factors influencing mortality and morbidity after a liver resection 35,36,37. In recent years the development of innovative instruments has significatively improved control of bleeding during parenchymal transection. Neverthless 50–80% of conversions to laparotomy 31,33 were still determined by uncontrolled bleeding. In our series mean blood loss was 280 ml; we never used either transfusions or Pringle's manoeuvre and never converted to laparotomy for an intraoperative haemorrhage. These good results could be attributed to careful selection criteria, to extensive experience in hepatic and laparoscopic surgery or even to the use of dedicated technologies, such as harmonic scalpel, argon beamer, endo-stapler and fibrin glue that greatly improved the ability to maintain haemostasis during laparoscopic surgery. These devices, correctly employed, determine a reduction of blood loss and of biliary leaks, so shortening the operative time and preventing intraoperative and postoperative complications 38.
The perfect sealing of the blood vessels (up to 3 mm) and of biliary ducts by the use of a harmonic scalpel associated with the haemostatic effect of the pneumo-peritoneum were responsible for the zero rate of Pringle's manoeuvre in our patients. The use of this technique 39 during laparoscopic hepatic resections is still debated. In a Multicenter European Study 31 portal triad clamping was performed in 6/37 patients (16%) but in 4/10 patients with HCC (40%); it was unilateral in one patient and total in five patients. The mean duration was 31 min (range 15–50). Some authors 10,26,34,40,41 usually apply intermittent clamping (15-min clamping and 5-min release periods) that is better tolerated than continuous clamping either in cirrhotic livers or in the case of clamping lasting >1 hour 42,43. We instead believe that the Pringle manoeuvre must be performed only in selected cases 44. In our opinion this clamping, especially in laparoscopic resections for HCC on cirrhosis (mostly atypical resections), is not useful and dangerous to apply for the severe portal hypertension associated with the underlying chronic liver disease. Furthermore, in literature it has been reported that clamping the portal triad during a pneumoperitoneum could have a significant impact on haemodynamics, causing deterioration of important cardiovascular variables (cardiac output, mean arterial pressure, portal venous flow) 45,46, even though a recent prospective study did not find any difference in haemodynamic changes during clamping in patients with pneumoperitoneum 47.
Satisfactory control of intraoperative bleeding has also been ensured by the use of intraoperative US that, in our opinion, is an indispensable procedure that should always precede a planned operation (with open or laparoscopic approach) aiming at hepatic resection, particularly in cirrhotic patients. This examination, thanks to the development of laparoscopic flexible probes, allows the surgeon not only to study the tumour relationships to the major vascular and biliary structures but also to confirm its three-dimensional location (useful in case of non-subcapsular lesions), its correct staging and its extension 18,28,48,49. Thus it is possible to perform smaller resections, with lower blood loss and shorter operative times but oncologically appropriate 31,50,51. In our series preoperative staging was always correct and laparoscopic US did not detect any new lesions; however, it was useful in identifying the sites of the intraparench-ymal lesions and their relationships with major surrounding structures.
In two patients (13.3%) we noticed a moderate postoperative ascites which persisted for 10 and 15 days, respectively, and was successfully treated with medical therapy.
In our group there was one death (6.6%) on the third postoperative day due to ARDS, but we do not think that this event could be attributed to the laparoscopic approach. In fact it was caused by a wrong preoperative evaluation of the respiratory function, and in retrospect the patient may not have been an appropriate candidate for surgery, either laparoscopic or open. On the other hand, in this case, the tumour was unsuitable for percutaneous ablation, being located close to the gallbladder fundus 52.
No other complications were noticed in our patients but it must be remembered that in the literature there are reports of other major complications such as liver failure, haemorrhage, pulmonary infection, pleural effusion, biliary leakage and, above all, two cases of gas embolism. Both of them were associated with the use of argon cautery, which causes an increase in intra-abdominal pressure 25. The opening of a trocar valve during the utilisation of an argon beamer 6 and the creation of positive pressure pulmonary insufflation are recommended when approaching the main hepatic veins 1,4,53.
Laparoscopic liver surgery for malignancies is still controversial, although there is no evidence in the literature that the use of laparoscopic techniques increases the risk of tumour dissemination 34. In our patients we have experienced neither port site metastases nor intra-abdominal seeding, but as in other published series 13,14,15,31,41 these data are inconclusive because of the limited number of patients and the short follow-up. In any case the use of a plastic bag for removing the specimen is mandatory.
Unlike the treatment of benign diseases, the aim of laparoscopic hepatic surgery for malignancy is not only the functional postoperative advantage but, above all, a 5- and 10-year disease-free survival rate similar to open surgery. Until now only one author 41 has provided information about the short- and long-term outcome of patients with HCC treated by the laparoscopic approach. This study reports a better short-term outcome (postoperative hospital stay and postoperative complications) after laparoscopic surgery than after conventional open hepatectomy. The long-term prognosis (overall survival rate and disease-free survival rate) was similar in the two groups as well as the local control of HCC. The same results were reported in 2003 by Cherqui et al., with some advantages in terms of morbidity, a same or better 3-year survival and a similar recurrence rate 15.
These results could be achieved only if the same oncologic rules as in open surgery are applied: ‘no-touch technique’, R0 radical resection, and achievement of a tumour-free surgical margin 31. Although the 1-cm tumour-free resection margin has been challenged recently 54,55, the achievement of a tumour-free surgical margin is the key point of any laparoscopic resection for HCC. Clearance margin does not seem to affect oncologic results provided that the resection is complete and tumour is not exposed 15. The difficulty in reaching this requirement is related to the lack of digital palpation, but the use of intraoperative US could solve this problem. In a multicentre European study the incidence of invaded or < 1 cm surgical margin was more frequent when laparoscopic US was omitted (3/5 patients, 60%) than when laparoscopic US was used (4/20 patients, 20%) 31.
In our series 13 patients (86%) had a surgical margin >1 cm while only 2 patients (14%) had a surgical margin <1 cm; in these cases tumour was surrounded by a capsule that was not infiltrated at histology.
Our results confirm that limited laparoscopic liver resections of the anterior segments or anatomical left lateral segmentectomies are feasible and safe even for malignancies in selected well compensated cirrhotic patients. With the laparoscopic approach several advantages can be obtained: cosmetic advantages, decreased postoperative pain, early mobilisation and feeding, reduction of respiratory and thrombo-embolic complications, shorter hospital stay, earlier commencement of adjuvant therapy, reduction in postoperative adhesions. Moreover, the absence of large abdominal incisions reduces the risk of parietal hernias and, most important, a lesser destruction of wall porto-systemic shunts may reduce the increase of portal hypertension and consequent postoperative bleeding and ascites 4. In fact ascites is present in 30–50% of the Child A patients undergoing open surgery but only in 1/13 patients (8%) undergoing laparoscopic resections 15. In our series only two patients developed ascites. Last but not least laparoscopy may be considered as a safe and effective bridge for patients with small HCC on a waiting list for orthotopic liver transplantation 56.
In conclusion we believe that limited laparoscopic liver resections in cirrhotic patients are technically feasible with a low complication rate when careful selection criteria are followed. In our opinion, at the moment, this approach should be considered the best option for the treatment of small esophitic or subcapsular HCC on cirrhosis and a valid option when it is necessary to perform a left lateral anatomical resection or non-anatomical resections. This surgery should be performed in highly specialised units by surgeons assisted by all requested technologies and with extensive experience in hepatobiliary and advanced laparoscopic surgery 14.
References
- 1.Samama G, Chiche L, Brefort JL, Le Roux Y. Laparoscopic anatomical hepatic resection. Report of four left lobectomies for solid tumors. Surg Endosc. 1998;12:76–8. doi: 10.1007/s004649900599. [DOI] [PubMed] [Google Scholar]
- 2.Hashizume M, Takenaka K, Yanaga K, et al. Laparoscopic hepatic resection for hepatocellular carcinoma. Surg Endosc. 1995;9:1289–91. doi: 10.1007/BF00190161. [DOI] [PubMed] [Google Scholar]
- 3.Azagra JS, Goergen M, Gilbart E, Jacobs D. Laparoscopic anatomical (hepatic) left lateral segmentectomy – technical aspects. Surg Endosc. 1996;10:758–61. doi: 10.1007/BF00193052. [DOI] [PubMed] [Google Scholar]
- 4.Huscher CGS, Napolitano C, Chiodini S, Recher A, Buffa PF, Lirici MM. Hepatic resections through the laparoscopic approach. Ann Ital Chir. 1997;6:791–7. [PubMed] [Google Scholar]
- 5.Yamanaka N, Tanaka T, Tanaka W, et al. Laparoscopic partial hepatectomy. Hepatogastroenterology. 1998;45:29–33. [PubMed] [Google Scholar]
- 6.Kaneko H, Takagi S, Shiba T. Laparoscopic partial hepatectomy and left lateral segmentectomy: technique and results of a clinical series. Surgery. 1996;120:468–75. doi: 10.1016/s0039-6060(96)80065-1. [DOI] [PubMed] [Google Scholar]
- 7.Marks J, Mouiel J, Katkhouda N, Gugenheim J, Fabiani P. Laparoscopic liver surgery. A report on 28 patients. Surg Endosc. 1998;12:331–4. doi: 10.1007/s004649900664. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe Y, Sato M, Ueda S, et al. Laparoscopic hepatic resection: a new and safe procedure by abdominal wall lifting method. Hepatogastroenterology. 1997;44:143–7. [PubMed] [Google Scholar]
- 9.Belli G, Fantini C, D'Agostino A. Laparoscopic liver resection without Pringle manoeuvre for hepatocellular carcinoma in cirrhotic patients. J Hepato-Biliary-Pancreatic Surgery. 2002;9:14. [Google Scholar]
- 10.Cherqui D, Husson E, Hammoud R, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000;232:753–62. doi: 10.1097/00000658-200012000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rau HG. Laparoscopic liver resections compared with conventional partial hepatectomy. A prospective analysis. Hepatogastroenterology. 1998;45:2333–8. [PubMed] [Google Scholar]
- 12.Lesurtel M, Cherqui D, Laurent A, Tayar C, Fagniez PL. Laparoscopic versus open left lateral hepatic lobectomy: a case-control study. J Am Coll Surg. 2003;196:236–42. doi: 10.1016/S1072-7515(02)01622-8. [DOI] [PubMed] [Google Scholar]
- 13.Morino M, Morra I, Rosso E, Miglietta C, Garrone C. Laparoscopic vs open hepatic resection. A comparative study. Surg Endosc. 2003;17:1914–18. doi: 10.1007/s00464-003-9070-4. [DOI] [PubMed] [Google Scholar]
- 14.Cherqui D. Laparoscopic liver resection. Br J Surg. 2003;90:644–6. doi: 10.1002/bjs.4197. [DOI] [PubMed] [Google Scholar]
- 15.Laurent A, Cherqui D, Lesurtel M, Brunetti F, Tayar C, Fagniez PL. Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg. 2003;138:763–9. doi: 10.1001/archsurg.138.7.763. [DOI] [PubMed] [Google Scholar]
- 16.Pugh RNH, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–9. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 17.Ishak KG. Pathologic feature of chronic hepatitis. A review and update. Am J Clin Pathol. 2000;113:40–5. doi: 10.1309/42D6-W7PL-FX0A-LBXF. [DOI] [PubMed] [Google Scholar]
- 18.Lo CM, Fan ST, Liu CL, et al. Determining resectability for hepatocellular carcinoma: the role of laparoscopy and laparoscopic ultrasonography. J Hepatobiliary Pancreat Surg. 2000;7:260–4. doi: 10.1007/s005340070046. [DOI] [PubMed] [Google Scholar]
- 19.Couinaud C. Masson; Paris: 1957. Le Foie. Etudes Anatomiques et Chirurgicales. [Google Scholar]
- 20.Belli G, Fantini C, D'Agostino A. Chirurgia resettiva epatica “open” e videolaparoscopica per carcinoma epato-cellulare: 15 anni di esperienza. Atti 104° Congresso Soc Ital Chir, Roma 13–16 Ottobre. 2002;2:133–44. [Google Scholar]
- 21.Belli G, D'Agostino A, Ciciliano F, Fantini C. Liver resection for hepatic metastases: a 15-year experience. J Hepat O-Biliary-Pancreatic Surg. 2002;9:607–13. doi: 10.1007/s005340200082. [DOI] [PubMed] [Google Scholar]
- 22.Grazi GL, Ercolani G, Pierangeli F, et al. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg. 2001;234:71–8. doi: 10.1097/00000658-200107000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farges O, Jagot P, Kirstetter P, Marty J, Belgiti J. Prospective assessment of the safety and benefit of laparoscopic liver resections. J Hepato-Biliary-Pancreatic Surg. 2002;9:242–8. doi: 10.1007/s005340200026. [DOI] [PubMed] [Google Scholar]
- 24.Gagner M, Rheault M, Dubuc J. Laparoscopic partial hepatectomy for liver tumor. Surg Endosc. 1992;6:97–8. [Google Scholar]
- 25.Biertho L, Waage A, Gagner M. Hepatectomies sous laparoscopic. Ann Chir. 2002;127:164–70. doi: 10.1016/s0003-3944(01)00709-x. [DOI] [PubMed] [Google Scholar]
- 26.Descottes B, Lachachi F, Durand-Fontanier S, Sodji M, Pech de Laclause B, Valleix D. Traitement laparoscopique des tumeurs solides et kystiques du foie. Etude de 33 cas. Ann Chir. 2000;125:941–7. doi: 10.1016/s0003-3944(00)00403-x. [DOI] [PubMed] [Google Scholar]
- 27.Morino M, De Giuli M, Festa V, Garrone C. Laparoscopic management of symptomatic nonparasitic cysts of the liver. Ann Surg. 1994;219:157–64. doi: 10.1097/00000658-199402000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbot DJ, Marks JH, Feld RI, Liu JB, Rosato FE. Improved staging of liver tumors using laparoscopic liver ultrasound. J Surg Oncol. 1997;64:63–7. doi: 10.1002/(sici)1096-9098(199701)64:1<63::aid-jso12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 29.Descottes B, Lachachi F, Sodji M, et al. Early experience with laparoscopic approach for solid liver tumors: initial 16 cases. Ann Surg. 2000;232:641–5. doi: 10.1097/00000658-200011000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouiel J, Katkhouda N, Gugenheim J, Fabiani P. Laparoscopic surgery for hepatobiliary pancreatic disease: possibilities of laparoscopic liver resection. J Hepato-Biliary-Pancreatic Surg. 2000;7:1–8. doi: 10.1007/s005340050146. [DOI] [PubMed] [Google Scholar]
- 31.Gigot JF, Glineur D, Azagra JS, et al. Laparoscopic liver resection for malignant liver tumors. Preliminary results of a multicenter European study. Ann Surg. 2002;236:90–7. doi: 10.1097/00000658-200207000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ker CG, Chen HY, Juan CC, et al. Laparoscopic subseg-mentectomy for hepatocellular carcinoma with cirrhosis. Hepatogastroenterology. 2000;47:1260–3. [PubMed] [Google Scholar]
- 33.Descottes B, Glineur D, Lachachi F, et al. Laparoscopic liver resection of benign liver tumors. Results of a multi-center European experience. Surg Endosc. 2003;17:23–30. doi: 10.1007/s00464-002-9047-8. [DOI] [PubMed] [Google Scholar]
- 34.Abdel-Atty MY, Farges O, Jagot P, Belghiti J. Laparoscopy extends the indications for liver resection in patients with cirrhosis. Br J Surg. 1999;86:1397–400. doi: 10.1046/j.1365-2168.1999.01283.x. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto J, Kosuge T, Takayama T, et al. Perioperative blood transfusions promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery. 1994;115:303–9. [PubMed] [Google Scholar]
- 36.Poon RTP, Fan ST, Lo CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwatsuki S, Startzl TE. Experience with resection of primary hepatic malignancy. Surg Clin North Am. 1989;69:315–22. doi: 10.1016/s0039-6109(16)44788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidbauer S, Hallfeldt K, Sitzmann G, Kantelhardt T, Trupka A. Experience with ultrasound scissors and blades (Ultracision) in open and laparoscopic liver resection. Ann Surg. 2002;235:27–30. doi: 10.1097/00000658-200201000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pringle JH. Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg. 1908;48:531–49. doi: 10.1097/00000658-190810000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huscher CGS. Laparoscopia nella chirurgia del fegato. Osp Ital Chir. 2000;6:113–28. [Google Scholar]
- 41.Shimada M, Hashizume M, Maehara S, et al. Laparoscopic hepatectomy for hepatocellular carcinoma. Surg Endosc. 2001;15:541–4. doi: 10.1007/s004640080099. [DOI] [PubMed] [Google Scholar]
- 42.Belghiti J, Noun R, Malafosse R, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369–75. doi: 10.1097/00000658-199903000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu CC, Hwang CR, Liu TJ, P'Eng FK. Effects and limitations of prolonged intermittent ischemia for hepatic resection of the cirrhotic liver. Br J Surg. 1996;83:121–4. doi: 10.1002/bjs.1800830139. [DOI] [PubMed] [Google Scholar]
- 44.Belli G, Ciciliano F, Iannelli A, D'Agostino A, Marano I. 84 consecutive hepatic resections for hepatocellular carcinoma (HCC). 4th World Congress of the International Hepato-Pancreato-Biliary Association – IHPBA, Brisbane, Australia. HPB Surgery. 2000;2:228. [Google Scholar]
- 45.Haberstroh J, Ahrens M, Munzar T, et al. Effects of the Pringle maneuver on hemodynamics during laparoscopic liver resection inthe pig. Eur Surg Res. 1996;28:8–13. doi: 10.1159/000129434. [DOI] [PubMed] [Google Scholar]
- 46.Jakimowicz J, Stultiens G, Smulders F. Laparoscopic insufflation of the abdomen reduces portal venous flow. Surg Endosc. 1998;12:129–32. doi: 10.1007/s004649900612. [DOI] [PubMed] [Google Scholar]
- 47.Decailliot F, Cherqui D, Leroux B, et al. Effects of portal triad clamping on haemodynamic conditions during laparoscopic liver resection. Br J Anaesth. 2001;87:493–6. doi: 10.1093/bja/87.3.493. [DOI] [PubMed] [Google Scholar]
- 48.Lo CM, Lai EC, Liu CL, Fan ST, Wong J. Laparoscopy and laparoscopic ultrasonography avoid exploratory laparotomy in patients with hepatocellular carcinoma. Ann Surg. 1998;227:527–32. doi: 10.1097/00000658-199804000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montorsi M, Santambrogio R, Bianchi P, Dapri G, Spinelli A, Podda M. Perspectives and drawbacks of minimally invasive surgery for hepatocellular carcinoma. Hepatogas-troenterology. 2002;49:56–61. [PubMed] [Google Scholar]
- 50.Tait IS, Yong SM, Cuschieri A. Laparoscopic in situ ablation of liver cancer with cryotherapy and radiofrequency ablation. Br J Surg. 2002;89:1613–19. doi: 10.1046/j.1365-2168.2002.02264.x. [DOI] [PubMed] [Google Scholar]
- 51.Podnos YD, Henry G, Ortiz JA, et al. Laparoscopic ultrasound with radiofrequency ablation in cirrhotic patients with hepatocellular carcinoma: technique and technical considerations. Am Surg. 2001;67:1181–4. [PubMed] [Google Scholar]
- 52.Belli G, Romano G, Iannelli A, D'Agostino A, Marano I. Hepatic resection and percutaneous alcohol injection for the treatment of selected patients with more than one hepatocellular carcinoma. Eur J Surg. 1999;165:647–51. doi: 10.1080/11024159950189690. [DOI] [PubMed] [Google Scholar]
- 53.Schmandra TC, Mierdl S, Bauer H, Gutt C, Hanisch E. Transoesophageal echocardiography shows high risk of gas embolism during laparoscopic hepatic resection under carbon dioxide pneumoperitoneum. Br J Surg. 2002;89:870–6. doi: 10.1046/j.1365-2168.2002.02123.x. [DOI] [PubMed] [Google Scholar]
- 54.Elias D, Cavalcanti A, Sabourin JC, et al. Results of 136 curative hepatectomies with a safety margin of less than 10 mm for colorectal metastases. J Surg Oncol. 1998;69:88–93. doi: 10.1002/(sici)1096-9098(199810)69:2<88::aid-jso8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 55.Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg. 2000;231:544–51. doi: 10.1097/00000658-200004000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poon RTP, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function. Ann Surg. 2002;235:373–82. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]