Abstract
Background
Laparoscopic treatment of hydatid disease of the liver can be performed safely in selected patients.
Methods
Six hundred and fifty patients were treated for hydatid disease of the liver between 1980 and 2003 at the Hepatopancreatobiliary Surgery Unit of Istanbul Medical Faculty, Istanbul University. Of these, 60 were treated laparoscopically between 1992 and 2000. A special aspirator-grinder apparatus was used for the evacuation of cyst contents. Ninety-two percent of the cysts were at stages I, II or III according to the ultrasonographic classification of Gharbi.
Results
Conversion to open surgery was necessary in eight patients due to intra-abdominal adhesions or cysts in difficult locations. There was no disease- or procedure-related mortality. Most of the complications were related to cavity infections (13.5%) and external biliary fistulas (11.5%) resulting from communications between the cysts and the biliary tree. There were two recurrences in a follow-up period ranging between 3.5 and 11 years.
Discussion
Laparoscopic treatment of hydatid disease of the liver is an alternative to open surgery in well-selected patients. Important steps are the evacuation of the cyst contents without spillage, sterilization of the cyst cavity with scolicidal agents and cavity management using classical surgical techniques. Our specially designed aspirator-grinder apparatus was safely used to evacuate the cyst contents without causing any spillage. Knowledge of the relationship of the cyst with the biliary tree is essential in choosing the appropriate patients for the laparoscopic technique. In our experience of 650 cases, the biliary communication rate was as high as 18%; half of these can be detected preoperatively. In the remaining, biliary communications are usually detected during or after surgery. Endoscopic retrograde cholangiopancreatography (ERCP) and sphincterotomy are helpful to overcome this problem. As hydatid disease of the liver is a benign and potentially recurrent disease, we advocate the use of conservative techniques in both laparoscopic and open operations.
Keywords: hydatid cysts, liver, laparoscopic treatment, biliary communication, conservative methods
Introduction
Hydatid disease is caused by Echinococcus granulosus and is endemic in many parts of the world, especially in Eastern Europe, the Mediterranean countries, South Africa, South America, Australia, and the Far East 1,2,3. Turkey is also an endemic area for this disease.
The liver is the most frequently affected organ, followed by the lungs. The typical lesion is a cystic cavity, filled with clear hydatid fluid containing live protoscoleces. The cyst increases its size while compressing the liver tissue. Classically, it is believed that the cyst consists of an outer fibrotic layer that is called the ectocyst or the pericyst and an inner layer that is called the endocyst or the germinative membrane from which brood capsules containing protoscoleces proliferate towards the cystic cavity. But in reality, the ectocyst consists of compressed liver cells and fibrotic tissue, which is a host reaction to the presence of the parasite and does not belong to the parasite but to the host liver. Therefore, the only live material, which should be the target of any treatment modality, is the germinative membrane and the fluid it contains together with live and infective protoscoleces or daughter cysts.
The natural history of liver hydatidosis in humans is poorly understood. Different morphological appearances observed by ultrasonography 4,5 indicate that the parasite has its own evolution from birth to death in a human liver. Incidental findings such as a fully calcified (=inactive; dead) cyst in some patients without anysymptoms, suggests that not all hydatid cysts need treatment 6. Studies have also failed to show any correlation between the presence of symptoms and the stage of disease 7,8.
The rationale for elective treatment of a hydatid lesion of the liver is based on the possibility that it may grow and cause symptoms and complications such as infection, jaundice or cholangitis from a biliary communication, rupture and anaphylaxis.
Although two alternative therapies such as medical (albendazole) and percutaneous treatment were introduced in recent years, treatment of hydatid disease of the liver is primarily surgical. With the developments in laparoscopic surgery, there have been various successful attempts to treat hydatid cysts of the liver with the added advantages of this new technique 9,10,11,12,13,14,15,16,17.
Between 1980 and 2003, 650 patients with hydatid cysts of the liver were treated at the Hepatopancreatobiliary Surgery Unit of Istanbul Medical Faculty. Sixty of them were treated laparoscopically between 1992 and 2000. The initial results were reported previously 11,12,13. This paper will discuss controversies in the laparoscopic treatment of the liver hydatid cysts in the light of our experience derived from the management of this large series and that of the literature.
The aim of surgery: can this be achieved by laparoscopy?
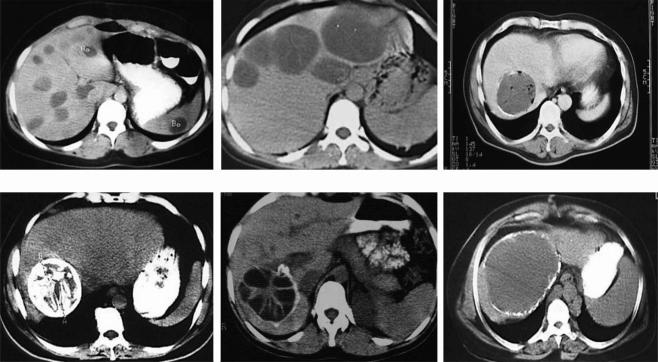
The aims of surgery are complete evacuation of the cyst without spillage, followed by sterilization and obliteration of the cavity. As there are various forms of the disease (Figure 1), a single type of operation cannot be suitable to fulfill all the aims listed above for all kinds of cysts. Therefore many surgical techniques such as simple drainage 18,19, deroofing 20, capitonnage 19, introflexion 21, omentoplasty 22,23, pericystectomy 24,25 and hepatic resection have been performed alone or in combination.
Figure 1. .
Examples of diverse presentations of hepatic hydatidosis.
In general, the laparoscopic approach is a suitable surgical technique to achieve these aims, but there are certain technical problems to overcome. In particular, intraparenchymal cysts, which do not reach the surface of the liver, are difficult to localize and manage laparoscopically. Multiplicity and location of the cysts are two other parameters to be considered while planning treatment. Multiple cysts were encountered in 37% of the cases in our series. Sixty percent of the cysts were located in the right lobe and 19% in the left. In 21% of cases the distribution was bilobar. In these cases, there is an increased possibility of having a cyst in the posterior localization with difficult laparoscopic access. Cysts in an advanced stage, such as those with calcified walls, may cause cavity-related problems after surgery. This problem can be solved with the use of more advanced laparoscopic techniques such as partial cystectomy or pericystectomy, which in return, may increase morbidity. Also, patients with recurrent disease may not be suitable candidates for laparoscopic surgery due to the possibility of dense intra-abdominal adhesions, as well as cysts with preoperatively recognized biliary communications.
Evacuation of the cyst material and prevention of spillage
The first important step in the surgical treatment of the hydatid cyst is the evacuation of the cavity without any spillage. Spillage of hydatid fluid is held responsible for recurrence and has been shown to result in anaphylactic reactions 26,27. The most popular method is to insert a needle into the cyst 16,28,29 for aspiration of the cyst fluid and to replace the same volume with a scolicidal agent. Although it seems to be logical, we know from our experience in open surgery that this has two main disadvantages. First, as there is high pressure in the cyst any attempt to puncture it even with a fine needle may result in a leak around the puncture site. Bickel et al.30 also concluded that simple needle aspiration fails to prevent spillage. Most surgeons take special measures to minimize the effects of this inevitable problem. For example, Khoury et al.14 recommended placing the patient in deep Trendelenburg position, filling the right upper quadrant with 1% cetrimide solution (an effective scolicidal agent) and irrigating the puncture site continuously. Second, the puncture needle gets obstructed very easily by the germinative membrane itself, or by the daughter cysts, necessitating withdrawal that causes further leakage. Ramachandran et al.16 inserted a 12-gauge aspiration needle into the cyst cavity and positioned a 5-mm suction cannula next to the aspirating needle to aspirate any spillage. This maneuver can be helpful in open surgery but might be dangerous during laparoscopy since vision and control over the operative field may be lost with sudden decrease in intra-abdominal pressure. Bickel and Eitan 12 proposed a beveled tip transparent cannula, attached to the surface of the cyst with suction, to evacuate the cyst contents.
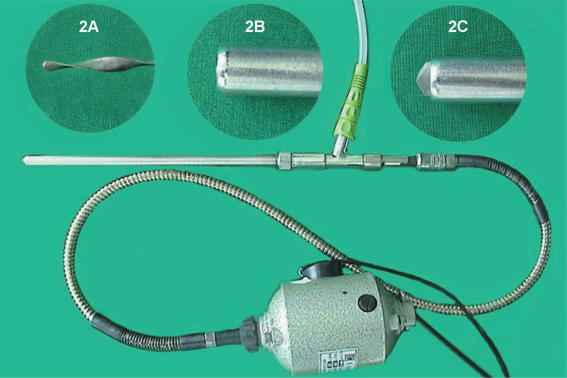
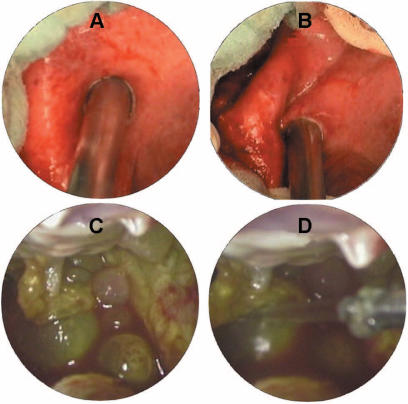
An aspirator-grinder apparatus was developed in our unit 11,12,13, which helps rapid and effective aspiration of the cyst contents, minimizing the possibility of spillage (Figure 2). A rotatable grinder-like device with a driller-tip is positioned in the inner section of the apparatus (Figure 2A), which opens a tight hole in the cyst wall to facilitate the entrance of the cannula into the cyst cavity. Connected to an aspirator for continuous suction, it grinds semi-solid cyst contents (germinative membrane, daughter cysts) (Figure 3) which otherwise may obstruct the aspirator (Figure 4). The apparatus, driven by an electrical spiral motor rotating the grinder, is activated by a foot-pedal. The outer sheath (metal cannula of 10 mm external diameter) is movable with a screw mechanism to bring the driller-tip in and out as needed (Figure 2B, C).
Figure 2. .
The aspirator-grinder apparatus. (A) The inner part (grinder-tike device with a driller-tip); (B) sharp tip in; (C) sharp tip out.
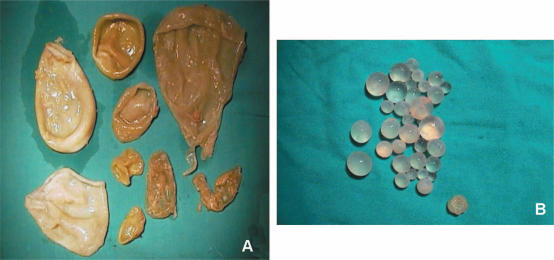
Figure 3. .
Cyst content (A) germinative membranes and (B) daughter cysts; both may obstruct a simple aspirator.
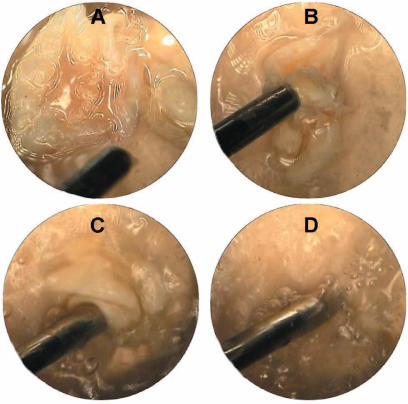
Figure 4. .
(A–D) Ex vivo demonstration of the apparatus performance.
The apparatus is introduced through a 10-mm trocar in the abdomen. At this point, the sharp tip of the inner part is positioned inside the outer sheath (Figure 2B). The tip of the cannula is firmly pressed against the cyst wall and suction is activated. This precaution is necessary to preserve the pneumoperitoneum and to prevent leakage during this step. Another aspirator cannula is kept ready for immediate aspiration if needed. Saline-soaked gauzes may also be placed around the cyst. Under continuous suction against the cyst wall and with the activated rotator, the outer sheath is screwed to bring the tip in contact with the cyst wall to penetrate into the cyst cavity (Figure 5A). Upon entry, the outer sheath is screwed back so that the sharp tip is re-encased in the outer sheath to avoid any damage to the liver parenchyma. The tight hole, active aspiration and grinding of cyst contents enables rapid and effective evacuation without leakage (Figure 5B). The cyst wall is then grasped with a forceps to prevent it from collapsing. If the aspirated material is not stained with bile, multiple irrigation-aspirations of the cavity with scolicidal agents (20% NaCl or 1% povidone iodine) are performed through the lateral inlet of the apparatus. After pulling the apparatus gently back under direct vision, the hole is enlarged with cautery scissors to introduce the telescope into the cavity for further exploration in search of remaining membranes or biliary communications (Figure 5C, D). The management of the residual cavity is achieved by simple drainage, unroofing, pericystectomy or other techniques. A drain is placed in all cavities through the nearest trocar port.
Figure 5. .
(A) The tip of the device, pressed against the cyst wall with continuous suaion. (B) Rapid evacuation of the cyst material and consequent cavity collapse after entry. (C, D) Exploration of the cavity under direct vision (C, daughter cysts in the cavity; D, irrigation).
Another question is whether spillage has any clinical importance. The only possible consequence of spillage might be recurrence at unusual locations such as the perihepatic space or the peritoneum. The fact that most recurrences occur in the liver indicates that the measures taken to prevent spillage are highly effective. Therefore, although spillage is considered a nightmare during hydatid cyst surgery, its relevance in practice is questionable.
An underestimated problem: biliary communications
Biliary communications are reportedly common in hydatid disease with variable frequencies between 3.5% 31 and 19% 32. Therefore, meticulous attention should be paid to their preoperative detection. Careful evaluation of blood biochemistry (cholestasis), radiological findings (bile duct dilatation) and the presence of jaundice or cholangitis in the clinical history of the patients are valuable clues. Patients with suspicion of any biliary communication should be excluded to prevent postoperative complications. Obviously, similar risks for postoperative external biliary fistulas exist with the open surgical techniques as well. But the difference, in our experience, lies in the fact that measures beyond simple suturing may be taken more easily and effectively in an open operation by the average general surgeon who may not be proficient in advanced laparoscopic techniques. In our whole series of 650 cases with hydatid disease of the liver, the frequency of biliary communication was 18%.
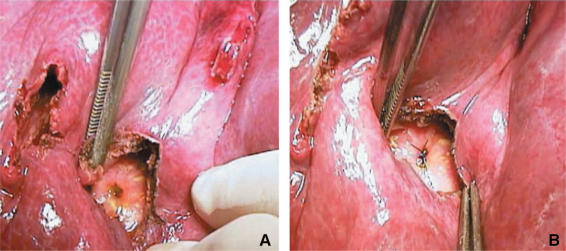
Two kinds of biliary communications can be differentiated: complicated and simple. Complicated biliary communication denotes the presence of rupture on both sides, namely on the bile duct and the membrane of the cyst. This situation causes a flow from a high-pressure zone (the cyst itself) towards to a low-pressure zone (the biliary system). If some particles such as a piece of membrane or small daughter cysts enter the biliary tree, this usually causes various degrees of cholestasis, biliary dilatation, marked jaundice and/or cholangitis. This situation, also called ‘frank intrabiliary rupture’ 33, can and should be diagnosed preoperatively based on clinical history and biochemical/ultrasonographic findings to design an appropriate treatment plan. Complicated biliary communications were found in 10% of our series and were one of the main reasons for the exclusion of these patients from laparoscopic treatment. Kornaros and Aboul-Nour 33 also found that 13% of their 208 patients with hydatid cysts of the liver had complicated biliary communications. Simple biliary communication indicates a rupture on the extended bile duct, with an intact membrane of the cyst. The assumption that it is characterized by the presence of bile in the cyst in otherwise normal findings may not be totally correct. Only half of our patients with simple biliary communication (8%) were diagnosed during surgery by the finding of a bile-stained cyst material or bile itself in the cavity after decompression of the cyst. But in the remaining 4% of the cases, although there was no evidence of a biliary communication before and during surgery, it became evident by the presence of bile in the drain immediately after surgery or on postoperative day one. This may explain the 11.5% (6 of 52 patients) biliary fistula rate in the laparoscopically treated patients in our series. It is believed that centrally located large cysts are more prone to this complication than the peripherally located smaller cysts. However, counterintuitive cases do occur (Figure 6). This complication may be solved with early endoscopic nasobiliary stenting or, as we prefer, with endoscopic sphincterotomy 34. In contrast to the opinions against the routine use of a closed suction drainage catheter 14,35, our experience in surgical treatment of hydatid disease of the liver urges us to routinely use short-term drainage in all cases.
Figure 6. .
A biliary communication in a peripherally located small cyst (open surgery). (A) Opening of a bile duct into the cavity; (B) an absorbable suture can prevent formation of a biliary fistula.
The role of medical treatment
Although some controversies exist regarding the efficacy of the albendazole treatment in liver hydatid disease 36, its use is routine in our unit for disseminated disease, inoperable cases and prophylaxis before surgery to sterilize the cyst and to minimize the effects of potential spillage during the operation 37,38,39. Nahmias et al.40 showed in their study that 800 mg albendazole/day in four 28-day courses separated by 14-day drug-free intervals resulted in cure in 41% of the cases (cysts disappeared) in a follow-up period of 3–7 years. Our aim is to start at least 10 days before any intervention and continue 3 months postoperatively at a dose of 10 mg/kg. The patient is kept under control for possible side effects, which sometimes require cessation of treatment. For prolonged use, intermittent treatment with 3–4-week courses and 1-week drug-free intervals is recommended.
Conservative vs radical surgery
Surgical methods to obliterate the cyst cavity can be separated as conservative (simple drainage, unroofing, introflexion, omentoplasty) or radical procedures (partial cystectomy, pericystectomy, hepatic resections). Although all these procedures have their own proponents, none of them can be considered as gold standard. A tailored approach is required in each patient due to variations in size, multiplicity, location and associated complications. Some authors advocate radical procedures, mainly pericystectomy 41,42. For this, usually smaller and peripherally located cysts are chosen. On the other hand, conservative methods are recommended in difficult cases. After performing an ‘easy’ laparoscopic pericystectomy, Sever and Skapin 10 recommended this technique as a less traumatic operation. Others find radical procedures time-consuming with increased blood loss and not justifiable for a benign disease 16,18. Dziri et al. found that intraoperative vascular injuries occurred more frequently in association with pericystectomy 43. In our approach, radical surgery is also employed only in selected cases. Pericystectomy should be considered a nonanatomical and potentially bloody hepatic resection. Its main advantage in open surgery is that it can be performed without opening the cyst cavity, thus avoiding the problems of spillage and cavity management. Manterola et al.17 performed eight pericystectomies laparoscopically without any complications. This successful result may be due to patient selection and small numbers of patients reported. The authors’ technique of evacuating the cyst before pericystectomy is controversial. This approach neutralizes the main advantages of pericystectomy. But, on the other hand, laparoscopic pericystectomy without prior evacuation of the cyst contents similar to the open surgical technique would carry the risk of perforation and dissemination of disease during dissection.
Considering that recurrence rates after operation for liver hydatid cysts range from 8% to 20% 44 especially in endemic areas, multiple operations may be necessary during the course of this benign disease. Thus, conservative procedures should be considered as the first choice in surgical treatment. Resectional procedures should only be performed in a limited number of well-selected patients by either the open or the laparoscopic approach.
Complications
In classical open surgery, overall postoperative mortality ranges between 0% 18 and as high as 7.5% 24 in the early period. Morbidity is also observed in 12–26% 45,46 of the cases. Dziri et al.43 found an overall complication rate of 33% in their multicenter, prospective, randomized trial. Yorganci and Sayek 41 have reported an even higher complication rate of 40%, which in their explanation was due to the presence of a higher number of complicated cases in their series.
Laparoscopic treatment of the hepatic cysts should not be regarded as a new surgical technique. Rather, it is a new and minimally invasive access to perform established surgical techniques. As is the case for other laparoscopic operations, laparoscopic hydatid surgery follows the basic surgical principles of treating hydatid cysts of the liver: evacuation of the live cyst content, prevention of spillage, sterilization of the cavity with scolicidal agents, and management of the residual cavity. It certainly eliminates the disadvantages of a surgical incision and shortens the hospital stay markedly. However, laparoscopy does not have the potential to eliminate disease-related complications.
Most of the reports on laparoscopic treatment of hydatid disease of the liver consist of case reports or small series 12,16,17,47,48,49,50 oriented to publish successful results with this technique. For example, Ramachandran et al.16 reported a series of six laparoscopically treated hydatid cysts with no complications. Similarly, Manterola et al.17 reported only one simple biliary communication in eight laparoscopic pericystectomy patients as a complication. Saglam 50 treated 11 cysts in 6 patients laparoscopically. Only in one patient was conversion to open surgery necessary due to severe intraoperative venous bleeding.
Comparing the complication rates of the two techniques, (surgery and laparoscopy) the difference detected in favor of the laparoscopic approach is likely to be misleading. This may be largely due to the limited number of patients treated so far by the new technique, as well as adherence to strict patient selection criteria protecting laparoscopy in the early phase of its development.
In our series of 60 patients, conversion to open surgery was necessary in 13% (8/60) due to difficult location of the cysts (intraparenchymal and/or posterior) or dense intra-abdominal adhesions. There was no disease- or procedure-related mortality. The most frequent complications in the postoperative period were cavity infection in 7 (13.5%) and biliary fistula in 6 (11.5%) patients. Most of these complications were treated with conservative procedures such as percutaneous drainage and ERCP. Complications can only be avoided with experience and better patient selection. With these measures we have been able to decrease the conversion rate to open surgery (7:1) and lower the frequency of postoperative biliary (4:2) and infectious complications (7:2).
Conclusion
Laparoscopic management of hydatid cysts of the liver can be performed safely and successfully with certain precautions. Careful patient selection is mandatory to achieve successful results. It is also important that surgeons are familiar with the surgical treatment of this disease as well as the laparoscopic techniques. Special devices are helpful to overcome technical problems. It must be borne in mind that hydatid disease of the liver is a benign disease and an indolent problem, despite the 10% risk of recurrence. Therefore, the approach should be safe and adequate for the particular patient.
References
- 1.Mentes A. Hydatid liver disease: a perspective in treatment. Dig Dis. 1994;12:150–60. doi: 10.1159/000171448. [DOI] [PubMed] [Google Scholar]
- 2.Meyers WC, Kim RD, Chari RS. Townsend CM, Beauchamp RD, Evers BM, Mattox KL. WB Saunders; Philadelphia: 2001. Echinococcal cysts, Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice; pp. 1053–5. [Google Scholar]
- 3.Sayek I, Yalin R, Sarac Y. Surgical treatment of hydatid disease of the liver. Arch Surg. 1980;115:847–50. doi: 10.1001/archsurg.1980.01380070035007. [DOI] [PubMed] [Google Scholar]
- 4.Gharbi HA, Hassine W, Brauner M, Dupuch K. Ultrasound examination of the hydatid liver. Radiology. 1981;139:459–63. doi: 10.1148/radiology.139.2.7220891. [DOI] [PubMed] [Google Scholar]
- 5.Caremani M, Benci A, Maestrini R, Rossi G, Menchetti D. Abdominal cystic hydatid diseases (CHD): classification of sonographic appearance and response to treatment. J Clin Ultrasound. 1996;24:491–500. doi: 10.1002/(SICI)1097-0096(199611/12)24:9<491::AID-JCU1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Frieder B, Larrieu E, Odriozola M. Long term outcome of asymptomatic liver hydatidosis. J Hepatology. 1999;30:228–31. doi: 10.1016/s0168-8278(99)80066-x. [DOI] [PubMed] [Google Scholar]
- 7.Macpherson CNL, Zeyhle E, Romig T, Rees PH, Were JB. Portable ultrasound scanner versus serology in screening for hydatid cysts in a nomadic population. Lancet. 1987;ii:259–61. doi: 10.1016/s0140-6736(87)90839-7. [DOI] [PubMed] [Google Scholar]
- 8.Frider B, Losada C, Larrieu E, Zavaleta O. Asymptomatic abdominal hydatidosis detected by ultrasonography. Acta Radiol. 1988;29:421–34. [PubMed] [Google Scholar]
- 9.Mompean JAL, Paricio PP, Campos RR, Ayllon JG. Laparoscopic treatment of a liver hydatid cyst. Br J Surg. 1993;80:907–8. doi: 10.1002/bjs.1800800736. [DOI] [PubMed] [Google Scholar]
- 10.Sever M, Skapin S. Laparoscopic pericystectomy of liver hydatid cyst. Surg Endosc. 1995;9:1125–6. doi: 10.1007/BF00189002. [DOI] [PubMed] [Google Scholar]
- 11.Alper A, Emre A, Hazar H, et al. Laparoscopic surgery of hydatid disease: initial results and early follow-up of 16 patients. World J Surg. 1995;19:725–8. doi: 10.1007/BF00295914. [DOI] [PubMed] [Google Scholar]
- 12.Bickel A, Eitan A. The use of a large, transparent cannula, with beveled tip, for safe laparoscopic management of hydatid cysts of liver. Surg Endosc. 1995;9:1304–5. doi: 10.1007/BF00190166. [DOI] [PubMed] [Google Scholar]
- 13.Alper A, Emre A, Acarli K, et al. Laparoscopic treatment of hepatic hydatid disease. J Laparoendosc Surg. 1996;6:29–33. doi: 10.1089/lps.1996.6.29. [DOI] [PubMed] [Google Scholar]
- 14.Khoury G, Jabbour-Khoury S, Bikhazi K. Results of laparoscopic treatment of hydatid cysts of the liver. Surg Endosc. 1996;10:57–9. doi: 10.1007/s004649910014. [DOI] [PubMed] [Google Scholar]
- 15.Verme GR, Bose SM. Laparoscopic treatment of hepatic hydatid cyst. Surg Laparosc Endosc. 1998;8:280–2. [PubMed] [Google Scholar]
- 16.Ramachandran CS, Goel D, Arora V. Laparoscopic surgery in hepatic hydatid cysts: a technical improvement. Surg Laparosc Endosc Percutan Tech. 2001;11:14–8. [PubMed] [Google Scholar]
- 17.Manterola C, Fernandez O, Munoz S, et al. Laparoscopic pericystectomy for liver hydatid disease: description of results observed during a long follow-up period. Surg Endosc. 2002;16:521–4. doi: 10.1007/s00464-001-8125-7. [DOI] [PubMed] [Google Scholar]
- 18.Karavias DD, Vagianos CE, Bouboulis N, et al. Improved techniques in the surgical treatment of hepatic hydatidosis. Surg Gynecol Obstet. 1992;174:176–80. [PubMed] [Google Scholar]
- 19.Romero-Torres R, Campbell JR. An interpretive review of the surgical treatment of hydatid disease. Surg Gynecol Obstet. 1965;121:851–64. [PubMed] [Google Scholar]
- 20.Sayek I, Yalin R, Sarac Y. Surgical treatment of hydatid disease of the liver. Arch Surg. 1980;115:847–50. doi: 10.1001/archsurg.1980.01380070035007. [DOI] [PubMed] [Google Scholar]
- 21.Ariogul O, Emre A, Alper A, Uras A. Introflexion as a method of surgical treatment for hydatid disease. Surg Gynecol Obstet. 1989;169:356–8. [PubMed] [Google Scholar]
- 22.Papadimitriou J, Mandrekas A. The surgical treatment of hydatid disease of the liver. Br J Surg. 1970;57:431–3. doi: 10.1002/bjs.1800570607. [DOI] [PubMed] [Google Scholar]
- 23.Pissidis A, Mandrekas A, Papadimitriou J, Androulakis J. The use of omentoplasty in the surgical treatment of hydatid disease of the liver. Bull Soc Intern Chir Belg. 1974;33:498–502. [PubMed] [Google Scholar]
- 24.Kayabali I. Sur la chirurgie des kystes hydatiques du foie. Lyon Chir. 1971;67:327–9. [PubMed] [Google Scholar]
- 25.Belli L, Del Favero E, Marni A, Romani F. Resection versus pericystectomy in the treatment of hydatidosis of the liver. Am J Surg. 1983;145:239–42. doi: 10.1016/0002-9610(83)90070-3. [DOI] [PubMed] [Google Scholar]
- 26.Gonzales EM, Selas PR, Martiner B, Garcia G, Carazo FP, Pascual MH. Results of surgical treatment of hepatic hydatidosis: current therapeutic modifications. World J Surg. 1991;15:254–60. doi: 10.1007/BF01659061. [DOI] [PubMed] [Google Scholar]
- 27.Khuroo MS, Zargar SA, Mahajan R. Echinococcus granulosus cysts of the liver: management with percutaneous drainage. Radiology. 1991;180:141–5. doi: 10.1148/radiology.180.1.2052682. [DOI] [PubMed] [Google Scholar]
- 28.Yucel O, Talu M, Unalmiser S, Ozdede S, Gurkan A. Videolaparoscopic treatment of liver hydatid cyst with partial cystectomy and omentoplasty. Surg Endosc. 1996;10:434–6. doi: 10.1007/BF00191634. [DOI] [PubMed] [Google Scholar]
- 29.Khoury G, Abiad F, Geagea T, Nabout G, Jabbour S. Laparoscopic treatment of hydatid cysts of the liver and spleen. Surg Endosc. 2000;4:243–5. doi: 10.1007/s004640000048. [DOI] [PubMed] [Google Scholar]
- 30.Bickel A, Daud G, Urbach D, Lefler E, Barasch EF, Eitan A. Laparoscopic approach to hydatid liver cysts: is it logical? Physical, experimental, and practical aspects. Surg Endosc. 1998;12:1073–7. doi: 10.1007/s004649900783. [DOI] [PubMed] [Google Scholar]
- 31.Bilge A, Sozuer EM. Diagnosis and surgical treatment of hepatic hydatid disease. HPB Surg. 1992;6:57–64. doi: 10.1155/1992/41956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berrada S, Essadki B, Zerouali NO. Kyste hydatique du foie. Traitement par resection du dome saillant. Notre experience a propos d'une serie de 495 cas. Arm Chir. 1993;47:510–12. [PubMed] [Google Scholar]
- 33.Kornaros SE, Aboul-Nour TA. Frank intrabiliary rupture of hydatid hepatic cyst: diagnosis and treatment. J Am Coll Surg. 1996;183:466–70. [PubMed] [Google Scholar]
- 34.Tekant Y, Bilge O, Acarli K, Alper A, Emre A, Ariogul O. Endoscopic sphincterotomy in the treatment of postoperative biliary fistulas of hepatic hydatid disease. Surg Endosc. 1996;10:909–11. doi: 10.1007/BF00188481. [DOI] [PubMed] [Google Scholar]
- 35.Demirci S, Eraslan S, Anadol E, Bozatli L. Comparison of the results of different surgical techniques in the management of the hydatid disease of the liver. World J Surg. 1989;13:88–91. doi: 10.1007/BF01671161. [DOI] [PubMed] [Google Scholar]
- 36.Chudhuri G, Prasad R, Tantry BV, et al. Poor response to long term albendazole therapy of hydatid liver cysts. Scand J Infect Dis. 1989;21:323–5. doi: 10.3109/00365548909035703. [DOI] [PubMed] [Google Scholar]
- 37.Horton RJ. Chemotherapy of Echinococcus infection in man with albendazole. Trans R Soc Trop Med Hyg. 1989;83:97–102. doi: 10.1016/0035-9203(89)90724-4. [DOI] [PubMed] [Google Scholar]
- 38.Todorov T, Vutova K, Mechkov G, et al. Chemotherapy of human cystic echinococcosis: comparative efficacy of mebendazole and albendazole. Arm Trop Med Parasitol. 1992;86:59–62. doi: 10.1080/00034983.1992.11812631. [DOI] [PubMed] [Google Scholar]
- 39.Cakmakci M, Sayek I. Prophylactic effect of albendazole in experimental peritoneal hydatidosis. Hepatogastroenterology. 1992;39:424–6. [PubMed] [Google Scholar]
- 40.Nahmias J, Goldsmith R, Soibelman M, El-On J. Three- to 7-year follow-up after albendazole treatment of 68 patients with cystic echinococcosis (hydatid disease) Arm Trop Med Parasitol. 1994;88:295–304. doi: 10.1080/00034983.1994.11812870. [DOI] [PubMed] [Google Scholar]
- 41.Yorganci K, Sayek I. Surgical treatment of hydatid cysts of the liver in the era of percutaneous treatment. Am J Surg. 2002;184:63–9. doi: 10.1016/s0002-9610(02)00877-2. [DOI] [PubMed] [Google Scholar]
- 42.Belli L, Aseni P, Rondinara GF, Bertini M. Improved results with pericystectomy in normothermic ischemia for hepatic hydatidosis. Surg Gynecol Obstet. 1986;163:127–32. [PubMed] [Google Scholar]
- 43.Dziri C, Paquet JC, Hay JM, et al. and the French Association for Surgical Research. Omentoplasty in the prevention of deep abdominal complications after surgery for hydatid disease of the liver: a multicenter, prospective, randomized trial. J Am Coll Surg. 1999;188:281–9. doi: 10.1016/s1072-7515(98)00286-5. [DOI] [PubMed] [Google Scholar]
- 44.Little JM, Hollands MJ, Ekberg H. Recurrence of hydatid disease of the liver. World J Surg. 1988;12:700–4. doi: 10.1007/BF01655892. [DOI] [PubMed] [Google Scholar]
- 45.Barros JL. Hydatid disease of the liver. Am J Surg. 1978;135:597–600. doi: 10.1016/0002-9610(78)90043-0. [DOI] [PubMed] [Google Scholar]
- 46.Rakas FS, El Mufti M, Mehta PM, et al. Omentoplasty or tube drainage for the management of the residual cavity following the removal of an hepatic hydatid cyst. Hepatogastroenterology. 1990;37:55–7. [PubMed] [Google Scholar]
- 47.Guibert L, Gayral F. Laparoscopic pericystectomy of a liver hydatid cyst. Surg Endosc. 1995;9:442–3. doi: 10.1007/BF00187171. [DOI] [PubMed] [Google Scholar]
- 48.Marks J, Mouiel J, Katkhouda N, Gugenheim J, Fabiani P. Laparoscopic liver surgery: a report on 28 patients. Surg Endosc. 1998;12:33–4. doi: 10.1007/s004649900664. [DOI] [PubMed] [Google Scholar]
- 49.Rogiers X, Bloechle C, Broelsch C. Safe decompression of hepatic hydatid cyst with laparoscopic surgiport. Br J Surg. 1995;82:1111. doi: 10.1002/bjs.1800820834. [DOI] [PubMed] [Google Scholar]
- 50.Saglam A. Laparoscopic treatment of liver hydatid cysts. Surg Laparosc Endosc. 1996;6:16–21. [PubMed] [Google Scholar]