Abstract
Background
Liver resection is reputed to be one of the most difficult procedures embraced in laparoscopy. This report shows that with adequate training, anatomical liver resection including major hepatectomies can be performed.
Methods
This is a retrospective study.
Results
From 1995 to 2004, among 84 laparoscopic liver resections, 46 (54%) anatomical laparoscopic hepatectomies were performed in our institution by laparoscopy. Nine (20%) patients had benign disease while 37 (80%) had malignant lesions. Among those with malignant lesions, 14 patients had hepatocellular carcinoma (HCC), 18 had colorectal metastasis (CRM), while 5 had miscellaneous tumours. For benign disease, minor (two Couinaud's segments or less) and major anatomic hepatectomies were performed in five and four patients, respectively. For malignant lesions, minor and major anatomic hepatectomies were performed in 15 and 22 patients, respectively. Overall, conversion to laparotomy was necessary in 7 (15%) patients. Blood transfusion was required in five (10%) patients. One patient died of cerebral infarction 8 days after a massive peroperative haemorrhage. The overall morbidity rate was 34% whatever the type of resection. Three patients required reoperation, either for haemorrhage (n=1) and/or biliary leak (n=2). For CRM (n=18), overall and disease-free survival at 24 months (mean follow-up of 17 months) were 100% and 56%, respectively. For HCC (n=14), overall and disease-free survival at 36 months (mean follow-up of 29 months) were 91% and 65%, respectively. No port site metastasis occurred in patients with malignancy.
Conclusions
After a long training with limited liver resection in superficial segments, laparoscopic anatomical minor and major resections are feasible. Short-term carcinological results seem to be similar to those obtained with laparotomy.
Introduction
Liver surgery is reputed to be one of the most difficult fields of digestive surgery and necessitates a long educational investment. This investment has to be even more extensive to obtain an adequate training in liver laparoscopic resection. However, despite these technical obstacles, the liver is a suitable organ for laparoscopy because of its deep location and, most of the time, the absence of need for reconstruction after hepatectomy. So, thanks to our double experience in laparoscopy and liver surgery, since 1995 we have explored the interest of laparoscopic liver surgery. This report describes our results concerning laparoscopic anatomical liver resection which best summarizes our very important educational investments in this field.
Materials and Methods
Selection of patients
Between January 1995 and January 2004, all patients admitted to our department for benign or malignant liver lesions were routinely assessed for laparoscopic resection. The decision to perform the operation laparoscopically was based on patient demographics, as well as on tumor and liver characteristics. Patient demographics included age, ASA score, previous abdominal surgery, associated surgical and/or medical pathologies. Tumor characteristics included their number, their size, their location, situation of hepatic veins with respect to the tumor and the suspected preoperative histological diagnosis. Liver-related variables included liver function tests, anatomical variations (especially of the hepatic vessels), and liver volume. Suspicion of cirrhosis, based on clinical data and/or morphological abnormalities, most often confirmed by liver biopsy, was always investigated.
The projected remnant liver volume, assessed with preoperative CT scan, was always >30% of the total liver volume. Whenever indicated, portal vein embolization was performed at least 3 weeks before surgery to increase the size of the opposite lobe before resection. Since 2000, portal vein embolization has been performed routinely before major hepatic resections in all Pugh-Child class A or B cirrhotic patients, irrespective of the projected remnant liver volume.
Indications for resection were similar to those in traditional surgery. Hepatic resection for benign disease was considered only in case of symptomatic disease and/or for uncertain diagnosis on biopsy specimens. Resection of metastatic deposits was entertained only in the absence of peritoneal carcinomatosis and/or inextirpable extra-hepatic localizations. Resection of hepatocellular carcinoma (HCC) was envisioned when there were no more than two nodules, without tumoral portal invasion involving the portal convergence, irrespective of the diameter of the lesions.
Exclusion criteria for laparoscopic resection included suspicion of gallbladder carcinoma, decompensated cirrhosis, and cardiac or respiratory failure. Until now, laparoscopic resection was excluded when there was a need for venous or biliary reconstruction.
All patients were informed of the innovative nature of the procedure and gave informed consent.
Surgical technique
Liver resections were defined according to Couinaud's segmental anatomical classification. Deep segments included segments 1, 4a, 7, and 8, while superficial segments included segments 2, 3, 4b, 5, and 6. Resection was considered ‘anatomic’ when at least one entire segment was removed, all other resections were defined as nonanatomic or wedge resections. Left lobectomy was defined as resection of segments 2 and 3. Extended right hepatectomy was defined as a right hepatectomy (removal of segments 5, 6, 7, 8) plus segment 4.
In all types of resections, the patient was placed supine, in the reverse Trendelenburg position, with the lower limbs spread apart. For right-sided resections, a foam pad was placed under the patient's right flank. A wide-screen monitor was located to the right of patient's head. A voice-controlled robot supporting a 0°, 10-mm laparoscope was used in all cases. The operating surgeon stood between the legs of the patient with one assistant on the left side of the patient.
After establishing carboperitoneum, abdominal pressure was monitored and maintained between 10 and 12 mmHg. The optical device was inserted through a 10-mm trocar introduced into the abdomen 3 cm above the umbilicus either on the mid-clavicular line or on the abdominal midline for right or left liver resections, respectively. A 12-mm trocar was then inserted, 10 cm above the initial trocar, slightly to the right, for the ultrasound probe. Four 5-mm trocars were disposed approximately in a square formation, centered on the optical trocar. According to the sites of dissection, the upper or the lower three trocars were used for the optical and operative devices: most often, the two lower trocars were operating ports, while the upper right 5-mm port was used for the smooth liver retractor, the assistant working through the left port. The 12-mm port was also used for the stapling device as needed.
The initial step was visual and ultrasound exploration of the liver. In case of malignancy, the abdomen was inspected for carcinomatosis and/or malignant lymph nodes. Once resection was decided, a vascular tape was placed around the hepatic pedicle should the Pringle maneuver become necessary. The lesser omentum was checked for the presence of a left hepatic artery.
For right resection, the cystic duct and artery were divided but the gallbladder itself was not completely removed to help mobilization of the liver as necessary. The hepatic pedicle was dissected from bottom to top, starting by locating the main bile duct including the biliary convergence. Portal and arterial branches anterior to the biliary tract were clipped and divided. The ipsilateral triangular ligament was divided, as high as possible for right hepatectomy, while the assistant elevated the liver with a retractor. The inferior vena cava was then dissected from bottom to top and the small retrohepatic veins were managed with bipolar coagulation or clips reinforced by stitches. The hepatic vein was dissected free last but not divided before parenchymal division. Whenever the right hepatic vein was difficult to approach extrahepatically, the vein was divided directly through the liver parenchyma. Hepatic transection was initiated according to the line of devascularization visualized on Glisson's capsule, directed to the axis of the previously located inferior vena cava, and whenever possible by the so-called Belghiti's maneuver 1 (i.e. opposite traction on a tape placed between the liver and the vena cava, between the right and the middle hepatic vein). Exposure, hemostasis, and parenchymal division were performed with the ultrasonic scissors held in the surgeon's dominant hand and the bipolar electrocoagulation instrument in the other, as necessary. Larger structures were secured with clips, ligatures, or stitches. The hepatic vein was divided with the linear stapler at the end of the hepatic resection. At the end of procedure and notably to divide the hepatic vein, a ‘HandPort’ system could be used to introduce the hand in the abdomen. This allowed the surgeon to mobilize the specimen, secure the procedure and extract the liver in some difficult cases of big liver and/or deep patient 2.
The resected specimen was always placed in a plastic bag and withdrawn through an infra-umbilical midline, a suprapubic horizontal incision or by a short subcostal incision used for the HandPort system. Bile leaks were searched for by a transcystic tube air-tightness test and/or an operative cholangiography. The surgical field was irrigated with serum or iodine solution in case of malignancy. Abdominal drainage was usually omitted and pneumoperitoneum was vented through the trocars in place.
Criteria studied
Intraoperative patient data included duration of surgery (overall and the clamping period), associated procedures, surgical mishaps, and necessity of peri-operative blood transfusion. Postoperative data included the largest diameter of specimen, size and number of each lesion, width of margins in case of malignancy, pathology reports, all medical or surgical postoperative complications, and length of hospital stay. For cancer, overall and disease-free survival at 12 and 36 months were analysed according to the Kaplan-Meyer method.
Results
Preoperative results
From January 1995 to January 2004, 180 liver resections were performed in the Montsouris Institute. Of these, a laparoscopic approach was attempted in 84 (46%) patients to perform a nonanatomical liver resection in 38 patients (28 wedge resections and 10 cyst resections) and an anatomical liver resection in 46 patients, the only ones analysed in this study. In all, 20% of the patients were ASA I, 56% ASA II, and 22% ASA III. Previous abdominal surgery had been performed in nine (22.5%) patients, including laparoscopic colorectal resection in five and hepatectomy by laparotomy in two.
Nine (20%) patients had benign disease while 37 (80%) had malignant lesions (Table 1). As regards the benign lesions, resections were performed with diagnostic intent in seven patients while two patients underwent resection for symptomatic hydatic cysts. Of the 14 patients with HCC, six had cirrhosis, four had fibrosteatosis, and four had normal livers. All patients had single nodules, with a median diameter of 6.7 cm (range 1–18). These patients had been treated preoperatively by chemoembolization (n=2) or alcohol injection (n=2). One of these patients had a tumoral thrombosis of the portal vein that partially reached the right portal branch. Of the 18 patients with colorectal metastases (CRM), 8 were metachronous (among them, 1 patient had resectable pulmonary metastases) while 10 were synchronous. Of these 10 patients, 2 had a laparoscopic colorectal resection performed during the same procedure, always before the liver resection. The median number of metastases per patient was 2 (range 1–4) with a median diameter of 3.5 cm (range 1.2–12). The other malignant lesions treated included intrahepatic cholangiocarcinoma (n=1), metastasis of a neuroendocrine tumor of the rectum (1), and metastasis originating from adenocarcinoma of Vater's ampulla (1), the pancreas (1), and ovary (1).
Table 1. Histological diagnosis.
| Histological diagnosis | n |
|---|---|
| Benign lesions | 9 (20%) |
| Focal nodular hyperplasia | 6 |
| Hydatid cyst | 2 |
| Biliary hamartoma (Von Mayenburg complex) | 1 |
| Malignant lesions | 37 (80%) |
| Hepatocellular carcinoma | 14 |
| Intrahepatic cholangiocarcinoma | 1 |
| Colorectal metastasis | 18 |
| Neuroendocrine tumor metastasis | 1 |
| Non-neuroendocrine noncolorectal metastasis | 3 |
| Total | 46 |
Preoperative liver biopsies were performed in 15 (34%) patients, notably to distinguish between liver adenoma and nodular focal hyperplasia in 2 patients. Preoperative diagnosis of liver cirrhosis was confirmed in 4 of 15 patients assessed. In all, six (13%) patients had liver cirrhosis (Child A). Preoperative portal vein embolization was performed in four (8%) patients, three before right hepatectomy for HCC in a pathologic liver and one before extended right hepatectomy for CRM in a noncirrhotic liver.
Intraoperative results
Of nine patients with benign disease, minor anatomic hepatectomy was performed in five patients, and a major anatomic hepatectomy in four patients. These resections concerned at least one deep segment in all patients in major hepatectomy and in no patients in minor hepatectomy.
Of 37 patients with malignant lesions, minor anatomic hepatectomy was performed in 15 patients and a major hepatectomy in 22 patients. These resections concerned at least one deep segment in all patients in major hepatectomy and in nine patients in minor hepatectomy.
Associated non-liver-related major surgical procedures were performed in 7 (15%) of 46 patients (proctectomy, left colectomy, small bowel dissection, and diaphragmatic localized resection).
The procedure was completed laparoscopically in 39 patients. Conversion to laparotomy was necessary in seven patients (15%) because of tight adhesions in two patients, because of an inadvertent opening of the diaphragm and because of peroperative hemorrhage in four patients. Details concerning the type of liver resections are summarized in Table 2. The Pringle maneuver (<30 min of intermittent portal triad clamping in each case) was used in seven patients, during segment 5–6 bisegmentectomy in one case, and during right hepatectomy in six cases. Blood transfusion was necessary intraoperatively in five patients during right hepatectomy. There were no signs suggestive of gas embolism in any of the patients.
Table 2. Types of liver resections performed.
| Resections | n |
|---|---|
| Minor anatomic liver resection | 20 (43%) |
| Segmentectomy 2 | 2* |
| Segmentectomy 3 | 1 |
| Segmentectomy 4 | 1* |
| Segmentectomy 6 | 5 |
| Segmentectomy 7 | 1 |
| Bisegmentectomy 2 and 3 | 7 |
| Bisegmentectomy 5 and 6 | 1 |
| Bisegmentectomy 6 and 7 | 1* |
| Major liver resections | 26 (60%) |
| Right hepatectomy | 19* |
| Left hepatectomy | 1 |
| Extended right hepatectomy | 3 |
| Bisegmentectomy 2 and 3 extended to 4a | 1 |
| Trisegmentectomy 5 + 6+7 | 1 |
| Trisegmentectomy 4a + 6+5 | 1 |
| Total | 64 |
*Resection performed on a cirrhotic liver.
For 26 major anatomical hepatectomies (19 right hepatectomies, 3 extended right hepatectomies, 1 left hepatectomy and 3 resections of more than 2 segments), median surgical time was 365 (210–515) min. For 20 minor procedures, median surgical time was 205 (95–480) min. Excluding associated non-liver-related major surgical procedures, median surgical time was 360 (210–480) min for major hepatectomy and 190 (95–360) min for minor hepatectomy.
Postoperative results
There was one postoperative death. This death occurred 8 days after a right hepatectomy for HCC complicated by a massive peroperative hemorrhage necessitating a conversion to laparotomy. The patient died because of cerebral infarction without any other organ failure. The postoperative complication rate was 34% for all patients (16/46). Excluding patients with liver-related major associated procedures, this rate was 23% (9/39). Regardless of the associated procedures, the postoperative complication rate was 34% after major anatomical resection (9/26) and 35% after minor anatomical resection (7/20) (Table 3). Overall, three patients required reoperation because of a biliary leak on the hepatic raw surface following respectively a left lobectomy and an extended right hepatectomy and because of a postoperative hemorrhage after a segmentectomy 7. Thus, the reoperation rate was 6% (3/46).
Table 3. Postoperative data.
| Overall (n = 46) | Series WMA (n = 39) | Minor resection (n = 20) | Major resections (n = 26) | |
|---|---|---|---|---|
| Mortality | 1 (2%) | 1 (2.5%) | – | 1 (3%) |
| Morbidity | 16 (34%) | 9 (23%) | 7 (35%) | 9 (34%) |
| Hemorrhage* (1) | Wall abscess (1) | |||
| Biliary leak* (2) | Subphrenic collection (4) | |||
| Infected collection (1) | Lethal hemorrhage (1) | |||
| Subphrenic collection (2) | Biliary leak (1) | |||
| Ileus (2) | Pleural effusion (1) | |||
| Ileus (1) | ||||
| Reoperation | 2 (3%) | 2 (4%) | 2(10%) | 1 (3%) |
| Hemorrhage (1) | Biliary leak (1) | |||
| Biliary leak (1) |
Series WMA, overall patients without patients with major associated procedure.
*Reoperation.
Two patients required medical treatment and repeated abdominal taps for ascites, in one case after extended right hepatectomy performed without preoperative portal vein embolization in a fibrous liver and in the other case after right hepatectomy in a cirrhotic liver, after preoperative embolization. Another patient presented a partially resolved vascular cerebral stroke during an involuntary catheterization of the carotid artery before an uncomplicated right hepatectomy for CRM.
The overall median hospital stay was 10 (3–36) days. Excluding those patients with major associated procedures, the median hospital stay was 9 (3–36) days. Details concerning the duration of hospital stay according to the type of resection are summarized in Table 4. The median hospital stay was 8 days (range 3–21) after minor hepatectomy and 11 days (range 6–36) after major hepatectomy.
The median width of specimen margins was 10 mm (range 1–40) in CRM and 11 mm (range 1–50) in HCC.
For the 37 patients with malignant lesions, mean follow-up was 22 months and no port site metastasis has been noted.
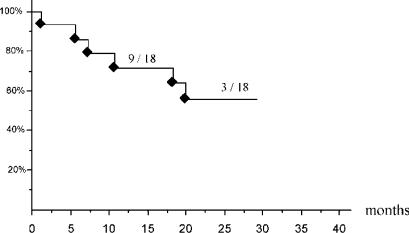
In CRM (n=18), with a mean follow-up of 17 months, overall survival at 24 months was 100%. Disease-free survival at 12 and 24 months was 71% and 56%, respectively (Figure 1). Because of the follow-up, the number of patients at risk at 12 months was 10/18 and 4/18 at 24 months. Recurrences occurred in seven patients without any significant differences noted according to the width of the surgical margin (p = 0.15, Fisher's exact test). Two recurrences were located in the liver; three in both chest and liver and one was only extrahepatic. Three of these six recurrences were treated surgically (two by laparotomy and one by laparoscopy). For one patient treated by laparotomy, a second recurrence was destroyed by radiofrequency.
Figure 1. .
Kaplan-Meyer curve of disease-free survival in patients with colorectal metastasis (n = 18) with the number of patients at risk at 12 months and 24 months.
In HCC (n=14), with a mean follow-up of 29 months, overall survival at 12 and 36 months was 91% and 65%, respectively. Disease-free survival at 12 and 36 months was 72% and 60%, respectively. The number of patients at risk at 12 months was 7/14 and 3/14 at 36 months. Recurrence occurred in four patients: three were located in the liver and one was found in both the chest and the liver. Of these, two have been treated by radiofrequency.
Discussion
With 46 laparoscopic anatomic liver resections (80% for cancer) including 26 major resections, to our knowledge this report is the most important single-center experience in this field. This report is the result of 10 years of experience and training in a team largely involved in laparoscopy involving a great deal of technology. Indeed, the morbidity of around 35% and especially the mortality rate of 2% provide factual data about this approach to liver surgery. As regards long-term survival after laparoscopic liver resection for malignancy, notably for HCC and CRM, it is difficult to draw conclusions about the effectiveness of our results because of the limited follow-up after 1 year. It should be noted that the absence of port site metastases among all these patients remains a key point of this report.
Even if laparoscopy is more audacious in liver than in other surgical fields because of specific difficulties, this approach will probably become very common in the future, as with the colon currently 3, because of expected advantages, notably in malignancy.
The specific difficulties associated with laparoscopic liver surgery include risks of hemorrhage, biliary wounds, and gas embolism 4. Hemorrhage and biliary wounds are directly related to the surgeon's experience and surgical skill. The surgeon must know how to avoid and control these problems: adequate intraoperative orientation during parenchymal transection and initial dissection of the biliary convergence before major hepatectomy is a prerequisite to safe surgery. For this reason, we place five to six trocars, as described in the methods section, high in the right flank, to allow better visualization and to control the very deep anatomical structures, notably the vena cava and hepatic veins. Fears of gas embolism as a major obstacle to liver resection by laparoscopy 5,6 were based on a hypothetic risk because of increased abdominal pressure. However, in an exhaustive review of the literature on hepatic surgery, only two cases of severe gas embolism could be found 7. Both cases were described with the use of the argon beam, which has since been specifically contraindicated 7. In addition, a recent experimental study, during left lobectomy by laparoscopy or by laparotomy in pigs 8, has compared the relative incidence and consequences of gas embolism. This study demonstrated that in spite of numerous gas embolisms occurring during laparoscopy detected by transesophageal echocardiography, there were few if any clinical consequences, most likely because of the high solubility of carbon dioxide used for pneumoperitoneum.
More than obvious cosmetic advantages and perhaps a slight reduction of length of hospitalization 9,10,11, liver laparoscopic surgery has particularly great potential in cancer. In malignancy, laparoscopy has been traditionally limited to ultrasonic assessment of patients with CRM or HCC 12,13,14, allowing the surgeon to avoid unnecessary laparotomy in patients in a palliative setting. Except this obvious proved advantage, for us, there are at least three other reasons to expect benefits from the use of this technique for malignancy. First, the postoperative course after hepatectomy is improved in the cirrhotic patient (mainly due to decreased postoperative ascites because of the preservation of abdominal wall allowing a better collateral venous drainage and improved postoperative kinetics of the diaphragm) 15,16,17. Second, minimal scarring and less adherences, the latter shown experimentally in pigs 18, leading to enhanced feasibility of repeat liver resection (not rare in malignancy), or transplantation whenever necessary. Third, improved postoperative immunity and especially, cellular immunity, largely involved in the anti-tumor response 19,20.
Currently for the majority of surgeons, practice of laparoscopy in the liver surgery is limited to minor resection and especially in superficial segments. If these rules must always guide the indication for laparoscopic liver resection at the start of experience, we suggest that major resections in deep segments could be planned and performed by an experienced team. This necessarily involves a good practice in traditional liver surgery, in sonographic exploration of the liver and of course in laparoscopy. A prior good experience in traditional liver surgery is necessary for the knowledge of precise intrahepatic anatomy to avoid pitfalls of magnification and notably errors in spatial orientation of the surgical plane. A good practice in laparoscopic ultrasound is also necessary to explore the parenchyma efficiently 21, to replace manual palpation and to look for vascular structures during liver section. Obviously, skills in laparoscopy must be sufficient to be able to work precisely with both hands such as, for example, a harmonic scalpel in the dominant hand and a bipolar forceps in the other hand to rescue hemostasis if necessary.
Besides these human factors, laparoscopic liver surgery requires a lot of technology like the harmonic scalpel (UltracisionR, Ethicon), auto-regulated bipolar forceps (LigasureR, Tyco), laparoscopic ultrasound probe and, in our team, a voice-controlled robot (AESOPR, Computer Motion) to mobilize easily and precisely the endoscope during these long surgical procedures.
If these conditions are met, anatomical and not only wedge resections could be considered. After excisional biopsies, cyst fenestrations and wedge resections in superficial segments (for instance in segment 3 or segment 2), the learning curve must go through the accomplishment of left lateral lobectomy. Recently, a case-control study has demonstrated the safety of this procedure which was associated with a decreased blood loss despite involving a slightly longer procedure 22.
Even if laparoscopic major liver resections and notably anatomical resections are feasible, as demonstrated in our report, some contraindications persist for the laparoscopic approach to the liver even for a specialized team. As well as contraindications regarding general anesthesia in the laparoscopic approach, some characteristics related to the tumor carry contraindications for this approach. First of all, gallbladder cancer is a traditional limitation of the laparoscopic approach because of the high incidence of port site metastases 23. Whatever the histological type, tumor location which could necessitate vascular resection or biliary reconstruction is currently a classical contraindication of laparoscopic resection. However, in the future, with laparoscopic computer-enhanced surgery that largely facilitates laparoscopic anastomoses, this technology will probably push back these limits 24.
Whatever the skill of the surgeon, one of the principal difficulties of laparoscopic liver resections is mobilization of the liver, notably to perform resection in a deep segment. To overcome this specific difficulty, we always locate our trocar as close as possible to the inferior limit of the liver to facilitate the surgical work in deep segments. However, some tumor locations, notably in segment 8 or close to the subhepatic vein confluence, are almost impossible to remove by a wedge resection or a limited anatomical resection. In this situation, the achievement of a laparoscopic major anatomical resection, like for instance a right hepatectomy, which removes the segment involved, is easier. Hence, one consequence of the development of the liver laparoscopy could be the increase of anatomical resections instead of wedge resections. This will be probably one of the more interesting problems related to the liver laparoscopic approach in future years. However, this problem has not been solved even by laparotomy, notably in colorectal metastases 25,26, although for hepatocellular carcinoma anatomical resections seem more suitable than wedge resections 27.
Conclusion
With a long period of training, which must start with excisional biopsies in superficial segments, a team largely involved in both hepatic surgery and laparoscopy can perform major and/or anatomical laparoscopic liver resections. In fact, these first short-term results seem comparable to those of traditional liver surgery. Hence, this report perhaps paves the way for a new surgical approach to liver malignancy if the long-term results confirm the early results of laparoscopy. Further prospective evaluations are required to assess the results of laparoscopy in the treatment of liver cancer and notably its place in major liver resection.
References
- 1.Belghiti J, Guevara OA, Noun R, Saldinger PF, Kianmanesh R. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 2001;193:109–11. doi: 10.1016/s1072-7515(01)00909-7. [DOI] [PubMed] [Google Scholar]
- 2.Fong Y, Jarnagin W, Conlon KC, DeMatteo R, Dougherty E, Blumgart LH. Hand-assisted laparoscopic liver resection: lessons from an initial experience. Arch Surg. 2000;135:854–9. doi: 10.1001/archsurg.135.7.854. [DOI] [PubMed] [Google Scholar]
- 3.Lacy AM, Garcia-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–9. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 4.Cherqui D, Husson E, Hammoud R, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000;232:753–62. doi: 10.1097/00000658-200012000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yacoub OF, Cardona I, Jr, Coveler LA, Dodson MG. Carbon dioxide embolism during laparoscopy. Anesthesiology. 1982;57:533–5. doi: 10.1097/00000542-198212000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Moskop RJ, Jr, Lubarsky DA. Carbon dioxide embolism during laparoscopic cholecystectomy. SouthMed J. 1994;87:414–5. doi: 10.1097/00007611-199403000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Hashizume M, Takenaka K, Yanaga K, et al. Laparoscopic hepatic resection for hepatocellular carcinoma. Surg Endosc. 1995;9:1289–91. doi: 10.1007/BF00190161. [DOI] [PubMed] [Google Scholar]
- 8.Schmandra TC, Mierdl S, Bauer H, Gutt C, Hanisch E. Transoesophageal echocardiography shows high risk of gas embolism during laparoscopic hepatic resection under carbon dioxide pneumoperitoneum. Br J Surg. 2002;89:870–6. doi: 10.1046/j.1365-2168.2002.02123.x. [DOI] [PubMed] [Google Scholar]
- 9.Rau HG, Buttler E, Meyer G, Schardey HM, Schildberg FW. Laparoscopic liver resection compared with conventional partial hepatectomy – a prospective analysis. Hepatogastroenterology. 1998;45:2333–8. [PubMed] [Google Scholar]
- 10.Shimada M, Hashizume M, Maehara S, et al. Laparoscopic hepatectomy for hepatocellular carcinoma. Surg Endosc. 2001;15:541–4. doi: 10.1007/s004640080099. [DOI] [PubMed] [Google Scholar]
- 11.Farges O, Jagot P, Kirstetter P, Marty J, Belghiti J. Prospective assessment of the safety and benefit of laparoscopic liver resections. J Hepatobiliary Pancreat Surg. 2002;9:242–8. doi: 10.1007/s005340200026. [DOI] [PubMed] [Google Scholar]
- 12.Rahusen FD, Cuesta MA, Borgstein PJ, et al. Selection of patients for resection of colorectal metastases to the liver using diagnostic laparoscopy and laparoscopic ultrasonography. Ann Surg. 1999;230:31–7. doi: 10.1097/00000658-199907000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montorsi M, Santambrogio R, Bianchi P, et al. Laparoscopy with laparoscopic ultrasound for pretreatment staging of hepatocellular carcinoma: a prospective study. J Gastrointest Surg. 2001;5:312–15. doi: 10.1016/s1091-255x(01)80053-6. [DOI] [PubMed] [Google Scholar]
- 14.Jarnagin WR, Conlon K, Bodniewicz J, et al. A clinical scoring system predicts the yield of diagnostic laparoscopy in patients with potentially resectable hepatic colorectal metastases. Cancer. 2001;91:1121–8. doi: 10.1002/1097-0142(20010315)91:6<1121::aid-cncr1108>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Atty MY, Farges O, Jagot P, Belghiti J. Laparoscopy extends the indications for liver resection in patients with cirrhosis. Br J Surg. 1999;86:1397–400. doi: 10.1046/j.1365-2168.1999.01283.x. [DOI] [PubMed] [Google Scholar]
- 16.Frazee RC, Roberts JW, Okeson GC, et al. Open versus laparoscopic cholecystectomy. A comparison of postoperative pulmonary function. Ann Surg 1991; 213: 651–31; discussion 653–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurent A, Cherqui D, Lesurtel M, Brunetti F, Tayar C, Fagniez PL. Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg 2003;138:763–9; discussion 769. [DOI] [PubMed] [Google Scholar]
- 18.Burpee SE, Kurian M, Murakame Y, Benevides S, Gagner M. The metabolic and immune response to laparoscopic versus open liver resection. SurgEndosc. 2002;16:899–904. doi: 10.1007/s00464-001-8122-x. [DOI] [PubMed] [Google Scholar]
- 19.Tsuchiya Y, Sawada S, Yoshioka I, et al. Increased surgical stress promotes tumor metastasis. Surgery. 2003;133:547–55. doi: 10.1067/msy.2003.141. [DOI] [PubMed] [Google Scholar]
- 20.Vittimberga FJ, Jr, Foley DP, Meyers WC, Callery MP. Laparoscopic surgery and the systemic immune response. Ann Surg. 1998;227:326–34. doi: 10.1097/00000658-199803000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catheline JM, Turner R, Champault G. Laparoscopic ultrasound of the liver. Eur J Ultrasound. 2000;12:169–77. doi: 10.1016/s0929-8266(00)00112-9. [DOI] [PubMed] [Google Scholar]
- 22.Lesurtel M, Cherqui D, Laurent A, Tayar C, Fagniez PL. Laparoscopic versus open left lateral hepatic lobectomy: a case-control study. J Am Coll Surg. 2003;196:236–42. doi: 10.1016/S1072-7515(02)01622-8. [DOI] [PubMed] [Google Scholar]
- 23.Weiland ST, Mahvi DM, Niederhuber JE, Heisey DM, Chicks DS, Rikkers LF. Should suspected early gallbladder cancer be treated laparoscopically? J Gastrointest Surg 2002; 6:50–6; discussion 56–7. [DOI] [PubMed] [Google Scholar]
- 24.Vibert E, Denet C, Gayet B. Major digestive surgery with a remote controlled robot: the next revolution. Arch Surg. 2003;138:1002–6. doi: 10.1001/archsurg.138.9.1002. [DOI] [PubMed] [Google Scholar]
- 25.Kokudo N, Tada K, Seki M, et al. Anatomical major resection versus nonanatomical limited resection for liver metastases from colorectal carcinoma. Am J Surg. 2001;181:153–9. doi: 10.1016/s0002-9610(00)00560-2. [DOI] [PubMed] [Google Scholar]
- 26.Nagakura S, Shirai Y, Yokoyama N, Wakai T, Suda T, Hatakeyama K. Major hepatic resection reduces the probability of intrahepatic recurrences following resection of colorectal carcinoma liver metastases. Hepatogastroenterology. 2003;50:779–83. [PubMed] [Google Scholar]
- 27.Regimbeau JM, Kianmanesh R, Farges O, Dondero F, Sauvanet A, Belghiti J. Extent of liver resection influences the outcome in patients with cirrhosis and small hepatocellular carcinoma. Surgery. 2002;131:311–17. doi: 10.1067/msy.2002.121892. [DOI] [PubMed] [Google Scholar]