Abstract
Trophinin and tastin form a cell adhesion molecule complex that potentially mediates an initial attachment of the blastocyst to uterine epithelial cells at the time of implantation. Trophinin and tastin, however, do not directly bind to each other, suggesting the presence of an intermediary protein. The present study identifies a cytoplasmic protein, named bystin, that directly binds trophinin and tastin. Bystin consists of 306 amino acid residues and is predicted to contain tyrosine, serine, and threonine residues in contexts conforming to motifs for phosphorylation by protein kinases. Database searches revealed a 53% identity of the predicted peptide sequence with the Drosophila bys (mrr) gene. Direct protein–protein interactions of trophinin, tastin, and bystin analyzed by yeast two-hybrid assays and by in vitro protein binding assays indicated that binding between bystin and trophinin and between bystin and tastin is enhanced when cytokeratin 8 and 18 are present as the third molecule. Immunocytochemistry of bystin showed that bystin colocalizes with trophinin, tastin, and cytokeratins in a human trophoblastic teratocarcinoma cell, HT-H. It is therefore possible that these molecules form a complex and thus are involved in the process of embryo implantation.
Embryo implantation is a process that depends on the coordinated development of the embryo and differentiation of the uterus to the receptive state (1–4). A two-way interaction between the blastocyst and uterus is essential for successful implantation and subsequent decidualization (5). In the mouse, the first conspicuous sign of the implantation is an increased endometrial vascular permeability at the site of blastocyst apposition, and this coincides with the initial attachment reaction (3, 6). This attachment is followed by adherence and penetration by trophoblasts cells through the underlying basement membrane and results in proliferation and differentiation of stromal cells into decidual cells. Numerous factors including growth factors (7), cytokines (8), homeotic genes (9), and prostaglandin (10, 11) have been implicated in implantation process. Among these, null mutations of leukemia inhibitory factor and Hoxa-10 genes result in defective implantation (8, 9), and a prostaglandin regulating cyclooxigenase 2 gene knock-out results in multiple failures of female reproductive processes including implantation (10, 11).
To understand the molecular mechanisms underlying embryo implantation, identification and characterization of specific molecules responsible for the initial attachment of the embryo and subsequent invasion of the trophoblasts to the uterus are essential. However, such analysis has been difficult because of the absence of appropriate in vitro models for implantation. In this regard two human cell lines, a trophoblastic teratocarcinoma, HT-H (12), and an endometrial adenocarcinoma, SNG-M (13), are noteworthy, because the interaction between these two cell types appears to mimic that of trophoblasts and endometrial epithelial cells participating in implantation. With these two cell lines, we have identified a unique cell adhesion molecule, trophinin, and a trophinin-assisting cytoplasmic protein, tastin (14).
Trophinin is an intrinsic plasma membrane protein containing 749 amino acids. The N-terminal region containing 66 amino acid residues is predicted to localize in the cytoplasm. The rest of the trophinin polypeptide contains eight hydrophobic stretches predicted to span the membranes.
Tastin is a cytoplasmic protein composed of 778 amino acid residues. Tastin is proline-rich and contains src homology 3 domains. It contains one tyrosine in a context favorable to phosphorylation by tyrosine kinases, and a total of 11 serine and threonine residues for potential phosphorylation by protein kinase C, casein kinase II, cAMP/cGMP-dependent protein kinase, and mitogen-activated protein kinase. When coexpressed in COS cells, tastin induces clustering of trophinin. Thus tastin is necessary for trophinin to function as a cell adhesion molecule by creating efficient adhesion sites on the cell surface.
Trophinin and tastin are not ubiquitously expressed in a variety of human tissues but rather are strongly expressed in cells involved in implantation, such as the trophectoderm cells of monkey blastocysts and the human endometrial epithelium at early secretory phase (14). In situ hybridization and immunohistology detected strong expression of trophinin and tastin at human embryo implantation sites (J. Nakayama, personal communication). Furthermore, the trophinin gene null mutation is found to be embryonic lethal, presumably at the implantation stage, demonstrating a critical role of trophinin in vivo (D. Nadano, and M.N.F., unpublished data).
Our preliminary yeast two-hybrid assay, however, revealed no direct binding between trophinin and tastin, indicating the interaction between these two molecules is indirect. This article describes identification of a cytoplasmic protein, bystin, and presents data indicating that bystin is the molecule bridging trophinin and tastin.
MATERIALS AND METHODS
cDNAs and cDNA Library.
A cDNA expression library was constructed from human trophoblastic teratocarcinoma HT-H cells (12) in the pcDNA1 vector. A custom ordered cDNA library was provided by Invitrogen. Each cDNA was inserted unidirectionally between the EcoRI and NotI sites of the pcDNA1 vector. The constructed library consists of 2 × 106 clones with insert sizes from 0.5 to 3.0 kb.
Transfection of COS-1 Cells and Cell Adhesion Assays.
COS-1 cells (0.5–1 × 106 cells per ml) were suspended in 0.02 M sodium phosphate (pH 7.4) containing 0.15 M NaCl and plasmid DNA (20–100 μg/ml). Transfection was performed by electroporation with the Gene-Pulser (Bio-Rad) at 0.4 kV with a capacitance of 125 μF. The transfected COS-1 cells were cultured for 48 h in DMEM. Before the cell adhesion assays, COS-1 cells were detached from the tissue culture plate with an enzyme-free cell-dissociation solution (Specialty Media, Lavallette, NJ) supplemented with 1 mM EDTA. Cells were suspended in DMEM containing 10% fetal bovine serum and were vitally stained with 0.01% neutral red at room temperature for 30 min. The cells were pelleted by centrifugation, suspended in Hepes/NaCl buffer (pH 7.4) containing 1 mM EDTA, and were overlaid on a fixed (1% paraformaldehyde in PBS, at room temperature for 15 min) monolayer of human endometrial adenocarcinoma SNG-M cells (13). After 20 min, nonadherent COS-1 cells were removed by washing the monolayer with buffer containing 1 mM EDTA. Cells attached to the surface of the SNG-M monolayer were recovered by flushing with medium through a Pasteur pipette, and cell numbers were counted with a hemocytometer.
Yeast Two/Three-Hybrid Assays.
The cDNAs encoding the N-terminal region of trophinin and full-length bystin and tastin were modified by PCR and subcloned in-frame into the yeast plasmid vectors pEG202, pJG4–5, and pVP-16 (15–17). Forward and reverse primers for trophinin were CTCGAATTCATGGATATCGACTGCCTAACA (EcoRI site underlined) and TGTCTCGAGCTAACTGGAGCTGGGTGCACCAT (XhoI site underlined); for bystin the the primers were CTCGAATTCATGGAGAAGCTGACTGAGAAG (EcoRI site underlined) and AGACTCGAGCGGCCGCTAGAATGGAAGAGGGAACCA (XhoI site underlined); for tastin they were CTCAGATCTTCATGACCACCCGGCAAGCC (BglII site underlined) and GAGGCGGCCGCTATCGTCCAGACGAGCCTCGTG (NotI site underlined). The plasmids pEG202-bcl-2 and pJG4-5-Bax (18) were provided by J. Reed (the Burnham Institute, La Jolla, CA) and used as positive controls. Plasmids without an insert were used as negative controls. Saccharomyces cerevisiae L40 [MATa his3D200 trp1–901 leu2–3, 112 ade2 LYS2∷(lexAop)4-HIS3 URA3∷(lexAop)8-lacZ) was grown in YPD medium containing 1% yeast extract, 2% polypeptone, and 2% glucose. Yeast nitrogen base (Difco) fortified with appropriate amino acids, adenine, and glucose was used unless otherwise specified. Plasmid DNA transformations were performed by the lithium acetate method (19). Double transfectants with pEG202 and pJG4-5 were selected in the medium supplemented with leucine and adenine, and triple transfectants with pEG202, pJG4-5, and pVP16 were selected in the medium supplemented with adenine alone. RNA encoding a fusion protein including the B42 transactivation domain, an simian virus 40 (SV40) nuclear localization signal, and cDNA was induced by galactose, as the pJG4-5 expression plasmid uses a galactose-dependent promoter. Expression of a LexA fusion protein encoded by the pEG202 vector was verified by Western blotting using mAb anti-LexA kindly provided by E. A. Golemis (Fox Chase Cancer Center). The expression of a fusion protein containing the SV40 nuclear localization signal, the acid blob B42, and the hemagglutinin (HA) HA-1 epitope tag encoded by the pJG4-5 vector was verified by Western blotting using the anti-HA mAb (clone 12CA5, Babco, Richmond, CA). Interactions between the proteins of interest were examined with β-galactosidase assays. Three yeast transformants were grown on filters in minimum medium containing either 2% glucose or galactose. The filters were treated with liquid nitrogen and placed on an agar plate containing 5-bromo-4-chloro-3-indoyl β-d-galactopyranoside (25 μg/ml). Colonies were monitored for blue reaction products at 0.5, 1, 2, 4, and 24 h.
Preparation of Glutathione S-Transferase (GST) Fusion Proteins.
A cDNA fragment encoding a full-length bystin polypeptide was prepared by PCR, using the same primer used for construction of yeast two-hybrid vector. A PCR product was ligated at the EcoRI and XhoI sites of the pGEX-4T-1 vector (Pharmacia) in-frame with the upstream sequence of GST (20). Escherichia coli A2022 cells were transformed with this plasmid vector and GST-bystin fusion protein was produced upon induction with 0.1 mM isopropyl β-d-thiogalactoside. The GST fusion protein was solubilized with 25 mM Tris⋅HCl (pH 7.5) containing 1.5% sodium sarcosyl sulfate, 10 mM EDTA, and lysozyme (5 mg/ml) on ice for 30 min (21). After brief sonication, insoluble materials were removed by centrifugation, and the supernatant was mixed with glutathione-Sepharose beads (Pharmacia). Beads were washed with 25 mM Tris⋅HCl (pH 7.5), and bound material was eluted with the buffer containing the reduced form of glutathione at 1 mM. Proteins were resolved by SDS/PAGE (22), and a GST-bystin fusion protein was identified as a band at the expected molecular mass (61 kDa) by staining the gel with Coomassie brilliant blue. The GST-bystin protein was dialyzed against PBS and stored at −20°C. A cDNA fragment encoding a full-length tastin was modified by PCR in the same manner as described for GST-bystin. The N-terminal cytoplasmic domain of trophinin fused with GST was prepared as described (14).
In Vitro Protein Binding Assays.
35S-labeled trophinin, tastin, bystin, cytokeratin 8, and cytokeratin 18 proteins were prepared by using the TNT-coupled in vitro translation system (Promega). Trophinin, tastin, and bystin were inserted into a mammalian expression vector, pcDNA1, and cytokeratin 8 and cytokeratin 18 (23) were inserted into a mammalian expression vector, pGEM. These vectors provide the promoter for T7 RNA polymerase for transcription. The transcribed RNAs were translated in the rabbit reticulocyte lysates in the presence of [35S]methionine. The labeled translation products were then incubated with each GST fusion protein, such as GST-bystin, and bound to glutathione-agarose beads in 0.02 M Tris⋅HCl (pH 7.5) containing 0.15 M NaCl, 1 mM DTT, and 0.1% Triton X-100. After washing the beads with the same buffer, bound materials were eluted with SDS sample buffer and analyzed by SDS/PAGE followed by autoradiography.
mAbs and Immunocytochemistry.
mAb anti-bystin (clone 19, mouse IgM) was prepared by immunizing BALB/c mice with a synthetic peptide GFRTEKREL (amino acid residues 215–223 of human bystin) conjugated to keyhole limpet hemocyanin (Imject, Pierce). Spleen cells from immunized mice were fused to P3X mouse myeloma cells. Hybridoma clones were selected by screening culture supernatants for antibodies reactive to HT-H cells. The hybridomas were further verified by immunostaining of COS cells transfected with bystin cDNA and also by Western blot of GST-bystin fusion protein. mAb anti-trophinin (clone 3–11, mouse IgM) was raised against the SIVGFSGGP epitope (amino acid residues 681–689 of human trophinin), and mAb anti-tastin (clone 38, mouse IgG3) was raised against the DQENQDPRR epitope (amino acid residues 41–49 of human tastin). mAb anti-cytokeratin (Troma-1, rat IgG) has been described (23). For double immunostaining of HT-H cells for trophinin and bystin, each IgM antibody was purified by using an ImmunoPure IgM purification kit (Pierce). Purified anti-trophinin antibody was conjugated with fluorescein isothiocyanate. Purified anti-bystin antibody was conjugated with rhodamine B isothiocyanate. HT-H cells were fixed with paraformaldehyde and reacted with fluorescein-conjugated anti-trophinin antibody. After washing, the cells were permeabilized with 0.1% saponin and reacted with rhodamine-conjugated anti-bystin antibody. All other immunocytochemistry was carried out by using fluorescent second antibodies as described (14, 24).
RESULTS
Molecular Cloning of Bystin.
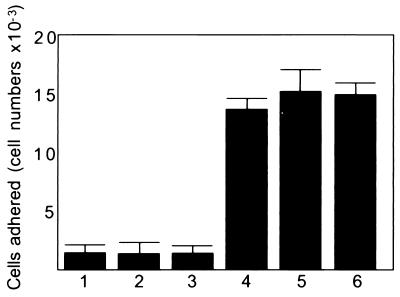
A cDNA library of human trophoblastic teratocarcinoma HT-H cells was constructed in a mammalian expression vector, and COS-1 cells were transfected with the library as was described (14). COS-1 cells that became adhesive to the monolayer of the SNG-M cells were selected by adhesion assay, and plasmid clones were rescued by transformation of E. coli MC1061/P3. After the second screen, 40 clones were randomly chosen and each clone was examined by a cell adhesion assay. When COS-1 cells were transfected with a cDNA clone named bystin (see below) and trophinin, cotransfected COS-1 cells adhered to the upper surfaces of the SNG-M cells (Fig. 1).
Figure 1.
Binding of transfected COS-1 cells to the surface of monolayers of SNG-M cells. COS-1 cells were transfected with pcDNAI vector (bar 1), trophinin cDNA alone (bar 2), bystin cDNA alone (bar 3), a mixture of trophinin and bystin cDNAs (bar 4), a mixture of trophinin and tastin cDNAs (bar 5), and a mixture of trophinin, bystin and tastin cDNAs (bar 6). Two days after transfection, COS-1 cells were subjected to a cell adhesion assay. Numbers presented are the averages obtained by duplicate counting.
The bystin cDNA clone consists of 1,224 nucleotides with an ORF encoding 306 amino acids (Fig. 2A). A Western blot of HT-H cell lysates with mAb anti-bystin showed a specific band at 35 kDa, which is consistent with the expected molecular weight of 35,169 for human bystin. Hydropathy analysis of the predicted bystin protein revealed no obvious signal sequences characteristic of secreted or membrane proteins, suggesting that bystin is likely to be a soluble cytoplasmic protein. Bystin contains tyrosine residues at positions 114, 183, and 199 that match the consensus sequence for tyrosine kinases (25). The sequences around the serine/threonine residues at positions 5, 46, 150, 212, and 301 conform to the sequence of a protein kinase C phosphorylation site, S(T)-X-R(K). The serine/threonine residues at positions 9, 60, 69, and 150 are putative phosphorylation sites for casein kinase II, as the consensus sequence for this kinase is S(T)-X-X-D(E). Bystin does not contain a kinase motif (26).
Figure 2.
Comparison of human bystin and putative polypeptides homologous to bystin in D. melanogaster, C. elegans, and S. cerevisiae. (A) Alignment of amino acid residues of human bystin and the Drosophila putative bys gene product. (B) Highly homologous regions of peptide sequences among human, fly, nematode, and yeast bystins (GenBank accession nos. L02076 for D. melanogaster, U13876 for C. elegans, and Z36116 for S. cerevisiae genes).
Database searches have revealed that the translated peptide sequence of bystin is homologous to that of the Drosophila bys (mrr) gene (Fig. 2A) (27, 28). Hence it is named bystin. Comparison of amino acid sequences between bystin and the Drosophila bys gene product indicate a 53% identity and an overall similarity of 75%, suggesting that the bystin identified in this study is the human orthologue of the Drosophila bys (mrr) gene product. A database search also showed the presence of a bystin-like gene in S. cerevisiae and Caenorhabditis elegans (Fig. 2B).
Northern blot analysis using mRNA from human teratocarcinoma HT-H cells and endometrial adenocarcinoma SNG-M cells showed 2.0-kb (major) and 3.6-kb (minor) bands (data not shown). Northern blot analysis using mRNA from a various human tissues showed very weak signal for bystin. Database search including expressed sequence tag (29) revealed no bystin homologues in human cDNA libraries. Immunohistology of several human tissues showed no definite immunostaining for human bystin.
Yeast Two/Three-Hybrid Assays of Bystin, Trophinin, Tastin, and Cytokeratins.
Interactions between trophinin, tastin, bystin and cytokeratin were examined by the yeast two-hybrid assay (30). As shown in Table 1, interactions between the proteins of interest were recorded as the intensity of blue color developed on the filter caused by the activation of reporter β-galactosidase gene. Because expression of a gene inserted in the pJG4–5 vector is inducible by galactose, protein–protein interactions are detected as the increase in blue color in the presence of galactose over the background color developed in the presence of glucose.
Table 1.
Interactions of trophinin, bystin, tastin, and cytokeratins in vivo in yeast
| Exp. | pEG202 | pJG4-5 | pVP16 | Results
|
||
|---|---|---|---|---|---|---|
| Glc | Gal | |||||
| 1 | No | No | — | − | − | (inducible two hybrid) |
| 2 | No | No | No | − | − | (inducible three hybrid) |
| 3 | No | — | No | − | (noninducible two hybrid) | |
| 4 | Tas | No | — | − | − | |
| 5 | Tas | Tas | — | ± | + | |
| 6 | Tas | Tro | — | − | − | |
| 7 | Tas | Tro | CK | ± | ± | |
| 8 | Tas | Bys | — | − | − | |
| 9 | Tas | Bys | CK | ± | + | |
| 10 | Tas | — | No | − | ||
| 11 | Tas | — | CK | ± | ||
| 12 | Bys | No | — | − | − | |
| 13 | Bys | Bys | — | + | + | |
| 14 | Bys | Tro | — | − | + | |
| 15 | Bys | Tro | CK | + | +++ | |
| 16 | Bys | Tas | — | ± | ++ | |
| 17 | Bys | Tas | CK | + | +++ | |
| 18 | Bys | — | No | − | ||
| 19 | Bys | — | CK | ++ | ||
| 20 | Bys | Tro | Tas | ± | + | |
Development of blue color in two/three-hybrid plasmids are indicated as ±, +, ++, or +++, depending on the relative strength of the color. Discernible blue color that developed after 4 h in β-galactosidase filter assays was recorded as positive (+). Three cotransformants with pEG202/pJG4-5 or with pEG202/pJG4-5/pVP16 were plated on either galactose- or glucose-containing medium to compare induced versus uninduced expression from the GAL-1 promotor of the pJG4-5 vector. Cotransfectant with pEG202/pVP16 vectors were plated in the medium containing glucose. The pEG202 plasmid expressing trophinin gave strong background color reaction, which makes it difficult to draw conclusions from this set of experiments. Tro, trophinin; Tas, tastin; Bys, bystin; CK, cytokeratin.
Two-hybrid assays show that trophinin and tastin do not directly interact with each other (Table 1, experiment 6). On the other hand, it was shown that bystin interacts directly with trophinin (Table 1, experiment 14), tastin (Table 1, experiment 16), and cytokeratins (Table 1, experiment 18). Interestingly, the strong interaction between bystin and tastin (Table 1, experiment 16) became stronger upon addition of cytokeratin as the third molecule (Table 1, experiment 17). Similarly, the positive interaction between bystin and trophinin (Table 1, experiment 14) was significantly increased upon addition of cytokeratin (Table 1, experiment 15). In these assays both cytokeratin 8 and cytokeratin 18 were equally effective.
Binding of Trophinin, Bystin, Tastin, and Cytokeratins in Vitro.
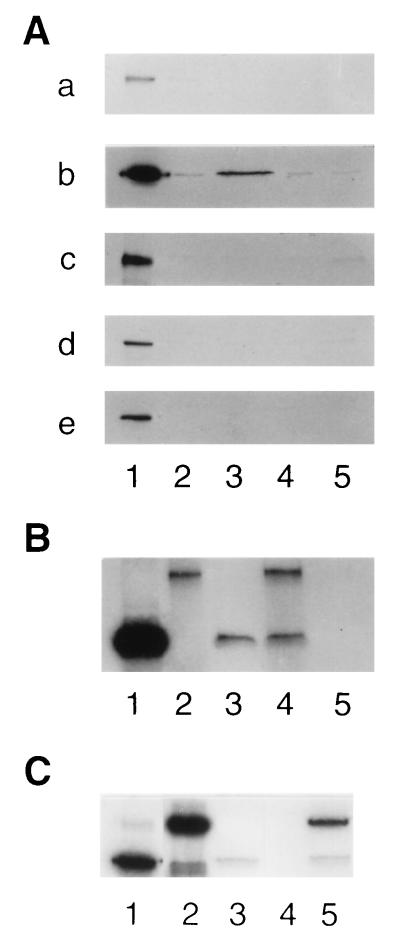
Protein–protein interactions were also examined by the in vitro protein binding assay using a GST fusion protein and 35S-labeled in vitro-translated protein. As shown in Fig. 3A, blot a, [35S]tastin did not bind to GST, GST-tastin, GST-bystin, or GST-trophinin. On the other hand [35S]bystin bound to GST-tastin (Fig. 3A, blot b, lane 3), a result consistent with that obtained by the yeast two-hybrid assay (Table 1, experiment 16). [35S]Bystin did not bind to GST, GST-bystin, or GST-trophinin (Fig. 3A, blot b, lanes 2, 4, and 5). Neither [35S]trophinin (Fig. 3A, blot c), [35S]cytokeratin 8, (Fig. 3A, blot d) nor [35S]cytokeratin 18 (Fig. 3A, blot e) bound to these GST fusion proteins.
Figure 3.
Autoradiographs of [35S]proteins bound to GST fusion proteins in in vitro protein binding assays. GST and GST fusion proteins were produced in bacteria, immobilized on glutathione-Sepharose, and incubated with 35S-labeled in vitro-translated polypeptides produced in rabbit reticulocyte lysates. (A) [35S]Tastin (blot a), [35S]bystin (blot b), [35S]trophinin (blot c), [35S]cytokeratin 8 (blot d), and [35S]cytokeratin 18 (blot e), prepared by in vitro translation (lanes 1) were incubated with GST (lanes 2), GST-tastin (lanes 3), GST-bystin (lanes 4), or GST-trophinin (lanes 5) immobilized on glutathione-beads. After washing the beads, the bound materials were analyzed by SDS/PAGE followed by autoradiography. (B) Materials bound to GST-tastin. [35S]Bystin alone (lane 3), [35S]trophinin and [35S]bystin (lane 4), and [35S]trophinin alone (lane 5) were incubated with GST-tastin beads. Lanes 1 and 2 show [35S]bystin and [35S]trophinin used for the binding assay. (C) Materials bound to GST-tastin. [35S]Bystin alone (lane 3), [35S]cytokeratin 18 alone (lane 4), and [35S]cytokeratin 18 and [35S]bystin (lane 5) were incubated with GST-tastin beads. Lanes 1 and 2 show [35S]bystin and [35S]cytokeratin 18 used for the binding assay.
The results obtained by the yeast three-hybrid assay (Table 1, experiments 17 and 20) were confirmed by triple binding assays. [35S]Bystin and [35S]trophinin were mixed together with GST-tastin beads (Fig. 3B). [35S]Trophinin, which did not bind to GST-tastin directly (Fig. 3B, lane 5), bound to GST-tastin in the presence of [35S]bystin (Fig. 3B, lane 4). This indicates that trophinin binds to tastin through bystin. Similarly, [35S]Cytokeratin, which did not bind to GST-tastin directly (Fig. 3A, blots d and e, lanes 3), bound to GST-tastin through [35S]bystin (Fig. 3C, lane 5).
Although the yeast two-hybrid assay detected weak interaction between trophinin and bystin (Table 1, experiment 14), binding of [35S]bystin to GST-trophinin was not demonstrated (Fig. 3A, blot b, lane 5). [35S]Trophinin appeared not to bind GST-bystin (Fig. 3A, blot c, lane 4). Such differences between the yeast two-hybrid assay and in vitro protein binding assay could be because of the fact that interactions of these proteins are dependent on posttranslational modifications that would likely not occur in a GST fusion protein produced in bacteria. Indeed, in vitro-translated trophinin and bystin produced in reticulocyte lysates were shown to bind with each other. Thus, [35S]trophinin bound to [35S]bystin, and together they bound to GST-tastin (Fig. 3B, lane 4).
Subcellular Localization and Potential Association of Bystin with Trophinin, Tastin, and Cytokeratin in Mammalian Cells.
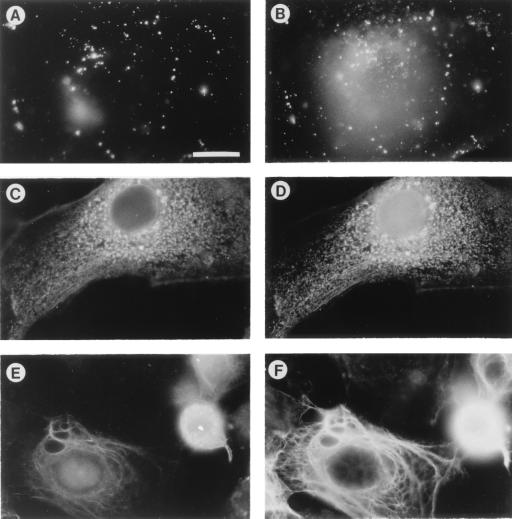
Bystin was localized in HT-H cells by using the mAb anti-bystin (Fig. 4). Interestingly, bystin in the HT-H cells alters its localization depending on the stage of cell culture. When HT-H cells are proliferating, bystin localizes in the cytoplasm as clustered granules (Fig. 4C). However, when HT-H cells cease growing and are apparently differentiated into syncytiotrophoblast (12), bystin appears to associate with the cytoskeleton (Fig. 4E).
Figure 4.
Immunofluorescence micrographs of HT-H cells stained with antibodies for trophinin, bystin, tastin, and cytokeratin. (A and B) Double immunostaining showing trophinin (A) and bystin (B). Unpermeabilized cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-trophinin antibody (A), permeabilized, and stained with rhodamine-conjugated anti-bystin antibody (B). These photographs focused on the upper cell surface where trophinin is present. Accordingly, only a fraction of bystin localized near upper plasma membranes is shown. (C and D) Double immunostaining showing bystin (C) and tastin (D). Cells were permeabilized and stained with unconjugated anti-bystin and anti-tastin antibodies, followed by FITC-conjugated goat anti-mouse IgM antibody for bystin (C) and rhodamine-conjugated goat anti-mouse IgG antibody for tastin (D). (E and F) Double immunostaining showing bystin (E) and cytokeratin 8 (F). Cells were permeabilized and stained with unconjugated anti-bystin and anti-cytokeratin antibodies, followed by FITC-conjugated goat anti-mouse IgM antibody for bystin (E) and rhodamine conjugated goat anti-rat IgG antibody for cytokeratin 8 (F). All photographs are presented at the same magnification. (Bar = 20 μm.)
Double immunostaining of HT-H cells with anti-trophinin and anti-bystin antibodies shows that, along with the upper plasma membranes, some of the bystin colocalizes with trophinin, but all of that fraction is in a large cluster (Fig. 4 A and B). Double immunostaining of HT-H cells with anti-bystin and anti-tastin antibodies also shows that bystin and tastin almost completely overlap with each other in the cytoplasm (Fig. 4 C and D). In some HT-H cells, bystin colocalizes with cytokeratin (Fig. 4 E and F).
DISCUSSION
We identified (14) a cell adhesion molecule complex containing trophinin and tastin that is potentially involved in implantation. The present study identifies a cytoplasmic protein, bystin, that binds directly to trophinin and tastin. Bystin may be involved in implantation and trophoblast invasion because bystin is found with trophinin and tastin in the cells at human implantation sites and also in the intermediate trophoblasts at invasion front in the placenta from early pregnancy (N.S., J. Nakayama, I.-M. Shih, D. Aoki, S. Nozawa, and M.N.F., unpublished data).
Our previous study suggested an association of trophinin and tastin with the cytoskeleton in HT-H and SNG-M cells (14). The present study indicates that bystin binds to cytokeratins (Table 1 and Figs. 3 and 4). Interactions between bystin and trophinin and between bystin and tastin become stronger when cytokeratin is present. During early embryogenesis, cytokeratin 8 and 18 are expressed in the trophectoderm of mouse blastocysts before and at the time of implantation (23). It is therefore possible that trophinin, bystin, and tastin interact with these cytokeratins in trophectoderm cells at the time of implantation.
The unique cell adhesion process of the trophoblast to endometrial epithelium and the subsequent invasion of the maternal tissue by the trophoblast is an essential element of embryo implantation. This process is often compared with metastasis and invasion of malignant tumors (31–33). Upon attachment of the blastocyst to endometrial epithelium, trophoblasts rapidly proliferate and invade the uterus. Apparently the initial attachment triggers a signal leading to trophoblast proliferation. A number of studies indicate that the functions of cell adhesion molecules are determined by their association with cytoplasmic proteins, including protein kinases and cytoskeletal proteins (34–37). It is possible that trophoblasts express specific cytoplasmic proteins that enhance cell–cell and cell–matrix adhesion. Such adhesiveness may be necessary for trophoblasts to attach to and invade the endometrial tissue. Trophoblasts at the implantation stage proliferate rapidly upon attachment and subsequently invade the endometrium, suggesting the existence of a signal transduction cascade triggered by adhesion leading to cell proliferation. Bystin and tastin, which contain potential phosphorylation sites for protein kinases, may play an important role in signal transduction linking adhesion to proliferation.
Acknowledgments
We thank Drs. Daita Nadano (The Burnham Institute), Rob Donell (Kansas State University), and Shiro Nozawa (Keio University, Japan) for their helpful discussions and Drs. Howard G. Damude (The Salk Institute), Shu-ichi Matsuzawa, and Carlos Caulin for providing us with plasmids. We also thank Ms. Grace Ceceña, Ms. Phuong Thai, and Mr. Kevin Lowitz for their excellent technical assistance. This study has been supported by Spaulding Memorial Fund to M.N.F. and R.G.O. and by National Institutes of Health Grants RO1 HD34108 to M.N.F., CA42302 to R.G.O., and GM55147 to T.S.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviation: GST, glutathione S-transferase.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. L36720 for human bystin).
References
- 1.Schlafke S, Enders A C. Biol Reprod. 1975;12:41–65. doi: 10.1095/biolreprod12.1.41. [DOI] [PubMed] [Google Scholar]
- 2.Denker H W. In: Trophoblast Research. Denker H W, Aplin J D, editors. New York: Plenum; 1990. pp. 3–20. [Google Scholar]
- 3.Psychoyos A. In: Handbook of Physiology. Greep R O, Astwood E G, Geige R G, editors. Washington, DC: Am. Physiol. Soc.; 1973. pp. 187–215. [Google Scholar]
- 4.Day S K. In: Reproductive Endocrinology, Surgery and Technology. Adashi E Y, Rock J A, Rosenwaks Z, editors. Philadelphia: Lippincott–Raven; 1996. pp. 421–434. [Google Scholar]
- 5.Paria B C, Huet-Hudson Y M, Dey S K. Proc Natl Acad Sci USA. 1993;90:10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huet Y M, Dey S K. J Reprod Fertil. 1987;81:453–458. doi: 10.1530/jrf.0.0810453. [DOI] [PubMed] [Google Scholar]
- 7.Das S K, Wang X-N, Paria B C, Damm D, Abraham J A, Klagsburn M, Andrews G K, Dey S K. Development (Cambridge, UK) 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 8.Stewart C L, Kaspar P, Brunet L J, Bhatt H, Gadi I, Kontgen F, Abbondanzo S J. Nature (London) 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 9.Benson G V, Lim H, Paria B C, Satokata I, Dey S K. Development (Cambridge, UK) 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- 10.Dinchuk J E, Car B D, Focht R J, Johnson J J, Jaffee B D, Covington M B, Contel N R, Eng V M, Collins R J, Cziernik P M, et al. Nature (London) 1995;378:406–409. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- 11.Lim H, Paria B C, Das S K, Dinchuk J E, Langenbach R, Trzaskos J M, Dey S K. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 12.Izhar M, Siebert P, Oshima R G, DeWolf W C, Fukuda M N. Dev Biol. 1996;116:510–518. doi: 10.1016/0012-1606(86)90151-x. [DOI] [PubMed] [Google Scholar]
- 13.Ishiwata I, Nozawa S, Inoue T, Okumura T. Cancer Res. 1977;37:1777–1785. [PubMed] [Google Scholar]
- 14.Fukuda M N, Sato T, Nakayama J, Klier G, Mikami M, Aoki D, Nozawa S. Genes Dev. 1995;9:1199–1210. doi: 10.1101/gad.9.10.1199. [DOI] [PubMed] [Google Scholar]
- 15.Golemis E, Gyuris J, Brent R. In: Current Protocols in Molecular Biology. Aushbel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Stuhl K, editors. New York: Wiley; 1994. pp. 20.1.1–20.1.27. [Google Scholar]
- 16.Gyuris J, Golemis E, Cherkov H, Brent R. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 17.Zervous A S, Gyuris J, Brent R. Cell. 1993;72:223–232. doi: 10.1016/0092-8674(93)90662-a. [DOI] [PubMed] [Google Scholar]
- 18.Sato T, Hanada M, Bodrug S, Irie S, Iwata N, Boise L H, Thompson C B, Golemis E, Fong L, Wang H G, Reed J. Proc Natl Acad Sci USA. 1994;91:9238–9242. doi: 10.1073/pnas.91.20.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gietz R D, Woods R A. Molecular Genetics of Yeast: Practical Approaches. London: Oxford Univ. Press; 1994. [Google Scholar]
- 20.Smith D B, Johnson K S. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 21.Frankel S, Sohn R, Leinwand L. Proc Natl Acad Sci USA. 1991;88:1192–1196. doi: 10.1073/pnas.88.4.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Oshima R G, Millan J L, Cecena G. Differentiation. 1986;33:61–68. doi: 10.1111/j.1432-0436.1986.tb00411.x. [DOI] [PubMed] [Google Scholar]
- 24.Aoki D, Lee N, Yamaguchi N, Dubois C, Fukuda M N. Proc Natl Acad Sci USA. 1992;89:4319–4323. doi: 10.1073/pnas.89.10.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kemp B E, Pearson R B. Trends Biochem Sci. 1990;15:342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- 26.Hanks S K, Quinn A M, Hunter T. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 27.Stewart M J, Denell R. Mol Cell Biol. 1993;13:2524–2535. doi: 10.1128/mcb.13.4.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson K L, Konrad K D, Woods D F, Bryant P J. Proc Natl Acad Sci USA. 1992;89:11302–11306. doi: 10.1073/pnas.89.23.11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 30.Fields S, Song O. Nature (London) 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 31.Kaufman M H. In: Biology of Trophoblast. Loke Y W, Whyte A, editors. Amsterdam: Elsevier Science; 1985. pp. 23–68. [Google Scholar]
- 32.Strickland S, Richards W G. Cell. 1992;71:355–357. doi: 10.1016/0092-8674(92)90503-5. [DOI] [PubMed] [Google Scholar]
- 33.McMaster M T, Bass K E, Fisher S J. Ann NY Acad Sci. 1994;734:122–131. doi: 10.1111/j.1749-6632.1994.tb21740.x. [DOI] [PubMed] [Google Scholar]
- 34.Clark E A, Brugge J S. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 35.Gumbiner B M. Neuron. 1993;11:551–564. doi: 10.1016/0896-6273(93)90068-3. [DOI] [PubMed] [Google Scholar]
- 36.Kemler R. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- 37.Takeichi M. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]