Abstract
Background. Perforation related to endoscopic retrograde cholangiopancreatography (ERCP) is a rare complication associated with significant morbidity and mortality. This study evaluated the management and outcomes of these perforations. Patients and methods. Between July 1996 and December 2002, a total of 6620 ERCPs were performed at our regional endoscopy unit serving the 1.5 million population of Southern Alberta. Thirty perforations (0.45%) were identified and retrospectively reviewed. Results. Seven of these 30 patients were found to have guidewire perforations of the bile duct, 11 perforations were peri-ampullary, 3 duodenal, 1 esophageal, and 1 patient had a perforation of an afferent limb of a Billroth II anastomosis. In seven patients the location of the perforation could not be determined (unknown). All patients with guidewire perforations were recognized during ERCP, and all were managed medically. Of the 11 peri-ampullary perforations, 7 of these patients had a pre-cut sphincterotomy, 5 underwent surgery and 4 patients died. Delay in diagnosis occurred in all patients that died. Of the three duodenal perforations, all required operation and one patient died. Of the seven ‘unknown’ retroperitoneal perforations, two patients required surgery and there was no mortality. The patients with esophageal and afferent limb perforations both recovered uneventfully after surgery. Most patients who required surgery had retroperitoneal fluid seen on CT scanning. Conclusions. We found that most guidewire perforations can be managed medically with little morbidity. Pre-cut sphincterotomy is a risk factor for perforation. Peri-ampullary and duodenal perforations have a high morbidity and mortality rate. In particular, retroperitoneal fluid collections on CT scans, delay in diagnosis and failure of medical therapy requiring salvage surgery are associated with poor outcomes. Early aggressive surgery may improve patient care.
Keywords: endoscopic retrograde cholangiopancreatography, perforation, management
Introduction
Endoscopic retrograde cholangiopancreatography (ERCP)-related perforations are a rare but serious complication. The incidence of perforation reported by recent series ranges from 0.3% to 1.3% 1,2,3,4,5,6,7. It is generally agreed that some ERCP-related perforations can be successfully managed without surgery 8,9,10,11; however, it is difficult to define these patients. Howard et al. 5 and Stapfer et al. 6 independently proposed a similar classification scheme for retroperitoneal perforations following ERCP, and selective management based on the type of injury. In this study we evaluate our experience with ERCP-related perforations at our regional referral centre.
Patients and methods
Between July 1996 and December 2002, a total of 6620 ERCPs were performed at our regional endoscopy unit serving the 1.5 million population of Southern Alberta. A total of 30 perforations (0.45%) were identified. These perforations were identified using two separate search strategies. First, 23 perforations were identified through our hospital medical records data system discharge diagnosis using the IC-9-CM code 998.2 (accidental puncture or laceration during a procedure). Second, to ensure that no perforation was missed by the ICD coding system, charts of those patients who had ERCP as a day procedure and were then admitted to any of the three adult hospitals in the Calgary Health Region within a week were hand searched. An additional seven perforations were identified.
Patient demographics including age, sex, and comorbidities such as coronary heart disease, chronic obstructive pulmonary disease, chronic renal failure, and malignancy were noted. The indication for ERCP, findings at ERCP, clinical presentation, radiographic findings, management, and outcomes were recorded and analyzed.
Results
Thirty perforations were identified. These included 1 esophageal perforation, 1 intra-peritoneal afferent limb perforation of a previously constructed Billroth II anastomosis, and 28 retroperitoneal perforations.
A patient who had undergone gastrectomy and Billroth II reconstruction for peptic ulcer disease presented with epigastric pain, mildly elevated liver function tests, and a dilated common bile duct (CBD) on ultrasound/MRCP examination. An initial ERCP was unsuccessful due to inability to reach the papilla. At the second ERCP attempt a perforation of the afferent limb occurred while trying to advance the endoscope to the papilla. The perforation was recognized during the procedure and the patient was referred for immediate surgery. The perforation was closed primarily at surgery. An intraoperative cholangiogram was performed and no distal obstruction was found. She did well postoperatively and was discharged home on postoperative day 8.
The remaining 28 retroperitoneal perforations were classified according to the scheme proposed by Howard et al. 5 and Stapfer et al. 6. They classified ERCP-related retroperitoneal perforations into guidewire perforations of the bile duct or pancreatic duct, peri-ampullary perforations related to sphincterotomy and duodenal perforations remote from the papilla. We found 7 guidewire perforations, 11 peri-ampullary perforations, and 3 duodenal perforations. The remaining seven perforations could not be classified based on available data and this group was designated as ‘unknown’. The demographics, ERCP indications, clinical presentation after ERCP perforation, management, and outcomes are presented in Table I.
Table I. Patient demographics, ERCP indications, and clinical outcomes.
| Parameter | Guidewire (n=7) | Peri-ampullary (n=11) | Duodenal (n=3) | Unknown (n=7) |
|---|---|---|---|---|
| Age (years) | 66±18 | 60±17 | 70±16 | 67±15 |
| Sex (% female) | 43% | 36% | 67% | 57% |
| Comorbidities | ||||
|
|
|
|
|
| ERCP indications | ||||
|
|
|
|
|
| Type of ES | ||||
|
|
|
|
|
| Diagnosis | ||||
|
|
|
|
|
| Clinical presentation | ||||
|
|
|
|
|
| Lab | ||||
|
|
|
|
|
| Biliary stents placed (including PTC) | 3 | 2 | 2 | 2 |
| Operations | 0 | 5 | 3 | 2 |
| Re-operations | 0 | 2 | 0 | 0 |
| Length of stay (days) | 4.4±2.1 | 26.5±25.8 | 13.7±15.3 | 12.6±5.6 |
| Death | 0 | 4 | 1 | 0 |
All guidewire perforations were recognized by extravasation of contrast dye on fluoroscopy during ERCP. All patients were treated with nothing by mouth, intravenous fluids, and parenteral antibiotics. The clinical course was uniformly benign as manifested by very mild abdominal pain, and rapid clinical improvement. One biliary stent was inserted during the same ERCP procedure after the guidewire perforation was discovered. After their perforation healed two patients received percutaneous trans-hepatic cholangiographic (PTC) drains placed to relieve jaundice secondary to malignancy.
Eleven peri-ampullary perforations were found. Two patients had biliary stents placed: one at the time of ERCP and the other 2 days later after extraction of a retained stone; both of these patients had successful medical management. No nasogastric or nasoduodenal tubes were used. Nine patients had post-ERCP CT scans. All had retroperitoneal air, and two patients had large retroperitoneal fluid collections. Both of these patients required surgery. Six patients had successful medical management. The clinical status of these patients improved rapidly, generally within 24–48 hours. Five patients required surgery, and their courses are summarized in Table II.
Table II. Surgical management of the peri-ampullary perforation group.
| Patient no./age/sex | Time to diagnosis/surgery | Presentation | Radiological findings | Surgical findings and management | Length of stay (days) |
|---|---|---|---|---|---|
| 1/58/F | 2/2 days | Peritonitis, pancreatitis, leukocytosis | Large retro-peritoneal fluid collection on CT | Retroperitoneal fluids, bile stain and sealed perforation. Drainage of retroperitoneum. Re-operation on POD 1: duodenostomy, sphincterotomy site sutured, T-tube, duodenostomy tube, JP drains | 18, death from sepsis |
| 2/77/M | Intra-ERCP/9 h | No pain | No retroperitoneal air or fluid | Bile-stained retroperitoneum, no perforation identified. Cholecystectomy, cholangiogram, CBDE, transduodenal sphincterotomy to remove CBD stone | 21 |
| 3/75/M | 20 h at 2nd ERCP/28 h | Severe abdominal pain, afebrile, leukocytosis | UGI study showed retro-peritoneal perforation at 22 h | Bile stain, no perforation identified. Cholecystectomy, and drainage of retroperitoneum. Re-operation on POD 30 for continuing sepsis. Drainage of retroperitoneum, duodenostomy tube, gastrostomy tube | 54, death from sepsis |
| 4/31/F | 25 h/36 h | Severe abdominal pain, afebrile, pancreatitis | Large retroperitoneal air and fluid collection on CT at 25 h | Bile stain, perforation not identified. Cholecystectomy, T-tube, feeding J-tube, drainage of retroperitoneum. Repeated percutaneous drainage of intra-abdominal abscess, and 2nd OR on POD 27 for open drainage of abdominal abscess | 60 |
| 5/69/M | 2 days/28 days | Mild abdominal pain, leukocytosis, febrile | Retroperitoneal air, liver abscess on CT on day 2. Normal UGI study on day 7 | Subhepatic abscess, which was drained at OR. Repeat ERCP on day 48 revealed pus in CBD, sphincterotomy and biliary stent placed | 80, death from sepsis |
Surgery for periampullary perforations was required in 5 of 11 patients; 2 patients required reoperation and in all of these patients their first operation was retroperitoneal drainage only. Both of these patients died of sepsis. The other two deaths in this group were after withdrawal of care and after an extended delay in surgery. All the patients who died had a delay in diagnosis of their perforation (approximately 2 days).
Three patients had duodenal perforations and all underwent surgery. Their clinical courses are summarized in Table III. The third patient returned to hospital following an elective laparoscopic cholecystectomy with abdominal pain, shock, and respiratory failure. An urgent ERCP through the afferent limb of a Billroth II reconstruction revealed a cystic duct leak. At surgery she was found to have a perforation in the second part of her duodenum. She died of sepsis.
Table III. Surgical management of the duodenal perforation group.
| Patient no./age/sex | Time to diagnosis/surgery | Presentation | Radiological findings | Surgical findings and management | Length of stay (days) |
|---|---|---|---|---|---|
| 1/52/M | 5 days/5 days | Localized peritonitis, fever, leukocytosis on day 1 | Retro-peritoneal fluids on CT on day 2. Extravasation of oral contrast at duodenum on 2nd CT on day 5. | Lateral duodenal perforation secondary to stent migration, which was oversewn with omental patch. G-tube and feeding J-tube. Postoperative intra-abdominal abscess requiring percutaneous drainage | 31 |
| 2/77/F | Intra-ERCP/2 h | Mild abdominal pain | Not done | Perforation at 2nd part of duodenum, which was oversewn with omental patch. Palliative chole-jejunostomy, gastro-jejunostomy, Roux-en-Y for unresectable pancreatic cancer. Drainage of retroperitoneum | 8 |
| 3/82/F | Intra-ERCP/6 h | Intubated for hemodynamic instability, massive subcutaneous emphysema on neck and chest | Not done | 1.5 cm duodenal wall perforation. Duodenostomy tube, drainage of retroperitoneum | 2, death from septic shock, multi-organ failure |
In the unknown group, only one perforation was identified during ERCP. The other six perforations were diagnosed by plain X-ray of the abdomen or CT scan for post-ERCP abdominal pain. All diagnoses were made within 36 hours post-ERCP. Only two patients required operation and the results of surgery are presented in Table IV. The rest were successfully managed with medical therapy.
Table IV. Surgical management of the unknown group.
| Patient no./age/sex | Time to diagnosis/surgery | Presentation | Radiological findings | Surgical findings and management | Length of stay (days) |
|---|---|---|---|---|---|
| 1/81/F | 28 h/31 h | Mild abdominal pain improved over 24 h | Pneumoperitoneum on X-ray at 28 h | No perforation identified. No bile leak. CBDE, removal of obstructing CBD stone, T-tube, drainage of retroperitoneum | 22 |
| 2/87/F | Intra-ERCP/5 h | Mild abdominal pain; afebrile | Retroperitoneal air on X-ray at 4 h | No perforation identified. Bile stains. CBDE, removal of CBD stones, T-tube, drainage of retroperitoneum | 18 |
Discussion
The management of ERCP-related perforations has been controversial. Some authors 12,13 have advocated early operations for all endoscopic sphincterotomy (ES) perforation. With increasing experience with this rare but potentially lethal complication, there is increasing evidence that most perforations may be managed without surgery 8,10,11. Thus, the difficulty lies with the early detection of those patients who will need surgery.
Guidewire perforations are benign and in general do not need surgery. Only one of the seven guidewire perforations had a biliary stent placed specifically for the perforation. Our experience suggests that these perforations seal quickly and biliary stenting may not be needed. This has the advantage of saving the patient from undergoing a subsequent ERCP to remove the stent.
Comparing our series of peri-ampullary perforations to that reported by Howard et al. 5 demonstrated a surprisingly high morbidity and mortality. Our group had an average length of hospital stay of 26.5 days vs 8.5 days, and our mortality rate was 36% vs 5% in Howard et al.'s series. We believe that the superior results in Howard et al.'s study were due to two factors: early diagnosis and aggressive endoscopic diversion of bile away from site of perforation. Twenty of 22 (91%) peri-ampullary perforations were recognized during ERCP compared with 4 of 11 (36%) in our series. If a perforation is not recognized or suspected during ERCP, it would be difficult to make the diagnosis early (i.e. within 12 hours), as a large percentage of patients have some abdominal symptoms after ES 3,9. The perforation is usually diagnosed with X-ray or CT scan after the patient's pain persists. The diagnosis is especially likely to be delayed if the patient has concurrent elevated lipase, and the pain is attributed to ERCP-induced pancreatitis.
Dunham et al. 10 first advocated repeat ERCP after perforation to ensure that residual CBD stones and blood clots from sphincterotomy are cleared and to ensure that bile is flowing into the duodenum rather than the retroperitoneum. However, they were against inserting a biliary stent because they felt that the presence of the foreign body might prevent the healing of the perforation. Twenty of 22 patients in Howard et al.'s series had aggressive bile diversion away from site of perforation in the form of biliary stents or naso-biliary tubes. Only 2 of 11 of the peri-ampullary perforations in our series had any biliary diversion in the form of endoscopic stents.
CT appears to be the diagnostic imaging method of choice 14. It is very sensitive in detecting retroperitoneal air. However, clinically asymptomatic retroperitoneal perforations are common. The presence of retroperitoneal air on post-ERCP CT scans in consecutive, asymptomatic patients has been reported to be 29% 15. CT findings of large retroperitoneal fluid collection also have important prognostic value. Several studies suggested that patients with retroperitoneal fluid collection have worse prognosis and require surgical intervention 6,9,14. Two patients in our peri-ampullary group had large retroperitoneal fluid collections on CT. Both required surgery: one died and the other had a prolonged hospital stay of 60 days. We also feel that the finding of retroperitoneal fluid collection suggests continuing bile leak from the site of perforation, and is an indication for surgical intervention.
The amount of retroperitoneal air is not correlated with clinical course, and is more a function of amount of air used during ERCP. Patients who were treated medically in our series had repeat CT scans, which all showed decreasing amounts of air, indicating that there was no continuing leak. Sometimes pneumomediastinum is seen in addition to the retroperitoneal air and this, in itself, is not an indication for surgery. We had three patients, one in the peri-ampullary group and two in the unknown group, who had pneumomediastinum, and none required an operation.
Peri-ampullary perforations are often difficult to identify in the operating room. The surgeon may only see a bile-stained retroperitoneum after kocherizing the duodenum. The fact that two of our deaths occurred after simple drainage procedures raises the question of whether diverting bile and succus entericus away from the perforation site is indicated. This could include the insertion of a T-tube in the CBD and placement of a duodenostomy tube. Duodenal diverticulization or pyloric exclusion may be a reasonable alternative. It is clear from our data that a delay in diagnosis and treatment was associated with significant morbidity and mortality.
The ‘unknown’ group in our series comprises a mix of guidewire and peri-ampullary perforations. Duodenal perforations require surgical intervention. The operation of choice depends on the size of perforation and the state of the duodenum at the time of operation. Simple oversewing with omental patch was successful in two patients in our series.
Other factors associated with a risk of ERCP-related perforations are Billroth II reconstruction following gastrectomy (present in 2 patients), and pre-cut sphincterotomy (present in 7 of 11 peri-ampullary perforations).
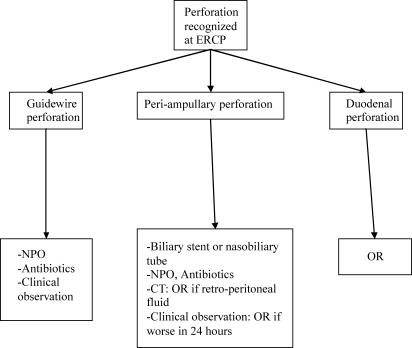
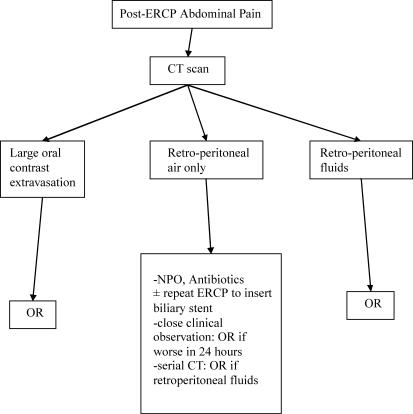
Based on our experience and that of others, we advocate a selective management algorithm for ERCP-related perforations, as shown in Figures 1 and 2. In summary, guidewire perforations are benign in nature, and can be treated with medical therapy. Peri-ampullary perforations are associated with a high morbidity and mortality, and should have aggressive endoscopic bile diversion from the site of perforation. Delay in diagnosis and surgery results in a worse outcome. As a result, there should be a high index of suspicion for perforation in any patient with significant abdominal pain following therapeutic ERCP, and an early CT scan should be considered in these patients. A CT finding of retroperitoneal fluid collection has a poor prognosis, and is an indication for immediate surgery. Duodenal perforation is the least common, and requires surgery.
Figure 1. .
Management algorithm for perforation recognized at ERCP.
Figure 2. .
Management algorithm for perforation not recognized at ERCP.
References
- 1.Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909–18. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 2.Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, et al. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48:1–10. doi: 10.1016/s0016-5107(98)70121-x. [DOI] [PubMed] [Google Scholar]
- 3.Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–93. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 4.Masci E, Toti G, Mariani A, Curioni S, Lomazzi A, Dinelli M, et al. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol. 2001;96:417–23. doi: 10.1111/j.1572-0241.2001.03594.x. [DOI] [PubMed] [Google Scholar]
- 5.Howard TJ, Tan T, Lehman GA, Sherman S, Madura JA, Fogel E, et al. Classification and management of perforations complicating endoscopic sphincterotomy. Surgery. 1999;126:658–63. [PubMed] [Google Scholar]
- 6.Stapfer M, Selby RR, Stain SC, Katkhouda N, Parekh D, Jabbour N, et al. Management of duodenal perforation after endoscopic retrograde cholangiopancreatography and sphincterotomy. Ann Surg. 2000;232:191–8. doi: 10.1097/00000658-200008000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enns R, Eloubeidi MA, Mergener K, Jowell PS, Branch MS, Pappas TM, et al. ERCP-related perforations: risk factors and management. Endoscopy. 2002;34:293–8. doi: 10.1055/s-2002-23650. [DOI] [PubMed] [Google Scholar]
- 8.Scarlett PY, Falk GL. The management of perforation of the duodenum following endoscopic sphincterotomy: a proposal for selective therapy. Aust N Z J Surg. 1994;64:843–6. doi: 10.1111/j.1445-2197.1994.tb04561.x. [DOI] [PubMed] [Google Scholar]
- 9.Sarr MG, Fishman EK, Milligan FD, Siegelman SS, Cameron JL. Pancreatitis or duodenal perforation after peri-Vaterian therapeutic endoscopic procedures: diagnosis, differentiation, and management. Surgery. 1986;100:461–6. [PubMed] [Google Scholar]
- 10.Dunham F, Bourgeois N, Gelin M, Jeanmart J, Toussaint J, Cremer M. Retroperitoneal perforations following endoscopic sphincterotomy; clinical course and management. Endoscopy. 1982;14:92–6. doi: 10.1055/s-2007-1021589. [DOI] [PubMed] [Google Scholar]
- 11.Chung RS, Sivak MV, Ferguson DR. Surgical decisions in the management of duodenal perforation complicating endoscopic sphincterotomy. Am J Surg. 1993;165:700–3. doi: 10.1016/s0002-9610(05)80791-3. [DOI] [PubMed] [Google Scholar]
- 12.Booth FV, Doerr RJ, Khalafi RS, Luchette FA, Flint LM., Jr Surgical management of complications of endoscopic sphincterotomy with precut papillotomy. Am J Surg. 1990;159:132–5. doi: 10.1016/s0002-9610(05)80618-x. [DOI] [PubMed] [Google Scholar]
- 13.Mustard R, Jr, Mackenzie R, Jamieson C, Haber GB. Surgical complications of endoscopic sphincterotomy. Can J Surg. 1984;27:215–17. [PubMed] [Google Scholar]
- 14.Zissin R, Shapiro-Feinberg M, Oscadchy A, Pomeranz I, Leichtmann G, Novis B. Retroperitoneal perforation during endoscopic sphincterotomy: imaging findings. Abdom Imaging. 2000;25:279–82. doi: 10.1007/s002610000033. [DOI] [PubMed] [Google Scholar]
- 15.Genzlinger JL, McPhee MS, Fisher JK, Jacob KM, Helzberg JH. Significance of retroperitoneal air after endoscopic retrograde cholangiopancreatography with sphincterotomy. Am J Gastroenterol. 1999;94:1267–70. doi: 10.1111/j.1572-0241.1999.00996.x. [DOI] [PubMed] [Google Scholar]