Abstract
The ability to diagnose pancreatic carcinoma has been rapidly improving with the recent advances in diagnostic techniques such as contrast-enhanced Doppler ultrasound (US), helical computed tomography (CT), enhanced magnetic resonance imaging (MRI), and endoscopic US (EUS). Each technique has advantages and limitations, making the selection of the proper diagnostic technique, in terms of purpose and characteristics, especially important. Abdominal US is the modality often used first to identify a cause of abdominal pain or jaundice, while the accuracy of conventional US for diagnosing pancreatic tumors is only 50–70%. CT is the most widely used imaging examination for the detection and staging of pancreatic carcinoma. Pancreatic adenocarcinoma is generally depicted as a hypoattenuating area on contrast-enhanced CT. The reported sensitivity of helical CT in revealing pancreatic carcinoma is high, ranging between 89% and 97%. Multi-detector-row (MD) CT may offer an improvement in the early detection and accurate staging of pancreatic carcinoma. It should be taken into consideration that some pancreatic adenocarcinomas are depicted as isoattenuating and that pancreatitis accompanied by pancreatic adenocarcinoma might occasionally result in the overestimation of staging. T1-weighted spin-echo images with fat suppression and dynamic gradient-echo MR images enhanced with gadolinium have been reported to be superior to helical CT for detecting small lesions. However, chronic pancreatitis and pancreatic carcinoma are not distinguished on the basis of degree and time of enhancement on dynamic gadolinium-enhanced MRI. EUS is superior to spiral CT and MRI in the detection of small tumors, and can also localize lymph node metastases or vascular tumor infiltration with high sensitivity. EUS-guided fine-needle aspiration biopsy is a safe and highly accurate method for tissue diagnosis of patients with suspected pancreatic carcinoma. 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) has been suggested as a promising modality for noninvasive differentiation between benign and malignant lesions. Previous studies reported the sensitivity and specificity of FDG-PET for detecting malignant pancreatic tumors as being 71–100% and 64–90%, respectively. FDG-PET does not replace, but is complementary to morphologic imaging, and therefore, in doubtful cases, the method must be combined with other imaging modalities.
Keywords: Pancreas, carcinoma, CT, MRI, ultrasonography, PET
Introduction
Pancreatic ductal adenocarcinoma has one of the most unfavorable prognoses. The only curative treatment is surgery, but the reported 5-year survival rates are only from 2 to 10% 1,2. To raise these survival rates, early and accurate diagnosis is required. There are three steps in the diagnosis of pancreatic carcinoma before deciding on the treatment approach. The first step is to detect the tumor. One of the reasons for the low survival rates is the difficulty in making an early diagnosis. The higher the sensitivity for detecting pancreatic tumors, the greater the number of patients with early pancreatic cancer can be expected to be. The next step is to differentiate pancreatic adenocarcinoma from other pancreatic diseases such as chronic pancreatitis, benign or malignant islet cell tumor, and intraductal papillary mucinous neoplasm. Finally, imaging should be able to permit staging of the tumor. In the case of pancreatic cancer, any infiltration of vessels and lymph nodes as well as possible distant metastases takes on special importance due to the impact on the assessment of resectability of the tumor or the decision to initiate chemotherapy.
In recent years, diagnostic imaging techniques such as multi-detector-row computed tomography (MDCT), magnetic resonance imaging (MRI) and endoscopic ultrasound (EUS) have been developed, elevating the ability to diagnose pancreatic carcinoma, although there are still inherent limitations. It is important to select the proper diagnostic technique in line with the purpose and characteristics of those procedures. This article reviews recent progress in the diagnosis of pancreatic adenocarcinoma.
Ultrasonography (US)
Abdominal US is often the first approach used in attempting to identify the cause of abdominal pain or jaundice, as it is a noninvasive and cost-effective method. Low echoic mass, dilatation of the pancreatic duct, and dilatation of the common bile duct on conventional US are signs of the presence of pancreatic tumor 3,4. By conventional US, most pancreatic tumors – including pancreatic adenocarcinoma, chronic pancreatitis, and endocrine cell tumors – are revealed as a hypoechoic area in the pancreas. In other words, there are no characteristic signs for the different pancreatic lesions. The accuracy of conventional US for diagnosing pancreatic tumors is only 50–70% 4.
Contrast-enhanced Doppler US has been proposed as a valuable technique for the diagnosis of pancreatic tumors. Characteristic signs of pancreatic tumors have been reported with the use of contrast-enhanced Doppler US 4. Pancreatic adenocarcinoma was found to be hypovascularized, whereas endocrine cell tumor is mostly hypervascularized and pancreatitis-associated mass is mostly isovascularized. Kitano et al. 5 assessed the usefulness of coded phase inversion harmonic imaging, a newly available sonographic technique based on a combination of phase inversion harmonics and coded technology, for the depiction and differential diagnosis of pancreatic tumors. They reported that the sensitivity and specificity of contrast-enhanced coded phase inversion harmonic US for pancreatic ductal carcinoma were 90% and 95%, respectively.
The presence of obscuring overlying bowel gas and the variable skill of the operator limit the sensitivity of US for identification and staging of pancreatic lesions. US may be an initial screening examination, generally followed by CT and MRI for more accurate diagnosis of pancreatic lesions.
CT
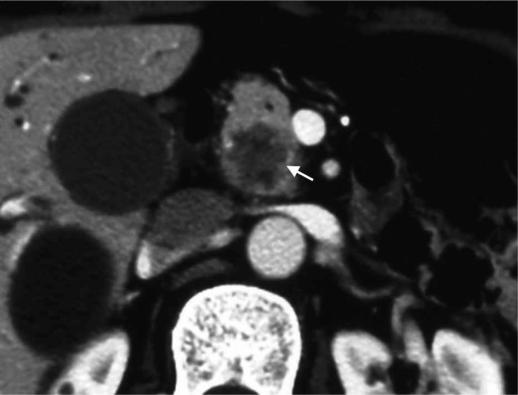
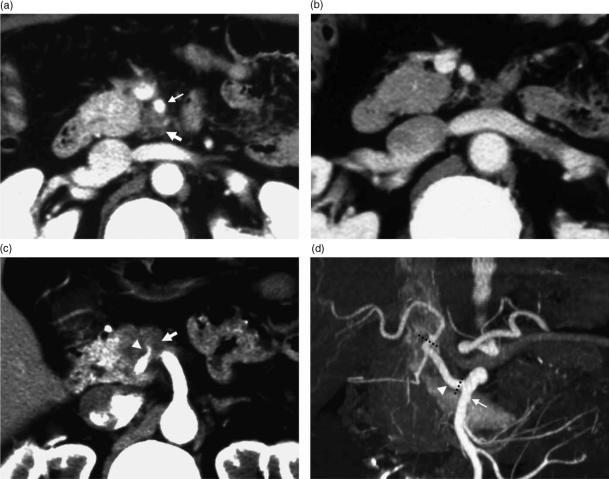
CT is the most widely used imaging examination for the detection and staging of pancreatic carcinoma. Pancreatic carcinoma is characterized by abundant fibrous stroma and hypovascularity (Figure 1), which accounts for the poor enhancement of the tumor compared with that of the surrounding pancreatic parenchyma, as seen in the early phase of dynamic CT and gradual enhancement at delayed CT. Lu et al. 6 reported that the mean tumor–pancreas contrast during the pancreatic phase (40–70 s after infusion of intravenous contrast material at 3 ml/s) was significantly greater than the hepatic phases (70–100 s after infusion) by implementing two-phase helical CT with scanning during both phases. Their technique has improved the detectability of pancreatic adenocarcinoma, which tends to be enhanced less than the surrounding parenchyma on pancreatic phase images. The reported sensitivity of helical CT in revealing pancreatic carcinoma is high, ranging between 89% and 97% 7. Pancreatic adenocarcinoma is generally depicted as a hypoattenuating area on contrast-enhanced CT, although some such tumors appear as isoattenuating areas. Prokesch et al. 8 reported that in 6 (11%) of 53 patients with pancreatic adenocarcinomas, the tumors were seen as isoattenuating. They also emphasized that indirect signs such as mass effect, atrophic distal parenchyma, and interrupted duct sign were important indicators of the presence of tumors with no visible tumor–pancreas contrast. It should also be kept in mind that pancreatitis accompanied by pancreatic adenocarcinoma might occasionally be the cause of staging overestimation. Thickening of the superior mesenteric artery is seen in both cancer invasion and infiltration of fat in acute or chronic pancreatitis 9 (Figure 2).
Figure 1. .
A 76-year-old man with pancreatic head carcinoma. CT revealed hypovascular tumor (arrow) in the head of the pancreas.
Figure 2. .
A 60-year-old man with pancreatic head carcinoma. (a) CT performed at a local hospital revealed a low-density area (thick arrow) surrounding the superior mesenteric artery (thin arrow). The tumor was diagnosed as inoperable due to invasion to the superior mesenteric artery. (b) CT performed 2 weeks later revealed that the low-density area surrounding the superior mesenteric artery had disappeared. A low-density area surrounding the superior mesenteric artery was considered as corresponding to the acute inflammation of pancreatitis accompanied by pancreatic carcinoma. Invasion to the superior mesenteric artery was ruled out. (c) CT at a more cranial slice revealed that a tributary of the superior mesenteric artery (arrowhead) was involved in the tumor (thick arrow). (d) Three-dimensional reconstruction CT revealed that the tributary of the superior mesenteric artery involved in the tumor was a replacement common hepatic artery (arrowhead). Pancreatoduodenectomy combined with resection of the replaced common hepatic artery was performed. The common hepatic artery was resected between its root and the root of the proper hepatic artery (dotted line) and reconstructed in an end-to-end fashion.
Several reports concluded that local extension of pancreatic cancer and invasion of adjacent vascular structures could be well depicted with helical CT, with the main limitations of this technique for preoperative staging being a difficulty in revealing unsuspected liver metastases and a low rate of revealing lymph node metastasis 7,10,11.
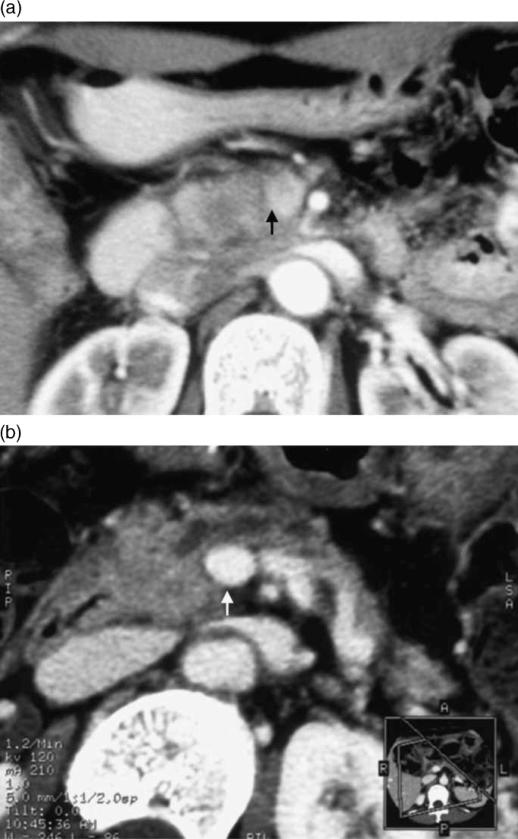
The recent development of MDCT allows the use of extremely thin collimation for the acquisition of high-resolution scans during multiple phases of contrast enhancement. Thus, greater parenchymal, arterial, and portal venous enhancement may be achieved when imaging the pancreas with MDCT, and this technique may offer an improvement in the early detection and accurate staging of pancreatic carcinoma 12,13,14. MDCT technology has allowed the acquisition of excellent three-dimensional images. Three-dimensional reconstruction techniques such as curved planar reformation, volume rendering, and maximum intensity projection can provide an excellent and quickly comprehensible overview of pertinent anatomy and structures (Figure 3) that are likely to be of great use to referring physicians and surgeons 12,13,14.
Figure 3. .
A 51-year-old woman with pancreatic head carcinoma. (a) Conventional CT performed at a local hospital revealed a low-density area in the head of the pancreas and the boundary to the portal vein was unclear (arrow). At that hospital, the tumor was diagnosed as inoperable due to portal vein invasion. (b) MPR (multi-planar reconstruction) images obtained by MDCT revealed that the portal vein was intact. Pancreatoduodenectomy combined with resection of the portal vein was performed. Histopathological examination showed no invasion to the portal vein (arrow).
Endoscopic retrograde cholangiopancreatography (ERCP)
Pancreatic carcinoma is detectable only if it impinges on the pancreatic duct, meaning that small early cancer and that situated in the uncinate process can be missed by this investigation. ERCP has the advantage of providing the opportunity both to sample for cytology or histology and to apply important therapy via biliary stenting for obstructive jaundice. The indication of ERCP for preoperative diagnosis of pancreatic cancer has been declining owing to advances of MR cholangiopancreatography (MRCP – see below). Diagnostic ERCP may not be indicated in patients with clinically evident pancreatic cancer, but it may be valuable if a tumor is suspected despite negative results on US and CT, or may be used as an additional aid to differentiate between chronic pancreatitis and cancer 11.
MRI
The ability of MRI to diagnose pancreatic carcinoma has improved in concert with improvements in the technology and its application. MRI offers several benefits for imaging of the pancreas. One is the better soft tissue contrast compared with CT before the administration of contrast material. Another is the possibility to examine the pancreatobiliary system noninvasively. MRCP obtained with long echo times on T2-weighted MR images can demonstrate the biliary and pancreatic ductal systems.
The typical appearance of pancreatic carcinoma on MRI is hypointense on T1-weighted images and hyperintense or isointense on T2-weighted images. The tumor shows diminished enhancement in the early phase of dynamic MR imaging and gradual enhancement in the late phase. T1-weighted spin-echo images with fat suppression and dynamic gradient-echo MR images enhanced with gadolinium have been reported to be superior to helical CT for detecting small lesions 15,16. Mangafodipir trisodium, a contrast agent originally designed for MRI of the liver, also shows uptake in the pancreatic parenchyma but not in pancreatic tumors. In a study comparing gadolinium- and mangafodipir trisodium-enhanced MR images of patients with suspected pancreatic tumor, gradient-recalled echo images enhanced with mangafodipir trisodium were significantly better at delineating pancreatic tumors than those enhanced with gadolinium chelates 17.
Chronic pancreatitis remains difficult to differentiate from pancreatic carcinoma on the basis of imaging criteria since both demonstrate low signal intensity on T1-weighted images and are associated with pancreatic and/or biliary ductal obstruction. Jenkins et al. 18 found no statistically significant difference in T1 and T2 between chronic pancreatitis and pancreatic carcinoma. Johnson and Outwater 19 assessed the ability of dynamic gadolinium-enhanced MRI for differentiating chronic pancreatitis and pancreatic carcinoma, concluding that they could not be distinguished on the basis of degree and time of enhancement. Ichikawa et al. 20 reported that the duct-penetrating sign on MRCP images was more helpful for distinguishing chronic pancreatitis from pancreatic carcinoma than were the enhancement patterns on CT and MR images.
Both gadolinium- and mangafodipir trisodium-enhanced MR images are useful for evaluating local tumor extension and vascular involvement of pancreatic carcinoma. Enhanced MRI has equal or better accuracy than helical CT in determination of local tumor extent and vascular involvement except for duodenal invasion and portal venous system involvement 20,21,22.
EUS
The typical features of pancreatic carcinoma seen by EUS include an inhomogeneous solid mass with irregular borders that appears hypoechoic compared with normal pancreatic parenchyma. EUS is highly sensitive in the detection of small tumors and invasion of major vascular structures. Thus, EUS is superior to spiral CT, MRI and PET in the detection of small tumors 23,24, and it can also locate lymph node metastases and vascular tumor infiltration with high sensitivity 25. The major drawbacks of the technique are operator dependence and limited field of visualization for detecting metastatic spread to the liver and peritoneum 23. EUS-guided fine needle aspiration biopsy is a safe and highly accurate method for tissue diagnosis of patients with suspected pancreatic carcinoma 26,27. While a positive diagnosis can be relied upon for a management decision on the basis of high specificity and a positive predictive value, a negative result cannot be completely reassuring. Considerable limitations of the EUS-guided fine needle aspiration biopsy in the diagnosis of pancreatic lesions are a relatively high number of inadequate specimens and false negative results 28.
Positron emission tomography (PET)
PET using the radiolabeled glucose analog 18F-fluorodeoxyglucose (FDG) has been suggested as a promising modality for noninvasive differentiation between benign and malignant lesions. Increased glucose utilization due to an increased number of glucose transporter proteins and increased hexokinase and phosphofructokinase activity is commonly found in malignant tumors. Previous studies reported the sensitivity and specificity of FDG-PET for detecting malignant pancreatic tumors to be 71–100% and 64–90%, respectively 29. There are certain limitations of FDG-PET imaging in the diagnosis of pancreatic carcinoma. Chronic and acute pancreatitis can accumulate FDG and result in false positive interpretations on PET imaging. It is well known that sensitivity in hyperglycemic patients tends to be lower than in euglycemic patients, as elevated serum glucose levels result in decreased FDG uptake in tumors by up to 50% due to competitive inhibition. False negative studies may also occur when the tumor is <1 cm 30. This functional imaging does not replace, but is complementary to morphologic imaging, and thus, in doubtful cases, the method must be combined with other imaging modalities.
There are other roles of FDG-PET in the management of patients with pancreatic carcinoma. It is useful for evaluating the prognostic value and for monitoring treatment. Nakata et al. 31 reported that patients with pancreatic adenocarcinoma with low FDG uptake survived longer than patients with high uptake. Maisey et al. 32 reported that the absence of FDG uptake at 1 month following chemotherapy for carcinoma of the pancreas is an indicator of improved overall survival.
Conclusion
Recent advances in diagnostic techniques such as contrast-enhanced Doppler US, helical CT, enhanced MRI, and EUS have led to improvements in sensitivity for identifying pancreatic carcinoma. However, differential diagnosis between pancreatic carcinoma and chronic pancreatitis still remains difficult. EUS-guided fine needle aspiration biopsy and FDG-PET may help to differentiate those diseases. For staging pancreatic carcinoma, CT, MRI, and EUS are all valuable, but their limitations must also be taken into account.
References
- 1.Nakagohri T, Kinoshita T, Konishi M, Inoue K, Takahashi S. Survival benefits of portal vein resection for pancreatic cancer. Am J Surg. 2003;186:149–53. doi: 10.1016/s0002-9610(03)00173-9. [DOI] [PubMed] [Google Scholar]
- 2.Kobari M, Matsuno S. Staging systems for pancreatic cancer: differences between the Japanese and UICC systems. J Hepatobiliary Pancreat Surg. 1998;5:121–7. doi: 10.1007/s005340050021. [DOI] [PubMed] [Google Scholar]
- 3.Karlson BM, Ekbom A, Lindgren PG, Kallskog V, Rostad J. Abdominal US for diagnosis of pancreatic tumor: prospective cohort analysis. Radiology. 1999;213:107–11. doi: 10.1148/radiology.213.1.r99oc25107. [DOI] [PubMed] [Google Scholar]
- 4.Rickes S, Unkrodt K, Neye H, Okran KW, Wermke W. Differentiation of pancreatic tumours by conventional ultrasound, unenhanced and echo-enhanced power Doppler sonography. Scand J Gastroenterol. 2002;37:1313–20. doi: 10.1080/003655202761020605. [DOI] [PubMed] [Google Scholar]
- 5.Kitano M, Kudo M, Maekawa K, Suetomi Y, Sakamoto H, Fukuta N, et al. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut. 2004;53:854–9. doi: 10.1136/gut.2003.029934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu DS, Vedantham S, Krasny RM, Kadell B, Berger WL, Reber HA. Two-phase helical CT for pancreatic tumors: pancreatic versus hepatic phase enhancement of tumor, pancreas, and vascular structures. Radiology. 1996;199:697–701. doi: 10.1148/radiology.199.3.8637990. [DOI] [PubMed] [Google Scholar]
- 7.Valls C, Andia E, Sanchez A, Fabregat J, Pozuelo O, Quintero JC, et al. Dual-phase helical CT of pancreatic adenocarcinoma: assessment of resectability before surgery. AJR Am J Roentgenol. 2002;178:821–6. doi: 10.2214/ajr.178.4.1780821. [DOI] [PubMed] [Google Scholar]
- 8.Prokesch RW, Chow LC, Beaulieu CF, Bammer R, Jeffrey RB., Jr Isoattenuating pancreatic adenocarcinoma at multi-detector row CT: secondary signs. Radiology. 2002;224:764–8. doi: 10.1148/radiol.2243011284. [DOI] [PubMed] [Google Scholar]
- 9.Schulte SJ, Baron RL, Freeny PC, Patten RM, Gorell HA, Maclin ML. Root of the superior mesenteric artery in pancreatitis and pancreatic carcinoma: evaluation with CT. Radiology. 1991;180:659–62. doi: 10.1148/radiology.180.3.1871275. [DOI] [PubMed] [Google Scholar]
- 10.Roche CJ, Hughes ML, Garvey CJ, Campbell F, White DA, Jones L, et al. CT and pathologic assessment of prospective nodal staging in patients with ductal adenocarcinoma of the head of the pancreas. AJR Am J Roentgenol. 2003;180:475–80. doi: 10.2214/ajr.180.2.1800475. [DOI] [PubMed] [Google Scholar]
- 11.Andersson R, Vagianos C, Williamson R. Preoperative staging and evaluation of resectability in pancreatic ductal adenocarcinoma. HPB. 2004;6:5–12. doi: 10.1080/13651820310017093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catalano C, Laghi A, Fraioli F, Pediconi F, Napoli A, Danti M, et al. Pancreatic carcinoma: the role of high-resolution multislice spiral CT in the diagnosis and assessment of resectability. Eur Radiol. 2003;13:149–56. doi: 10.1007/s00330-002-1473-4. [DOI] [PubMed] [Google Scholar]
- 13.Prokesch RW, Chow LC, Beaulieu CF, Nino-Murcia M, Mindelzun RE, Bammer R, et al. Local staging of pancreatic carcinoma with multi-detector row CT: use of curved planar reformations initial experience. Radiology. 2002;225:759–65. doi: 10.1148/radiol.2253010886. [DOI] [PubMed] [Google Scholar]
- 14.Vargas R, Nino-Murcia M, Trueblood W, Jeffrey RB., Jr MDCT in pancreatic adenocarcinoma: prediction of vascular invasion and resectability using a multiphasic technique with curved planar reformations. AJR Am J Roentgenol. 2004;182:419–25. doi: 10.2214/ajr.182.2.1820419. [DOI] [PubMed] [Google Scholar]
- 15.Gabata T, Matsui O, Kadoya M, Yoshikawa J, Miyayama S, Takashima T, et al. Small pancreatic adenocarcinomas: efficacy of MR imaging with fat suppression and gadolinium enhancement. Radiology. 1994;193:683–8. doi: 10.1148/radiology.193.3.7972808. [DOI] [PubMed] [Google Scholar]
- 16.Vellet AD, Romano W, Bach DB, Passi RB, Taves DH, Munk PL. Adenocarcinoma of the pancreatic ducts: comparative evaluation with CT and MR imaging at 1.5 T. Radiology. 1992;183:87–95. doi: 10.1148/radiology.183.1.1312736. [DOI] [PubMed] [Google Scholar]
- 17.Diehl SJ, Lehmann KJ, Gaa J, McGill S, Hoffmann V, Georgi M. MR imaging of pancreatic lesions. Comparison of manganese-DPDP and gadolinium chelate. Invest Radiol. 1999;34:589–95. doi: 10.1097/00004424-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins JP, Braganza JM, Hickey DS, Isherwood I, Machin M. Quantitative tissue characterisation in pancreatic disease using magnetic resonance imaging. Br J Radiol. 1987;60:333–41. doi: 10.1259/0007-1285-60-712-333. [DOI] [PubMed] [Google Scholar]
- 19.Johnson PT, Outwater EK. Pancreatic carcinoma versus chronic pancreatitis: dynamic MR imaging. Radiology. 1999;212:213–18. doi: 10.1148/radiology.212.1.r99jl16213. [DOI] [PubMed] [Google Scholar]
- 20.Ichikawa T, Sou H, Araki T, Arbab AS, Yoshikawa T, Ishigame K, et al. Duct-penetrating sign at MRCP: usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology. 2001;221:107–16. doi: 10.1148/radiol.2211001157. [DOI] [PubMed] [Google Scholar]
- 21.Schima W, Fugger R, Schober E, Oettl C, Wamser P, Grabenwager F, et al. Diagnosis and staging of pancreatic cancer: comparison of mangafodipir trisodium-enhanced MR imaging and contrast-enhanced helical hydro-CT. AJR Am J Roentgenol. 2002;179:717–24. doi: 10.2214/ajr.179.3.1790717. [DOI] [PubMed] [Google Scholar]
- 22.Romijn MG, Stoker J, van Eijck CH, van Muiswinkel JM, Torres CG, Lameris JS, et al. MRI with mangafodipir trisodium in the detection and staging of pancreatic cancer. J Magn Reson Imaging. 2000;12:261–8. doi: 10.1002/1522-2586(200008)12:2<261::aid-jmri8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 23.Muller MF, Meyenberger C, Bertschinger P, Schaer R, Marincek B. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology. 1994;190:745–51. doi: 10.1148/radiology.190.3.8115622. [DOI] [PubMed] [Google Scholar]
- 24.Legmann P, Vignaux O, Dousset B, Baraza AJ, Palazzo L, Dumontier I, et al. Pancreatic tumors: comparison of dual-phase helical CT and endoscopic sonography. AJR Am J Roentgenol. 1998;170:1315–22. doi: 10.2214/ajr.170.5.9574609. [DOI] [PubMed] [Google Scholar]
- 25.Rosewicz S, Wiedenmann B. Pancreatic carcinoma. Lancet. 1997;349:485–9. doi: 10.1016/s0140-6736(96)05523-7. [DOI] [PubMed] [Google Scholar]
- 26.Chang KJ, Nguyen P, Erickson RA, Durbin TE, Katz KD. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45:387–93. doi: 10.1016/s0016-5107(97)70149-4. [DOI] [PubMed] [Google Scholar]
- 27.Vander Noot MR, 3rd, Eloubeidi MA, Chen VK, Eltoum I, Jhala D, Jhala N, et al. Diagnosis of gastrointestinal tract lesions by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer. 2004;102:157–63. doi: 10.1002/cncr.20360. [DOI] [PubMed] [Google Scholar]
- 28.Brand B, Pfaff T, Binmoeller KF, Sriram PV, Fritscher-Ravens A, Knofel WT, et al. Endoscopic ultrasound for differential diagnosis of focal pancreatic lesions, confirmed by surgery. Scand J Gastroenterol. 2000;35:1221–8. doi: 10.1080/003655200750056736. [DOI] [PubMed] [Google Scholar]
- 29.Sendler A, Avril N, Helmberger H, Stollfuss J, Weber W, Bengel F, et al. Preoperative evaluation of pancreatic masses with positron emission tomography using 18F-fluorodeoxyglucose: diagnostic limitations. World J Surg. 2000;24:1121–9. doi: 10.1007/s002680010182. [DOI] [PubMed] [Google Scholar]
- 30.Delbeke D, Pinson CW. Pancreatic tumors: role of imaging in the diagnosis, staging, and treatment. J Hepatobiliary Pancreat Surg. 2004;11:4–10. doi: 10.1007/s00534-002-0775-x. [DOI] [PubMed] [Google Scholar]
- 31.Nakata B, Chung YS, Nishimura S, Nishihara T, Sakurai Y, Sawada T, et al. 18F-fluorodeoxyglucose positron emission tomography and the prognosis of patients with pancreatic adenocarcinoma. Cancer. 1997;79:695–9. [PubMed] [Google Scholar]
- 32.Maisey NR, Webb A, Flux GD, Padhani A, Cunningham DC, Ott RJ, et al. FDG-PET in the prediction of survival of patients with cancer of the pancreas: a pilot study. Br J Cancer. 2000;83:287–93. doi: 10.1054/bjoc.2000.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]