Abstract
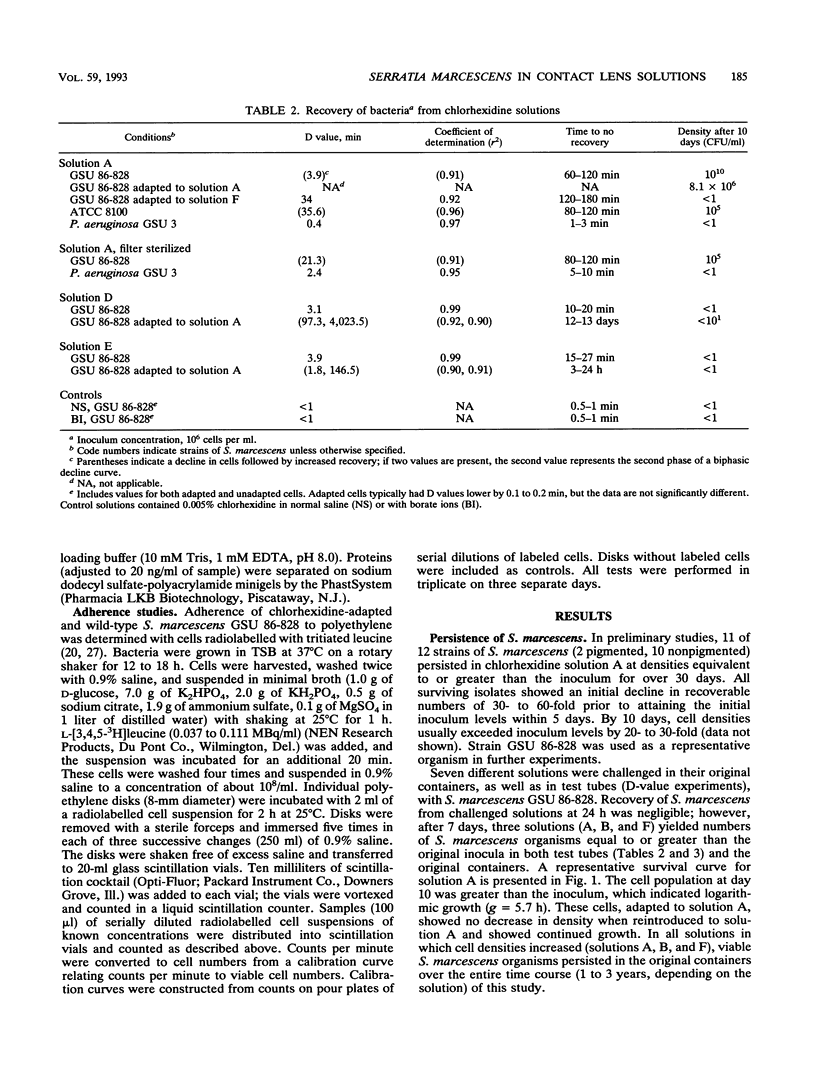
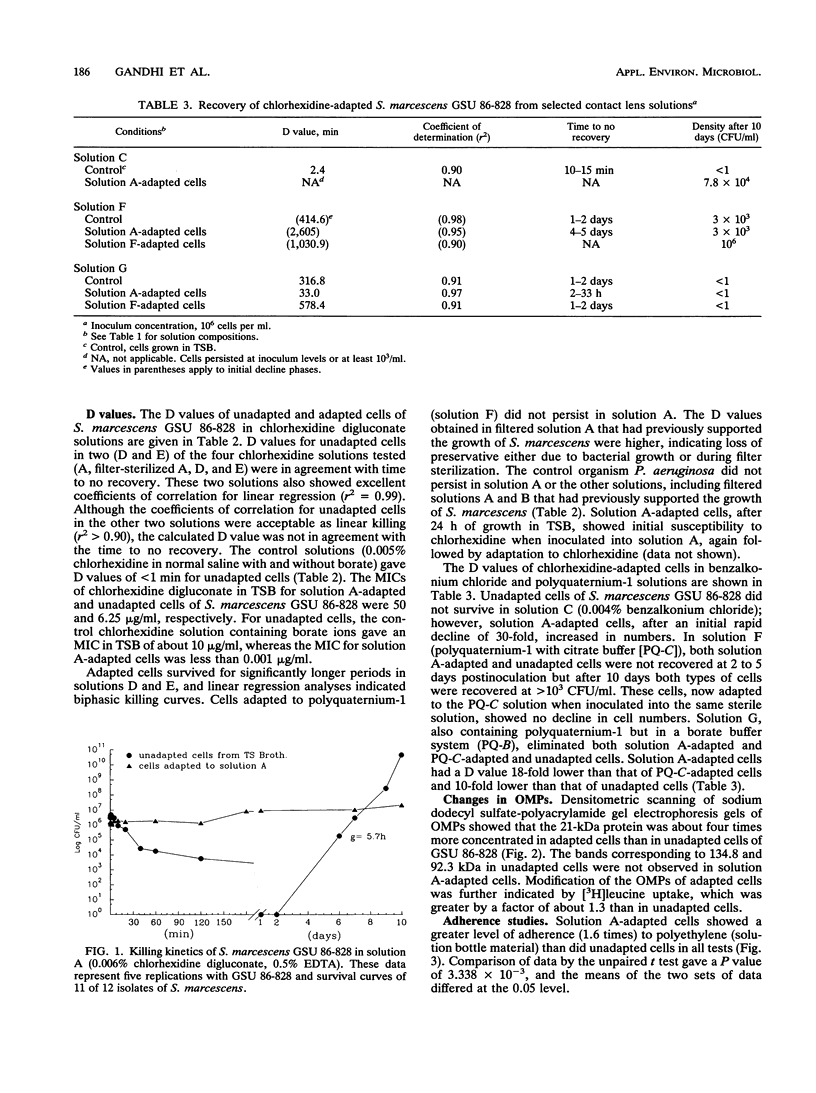
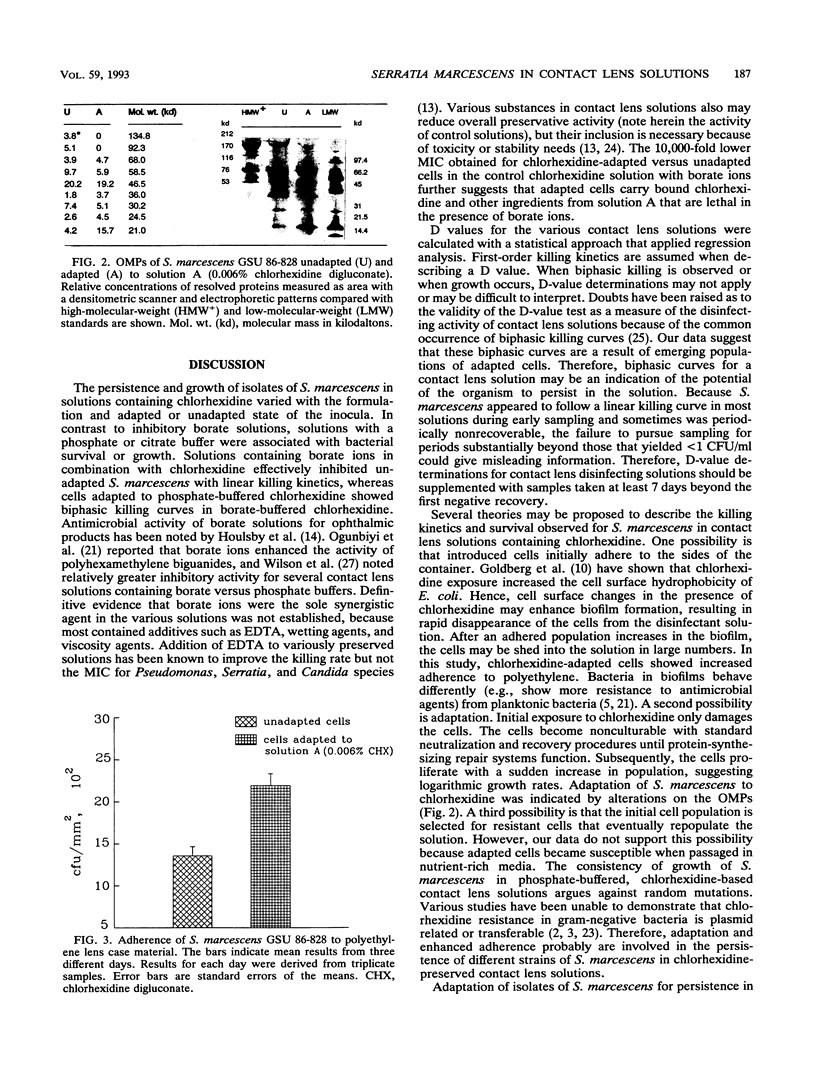
Serratia marcescens (11 of 12 strains) demonstrated an ability to grow in certain chlorhexidine-based disinfecting solutions recommended for rigid gas-permeable contact lenses. For a representative strain, cells that were grown in nutrient-rich medium, washed, and inoculated into disinfecting solution went into a nonrecoverable phase within 24 h. However, after 4 days, cells that had the ability to grow in the disinfectant (doubling time, g = 5.7 h) emerged. Solutions supporting growth of S. marcescens were filter sterilized. These solutions, even after removal of the cells, showed bactericidal activity against Pseudomonas aeruginosa and a biphasic survival curve when rechallenged with S. marcescens. Adaptation to chlorhexidine by S. marcescens was not observed in solutions formulated with borate ions. For chlorhexidine-adapted cells, the MIC of chlorhexidine in saline was eightfold higher than that for unadapted cells. Cells adapted to chlorhexidine showed alterations in the proteins of the outer membrane and increased adherence to polyethylene. Cells adapted to chlorhexidine persisted or grew in several other contact lens solutions with different antimicrobial agents, including benzalkonium chloride.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahearn D. G., Penley C. A., Wilson L. A. Growth and survival of Serratia marcescens in hard contact lens wetting solutions. CLAO J. 1984 Apr-Jun;10(2):172–174. [PubMed] [Google Scholar]
- Al-Najjar A. R., Quesnel L. B. Synergism between chlorhexidine and polymyxins against Pseudomonas aeruginosa. J Appl Bacteriol. 1979 Dec;47(3):469–476. doi: 10.1111/j.1365-2672.1979.tb01208.x. [DOI] [PubMed] [Google Scholar]
- Donzis P. B., Mondino B. J., Weissman B. A., Bruckner D. A. Microbial contamination of contact lens care systems. Am J Ophthalmol. 1987 Oct 15;104(4):325–333. doi: 10.1016/0002-9394(87)90219-4. [DOI] [PubMed] [Google Scholar]
- Goldberg S., Konis Y., Rosenberg M. Effect of cetylpyridinium chloride on microbial adhesion to hexadecane and polystyrene. Appl Environ Microbiol. 1990 Jun;56(6):1678–1682. doi: 10.1128/aem.56.6.1678-1682.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein G., Berman C., Jaffin R. Chlorhexidine. An adjunct to periodontal therapy. J Periodontol. 1986 Jun;57(6):370–377. doi: 10.1902/jop.1986.57.6.370. [DOI] [PubMed] [Google Scholar]
- HUGO W. B., LONGWORTH A. R. CYTOLOGICAL ASPECTS OF THE MODE OF ACTION OF CHLORHEXIDINE DIACETATE. J Pharm Pharmacol. 1965 Jan;17:28–32. doi: 10.1111/j.2042-7158.1965.tb07562.x. [DOI] [PubMed] [Google Scholar]
- HUGO W. B., LONGWORTH A. R. SOME ASPECTS OF THE MODE OF ACTION OF CHLORHEXIDINE. J Pharm Pharmacol. 1964 Oct;16:655–662. doi: 10.1111/j.2042-7158.1964.tb07384.x. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlsby R. D., Ghajar M., Chavez G. O. Antimicrobial activity of borate-buffered solutions. Antimicrob Agents Chemother. 1986 May;29(5):803–806. doi: 10.1128/aac.29.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlsby R. D., Ghajar M., Chavez L. Effects of preservatives, steroids, and ethylenediaminetetraacetate on the antimicrobial activity of sulfacetamide. J Pharm Sci. 1983 Dec;72(12):1401–1403. doi: 10.1002/jps.2600721208. [DOI] [PubMed] [Google Scholar]
- Lowe R., Vallas V., Brennan N. A. Comparative efficacy of contact lens disinfection solutions. CLAO J. 1992 Jan;18(1):34–40. [PubMed] [Google Scholar]
- Manning P. A., Beutin L., Achtman M. Outer membrane of Escherichia coli: properties of the F sex factor traT protein which is involved in surface exclusion. J Bacteriol. 1980 Apr;142(1):285–294. doi: 10.1128/jb.142.1.285-294.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo M. S., Schlitzer R. L., Ward M. A., Wilson L. A., Ahearn D. G. Association of Pseudomonas and Serratia corneal ulcers with use of contaminated solutions. J Clin Microbiol. 1987 Aug;25(8):1398–1400. doi: 10.1128/jcm.25.8.1398-1400.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. J., Ahearn D. G. Adherence of Pseudomonas aeruginosa to hydrophilic contact lenses and other substrata. J Clin Microbiol. 1987 Aug;25(8):1392–1397. doi: 10.1128/jcm.25.8.1392-1397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parment P. A., Rönnerstam R., Walder M. Persistence of Serratia marcescens, Serratia liquefaciens and E. coli in solutions for contact lenses. Acta Ophthalmol (Copenh) 1986 Aug;64(4):456–462. doi: 10.1111/j.1755-3768.1986.tb06953.x. [DOI] [PubMed] [Google Scholar]
- Russell A. D., Furr J. R. Susceptibility of porin- and lipopolysaccharide-deficient strains of Escherichia coli to some antiseptics and disinfectants. J Hosp Infect. 1986 Jul;8(1):47–56. doi: 10.1016/0195-6701(86)90104-0. [DOI] [PubMed] [Google Scholar]
- Russell A. D. The role of plasmids in bacterial resistance to antiseptics, disinfectants and preservatives. J Hosp Infect. 1985 Mar;6(1):9–19. doi: 10.1016/s0195-6701(85)80013-x. [DOI] [PubMed] [Google Scholar]
- Sutton S. V., Franco R. J., Porter D. A., Mowrey-McKee M. F., Busschaert S. C., Hamberger J. F., Proud D. W. D-value determinations are an inappropriate measure of disinfecting activity of common contact lens disinfecting solutions. Appl Environ Microbiol. 1991 Jul;57(7):2021–2026. doi: 10.1128/aem.57.7.2021-2026.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L. A., Sawant A. D., Ahearn D. G. Comparative efficacies of soft contact lens disinfectant solutions against microbial films in lens cases. Arch Ophthalmol. 1991 Aug;109(8):1155–1157. doi: 10.1001/archopht.1991.01080080115043. [DOI] [PubMed] [Google Scholar]
- el Moug T., Rogers D. T., Furr J. R., el-Falaha B. M., Russell A. D. Antiseptic-induced changes in the cell surface of a chlorhexidine-sensitive and a chlorhexidine-resistant strain of Providencia stuartii. J Antimicrob Chemother. 1985 Dec;16(6):685–689. doi: 10.1093/jac/16.6.685. [DOI] [PubMed] [Google Scholar]




