Abstract
Background. Obesity is often associated with increased biliary cholesterol secretion resulting in cholesterol gallstone formation. We have previously demonstrated that leptin-deficient C57Bl/6J Lep ob obese mice have abnormal biliary motility and are prone to cholesterol crystal formation. In addition, others have demonstrated that leptin-deficient mice when fed a lithogenic diet for eight weeks are not prone to gallstone formation. However, the biliary lipid and in vivo cholesterol crystal response of homozygous and heterozygous leptin-deficient mice to four weeks on a lithogenic diet has not been studied. Therefore, we tested the hypothesis that lithogenic diets influence gallbladder bile composition, serum lipids and cholesterol crystal formation in homozygous and heterozygous leptin-deficient mice compared to normal lean controls. Methods. 319 female lean control mice, 280 heterozygous lep ob obese mice and 117 homozygous lep ob obese mice were studied. Mice were fed either a lithogenic or control non-lithogenic chow diet for four weeks. Gallbladder volumes were measured, and bile was pooled to calculate cholesterol saturation indices. Serum cholesterol, glucose, and leptin levels were determined. Hepatic fat vacuoles were counted, and bile was observed microscopically for cholesterol crystal formation. Results. The lithogenic diet and mouse strain influenced body and liver weights, gallbladder volume, cholesterol crystal formation, serum cholesterol, glucose and leptin levels and hepatic fat vacuole numbers. However, only diet, not strain, altered biliary cholesterol saturation. Conclusion. The association among obesity, leptin, and gallstone formation may be primarily related to altered gallbladder motility and cholesterol crystal formation and only secondarily to biliary cholesterol saturation.
Keywords: obesity, diabetes, gallbladder, bile, cholesterol, strain
Introduction
Cholesterol gallstone disease is the most widespread digestive disorder in Western countries. In the United States, approximately 12% of the adult population have gallstones, and the cost of caring for these patients is between 8 and 10 billion dollars annually 1,2. Cholesterol gallstones are a multifactorial disease involving a complex interaction between both environmental and genetic factors including age, race, gender, parity, diet, diabetes, family history, and obesity. The formation of cholesterol gallstones requires three pathophysiological conditions including increased biliary secretion of cholesterol producing cholesterol supersaturated bile, nucleation and growth of cholesterol monohydrate crystals, and altered biliary motility.
Obesity, which is also a polygenic and multifactorial disease, has been well documented as a risk factor for gallstone formation especially in women 3. Obese individuals have been shown to have supersaturated bile (4,5), larger gallbladder fasting volumes 6, and impaired gallbladder emptying 7. Leptin, the protein product of the ob gene, is a small peptide hormone produced primarily by adipose tissue and is highly correlated with total body mass 8. Recessive mutations in the mouse obese (ob) and diabetes (db) genes result in weight gain and diabetes conditions similar to human obesity. Leptin–deficient (ob/ob) and leptin-receptor deficient (db/db) mice have similar phenotypes, weighing 2.5-3 times more than normal mice and have five times the body fat content. In addition to obesity, ob/ob mice exhibit hyperphagia, hyperglycemia, abnormal reproductive functions, hormonal imbalances and decreased immune function. These mice are also hypometabolic and hypothermic.
We have recently reported that leptin-deficient mice have increased resting gallbladder volume, an indication of gallbladder stasis 9 and shortened cholesterol crystal observation time, suggesting the presence of increased cholesterol crystal pronucleators 10,11. In addition, we have shown that leptin-deficient mice have decreased gallbladder smooth muscle response to excitatory stimuli such as, acetylcholine, neuropeptide Y and cholecystokinin 9, and that leptin administration ameliorates this response 12. Thus, these data suggest that decreased gallbladder motility and enhanced crystal formation may play a role in gallstone pathogenesis associated with obesity. Thus, the aim of this study was to determine if genetically obese, leptin-deficient mice have altered biliary lipids and enhanced biliary cholesterol crystal formation in response to a lithogenic diet.
Materials and Methods
Animals and Diet
Female C57Bl/6J lean control female mice (n = 319), C57Bl/6Jlepob heterozygous mice (n = 280) and C57Bl/6Jlepob homozygous mice (n = 117) were obtained from The Jackson Laboratory (Bar Harbor, ME). The mice, aged 7–8 weeks, were housed 4–5 per cage in a light (0600–1800) and temperature (22° C) controlled room. Mice from each strain were randomly divided into two groups and given free access to either a control non-lithogenic chow diet (contains trace amounts of cholesterol) or a semi-synthetic lithogenic diet containing 1% cholesterol and 0.5% cholic acid (Dyets Inc., Bethlehem, PA) for four weeks. The Medical College of Wisconsin Animal Care and Use Committee approved the experiments described herein.
Tissue Procurement
At 12 weeks of age the mice were deprived of food overnight. The next morning the mice were anesthetized with ketamine-xylazine (15 mg/Kg xyl, 50 mg/Kg ket., IP) and then underwent a laparotomy, cholecystectomy and hepatectomy. The liver weights were measured, and a portion of the right lobe of the liver was fixed in Bouin's solution for histology. Bile was aspirated from the gallbladders, and the volumes were determined. An aliquot of bile was immediately removed and observed for cholesterol crystal determination. Whole blood was aspirated from the heart and centrifuged to isolate serum. Serum and bile were pooled on the basis of known average fluid volumes to obtain sufficient amounts for analyses as described below.
Biliary Lipid Analysis
Pooled gallbladder bile samples were analyzed for total bile acids, phospholipids, and cholesterol, as previously described 13 and cholesterol saturation indices were determined by Carey's critical tables 14.
Cholesterol Crystal Counts
Immediately following collection, 5 µL of gallbladder bile were examined microscopically for the presence of cholesterol crystals. Cholesterol crystals were identified by their characteristic shape and by birefringence using polarized light microscopy 15. Ten high power fields (400×) were examined, and the mean number of crystals per high power field are reported.
Serum Analysis
Serum total cholesterol, HDL cholesterol and glucose were determined using commercially available diagnostic kits as per the manufacturer's instructions (Sigma Procedure Nos. 352, 352-7 and 635, respectively, Sigma Chemical Co., St. Louis, MO). Serum leptin levels were determined using a commercially available radioimmunoassay (Linco Research, Inc., St. Charles, MO).
Fat Vacuoles
As a measure of hepatic steatosis, liver fat vacuoles were identified and counted in hematoxylin and eosin stained paraffin sections. Ten high power fields (400×) were examined, and the mean number of vacuoles per high power field are reported.
Statistical Analysis
Data are expressed as means ±SEM. Statistical differences were determined by two-way ANOVA, followed by pairwise comparisons utilizing Mann-Whitney test using SigmaStat Statistical Software version 2.03 (SPSS Inc., Chicago, IL). p<0.05 was employed as the nominal criterion of statistical significance.
Results
Strain and Diet Influence Body and Liver Weight
As expected, regardless of the experimental diet, significant differences in final mean body and liver weights were observed among the strains at the time of surgery (Table 1). Homozygous leptin-deficient mice had significantly greater (p<0.001) mean body and liver weights than either the heterozygous or lean control mice, with the heterozygotes being intermediate. Not surprisingly, significant differences (p<0.05) were observed in mean body and liver weights between dietary treatments.
Table 1. Comparison of Body Weight, Liver Weight, and Gallbladder Volume.
| n | Diet | Body Wt. | Liver Wt. | GBV | |
|---|---|---|---|---|---|
| Lean | 191 | chow | 18.3±0.1a | 0.9±0.01a | 14.2±0.5a |
| Het-Ob | 160 | chow | 20.8±0.1b | 1.0±0.01b | 17.6±0.5b |
| Homo-Ob | 68 | chow | 49.9±0.4c | 2.9±0.1c | 34.7±2.2c |
| Lean | 128 | xol | 18.3±0.1d | 1.2±0.01*d | 19.8±0.8*d |
| Het-Ob | 120 | xol | 21.5±0.2*e | 1.4±0.02*e | 18.7±0.6d |
| Homo-Ob | 49 | xol | 45.9±0.7*f | 3.3±0.09*f | 59.2±3.0*e |
| Two-Way ANOVA p-value | |||||
| Strain Effects | <0.001 | <0.001 | <0.001 | ||
| Diet Effects | <0.001 | <0.001 | <0.001 | ||
| Strain-Diet Interaction | <0.001 | 0.128 | <0.001 | ||
NOTE: Data represent mean±SEM.
Different superscript letters among strains on same diet indicate significant difference from one another, p < 0.001. * p < 0.05 vs same strain on chow diet.
Abbreviations: Wt.=weight, GBV = gallbladder volume.
Strain and Diet Influence Gallbladder Volumes
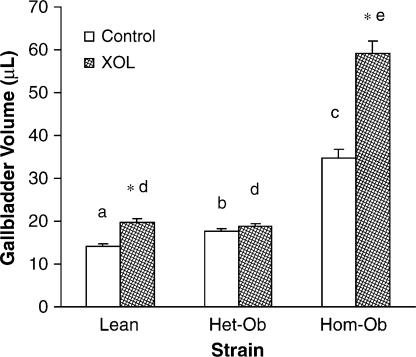
Mean gallbladder volumes were significantly greater (p<0.001) in homozygous leptin-deficient mice compared to either the heterozygous leptin-deficient or lean control mice regardless of experimental diet (Table 1, Figure 1). Feeding lean control and homozygous leptin-deficient mice a lithogenic diet for four weeks significantly increased (p<0.05) their gallbladder volumes compared to mice given the control non-lithogenic chow diet. However, heterozygous leptin-deficient mice did not exhibit increased gallbladder volumes.
Figure 1. .
Lithogenic diet (XOL) increases gallbladder volume in lean and to a greater extent obese mice. Different letters above error bars indicate means are significantly different from one another, p<0.001. *p<0.05 vs. same strain on chow diet.
Diet but not Strain Alters Biliary Lipids
Interestingly, two-way ANOVA indicated that strain had no effect on biliary lipids; but as expected, diet did significantly (p < 0.05) alter biliary lipids (Table 2). When fed a lithogenic diet, homozygous leptin-deficient obese mice had decreased molar percent total bile acids and increased molar percent phospholipids, but molar percent cholesterol and CSI were not different from lean control or heterozygous leptin-deficient mice. However, two-way ANOVA did indicate significant interactions between strain and diet for total bile acids, phospholipids, cholesterol and total lipids, but not for CSI.
Table 2. Effect of a Lithogenic Diet on Biliary Lipids.
| No. of pools | Diet | TBA mM | PPL mM | XOL mg/ml | TBA mol% | PPL mol% | XOL mol% | TL g/dl | CSI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lean | 14 | chow | 216.1±9.8a | 43.6±3.2a | 3.5±0.5a | 81.1±1.1a | 16.2±0.9a | 3.4±0.4a | 14.1±0.6a | 0.6±0.1a |
| Het-Ob | 13 | chow | 185.3±7.0b | 32.6±1.7b | 2.1±0.2b | 83.0±0.5a | 14.6±0.5ab | 2.4±0.1a | 11.8±0.5b | 0.4±0.02a |
| Homo-Ob | 11 | chow | 272.8±18.1c | 50.6±4.5a | 3.6±0.6a | 83.9±1.3a | 12.9±0.7b | 3.2±0.6a | 16.9±1.0c | 0.6±0.1a |
| Lean | 10 | xol | 210.5±10.7d | 38.4±1.5d | 6.3±0.5*c | 78.6±1.5bc | 14.7±0.9c | 7.0±0.7*b | 13.9±0.4d | 1.2±0.1*b |
| Het-Ob | 10 | xol | 230.7±4.1*d | 37.1±1.9d | 6.7±0.5*c | 80.8±0.7c | 13.0±0.5c | 6.1±0.4*b | 14.8±0.4*d | 1.1±0.05*b |
| Homo-Ob | 8 | xol | 171.5±10.7*e | 40.5±2.4d | 4.8±0.4d | 74.0±1.4*b | 17.3±1.5*d | 5.8±0.3*b | 12.5±0.6*d | 0.9±0.1*b |
| Two-Way ANOVA p-value | ||||||||||
| Strain Effects | 0.447 | <0.001 | 0.367 | 0.126 | 0.396 | 0.53 | 0.033 | 0.61 | ||
| Diet Effects | 0.024 | 0.003 | 0.001 | <0.001 | 0.052 | <0.001 | 0.056 | <0.001 | ||
| Strain-Diet Interaction | <0.001 | <0.001 | 0.002 | 0.016 | <0.001 | 0.326 | <0.001 | 0.252 | ||
NOTE: Data represent mean ±SEM.
Different superscript letters among strains on same diet indicate significant difference from one another, p < 0.001.*p < 0.05 vs same strain on chow diet.
Abbreviations: TBA = Total Bile Acids, PPL = Phospholipids, XOL = Cholesterol, TL = Total lipids, CSI = Cholesterol Saturation Index.
Strain and Diet Influence Cholesterol Crystals
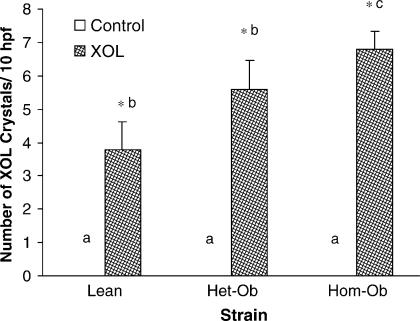
When fed a lithogenic diet, homozygous leptin-deficient mice had significantly greater (p<0.001) number of biliary cholesterol crystals than the lean controls (Figure 2). Heterozygous leptin deficient mice had intermediate number of crystals. No crystals were detected in any strains when fed a control non-lithogenic diet.
Figure 2. .
Lithogenic diet (XOL) increases the number of biliary cholesterol crystals/10 high-powered fields (hpf) (400×) in lean and to a greater extent, obese mice. The number of cholesterol crystals represents the total of 10 high-powered fields evaluated. Different letters above error bars indicate means are significantly different from one another, p<0.001. *p<0.05 vs. same strain on chow diet.
Strain and Diet Influence Hepatic Fat Vacuoles
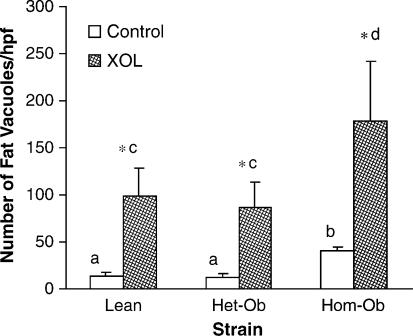
The number of hepatic fat vacuoles per high power field was determined as an indicator of hepatic fat storage. Regardless of dietary treatment, the number of fat vacuoles was significantly greater (p<0.001) in the homozygous leptin-deficient mice compared either the heterozygous or lean control mice (Figure 3). As expected, the number of vacuoles significantly increased (p<0.05) in all strains upon feeding a lithogenic, high cholesterol diet compared to the control non-lithogenic chow diet.
Figure 3. .
Lithogenic diet (XOL) increases hepatic steatosis in lean and to a greater extent, obese mice. The number of hepatic fat vacuoles represents the mean of 10 high-powered field (hpf) (400x) evaluated. Different letters above error bars indicate means are significantly different from one another, p<0.001. *p<0.05 vs. same strain on chow diet.
Strain and Diet Alter Serum Cholesterol, HDL Cholesterol, Glucose and Leptin
As expected, serum cholesterol was significantly higher (p<0.001) in homozygous leptin-deficient mice compared to either the heterozygous or lean control mice, regardless of the experimental diet (Table 3). Feeding a lithogenic, high cholesterol diet significantly increased (p<0.05) serum cholesterol in all strains compared to the control chow diet. Similar results were obtained for serum HDL cholesterol. Serum glucose levels were significantly higher (p<0.001) and serum leptin levels were significantly lower (p<0.001) in homozygous leptin-deficient mice compared to either the heterozygous or lean control mice with both diets. The high cholesterol diet significantly increased (p<0.05) serum leptin levels only in the lean control but not in the leptin-deficient mice.
Table 3. Measurement of Serum Lipid and Leptin Levels.
| No. of pools | Diet | total | leptin | |||
|---|---|---|---|---|---|---|
| XOL mg/dl |
HDL mg/dl |
glucose mg/dl |
ng/ml |
|||
| Lean | 14 | chow | 68.9±2.3a | 68.2±2.3a | 206.7±10.1a | 3.0±0.6a |
| Het-Ob | 13 | chow | 62.8±4.1a | 63.6±3.8a | 116.4±9.4b | 1.7±0.3b |
| Homo-Ob | 11 | chow | 146.7±4.9b | 143.2±9.1b | 428.0±23.3c | 0.4±0.03b |
| Lean | 10 | xol | 88.8±10.7c | 70.3±5.8c | 221.1±8.1d | 4.9±0.5*c |
| Het-Ob | 10 | xol | 139.5±3.0*d | 97.7±2.1*d | 200.1±20.8*d | 2.7±0.4d |
| Homo-Ob | 8 | xol | 221.5±14.6*e | 192.0±9.7*e | 395.1±18.9*e | 0.6±0.05e |
| Two Way ANOVA p-value | ||||||
| Strain Effects | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Diet Effects | <0.001 | <0.001 | 0.025 | 0.015 | ||
| Strain-Diet Interaction | <0.001 | <0.001 | <0.001 | 0.295 | ||
NOTE: Data represent mean ±SEM.
Different superscript letters among strains on same diet indicate significant difference from one another, p < 0.001. *p < 0.05 vs same strain on chow diet.
Abbreviations: XOL = Cholesterol, HDL = High Density Lipoprotein
Discussion
Although clinical studies have suggested that obesity is an important risk factor for gallstone disease, little is known regarding the exact role that obesity plays in the pathogenesis of this disease. Obesity has been associated with excessive hepatic secretion of cholesterol, larger gallbladder fasting volumes and impaired gallbladder emptying 4,5,6,7. We have previously demonstrated that homozygous leptin-deficient Lep ob mice have increased resting gallbladder volumes, diminished in vitro response to neurotransmitters and shorter crystal observation time compared to lean controls when fed a non-lithogenic diet 9,10,11. The present study addresses the hypothesis that a lithogenic diet will alter biliary and serum lipids and increase the number of biliary cholesterol crystals in heterozygous and homozygous leptin-deficient Lep ob compared to lean controls. We have shown that a lithogenic diet and mouse strain influence body and liver weights, gallbladder volume, cholesterol crystal formation, serum cholesterol, glucose and leptin levels and hepatic fat vacuole numbers. However, only diet altered the cholesterol saturation index suggesting that gallstone formation in leptin-deficient obesity may be due to altered biliary motility and crystal formation.
Impaired gallbladder emptying has been implicated in the pathogenesis of gallstones. In this and previous studies from our laboratory, leptin-deficient Lep ob mice had very large gallbladder volumes which became even larger when fed a high cholesterol lithogenic diet, while heterozygous leptin-deficient lean mice have more normal gallbladder volumes 9,16. These data are consistent with those reported by Bouchard et al. 17 in which female Lep ob mice, fed the same lithogenic diet for eight weeks, had larger gallbladder volumes compared to controls. However, human studies examining ultrasound measurements of gallbladder volume and emptying in obese patients have been conflicting. In most reports larger resting gallbladder volumes have been reported in obese patients; however, this observation may be proportional to body size and fat mass 6,7. While some human studies report that obesity is associated with decreased gallbladder emptying 18, many studies report normal emptying but an increased residual volume due to the increased resting volume 19,20.
We have previously reported that homozygous leptin-deficient Lep ob and leptin-resistant Lep db obese mice have impaired in vitro gallbladder muscle responses to acetylcholine, neuropeptide Y and cholecystokinin compared to lean controls, while heterozygous leptin-deficient lean mice had intermediate responses 9,16,21. In addition, agouti yellow Ay obese mice, which have a defect in melanocortin 4 receptor function and normal leptin metabolism, have a normal gallbladder response to biliary neurotransmitters similar to lean controls 21. Our findings demonstrate that leptin dysfunction plays an integral role in obesity-associated biliary stasis. Surprisingly, this “dose-response” effect did not apply to our observations with respect to biliary lipids.
In addition to biliary stasis, human obesity has also been associated with altered cholesterol metabolism including excess secretion of cholesterol into bile 4,5. In our murine model of obesity, homozygous and heterozygous leptin-deficient Lep ob mice fed a lithogenic diet for four weeks increased biliary cholesterol and CSI levels regardless of the mouse strain. In contrast, Bouchard et al. 17 reported that female Lep ob mice fed the same lithogenic diet for 8 weeks, had decreased biliary cholesterol and CSI levels compared to C57Bl/6J controls. This difference may reflect the longer feeding times with gallstone formation in the study by Bouchard and associates 17. However, with respect to biliary lipid analysis the present study has the advantage that 8–14 bile pools were measured so that statistical analyses could be preformed. In the study by Bouchard et al. 17 only one bile pool was available in each group.
Currently, no data are available on biliary lipids in leptin-deficient humans. In fact, spontaneous mutations in leptin are rarely a cause of human obesity, and most obese humans are leptin-resistant. Therefore, we have also investigated leptin-resistant obese mice (Lep db) which more closely parallel human obesity. We have recently reported that Lep db mice have low biliary cholesterol levels and CSI compared to lean controls 22 as well as a diminished tendency to form cholesterol crystals when fed a high cholesterol diet. However, Lep db, like Lep ob, mice have increased gallbladder volumes and a diminished in vitro response to neurotransmitters, supporting a role for biliary stasis in leptin-resistant obesity-associated gallstone pathogenesis.
In contrast to the leptin-resistant mice, in this study of leptin-deficient mice, cholesterol saturation indices increased in all strains when fed a lithogenic diet. Thus, biliary cholesterol crystals also significantly increased in all strains when fed a lithogenic diet. However, homozygous leptin-deficient mice had significantly greater number of biliary cholesterol crystals than the lean controls, while heterozygous leptin-deficient mice had an intermediate number of crystals, suggesting that cholesterol crystal formation is strain dependent. These data are consistent with our previous findings that crystal observation time (COT) is shortened in Lep ob mice compared to controls (7.0±0.4 vs 10.2±0.6 days), and that crystal growth and crystal mass are both increased 10. However, in contrast, Hyogo et al. 23 reported no crystal formation in male ob mice fed a similar lithogenic diet for 4 weeks. In addition, we have also identified carboxylesterase as a cholesterol crystal pronucleator in the bile of Lep ob mice 11,24. Thus, leptin-deficient mice have both altered gallbladder motility and enhanced cholesterol crystal formation.
Hepatic metabolism of fatty acids is disrupted in leptin-deficient obese mice leading to steatosis and hepatic insulin resistance 25. As expected, obese leptin-deficient mice had increased number of hepatic fat vacuoles compared to controls, consistent with their obesity. The number of fat vacuoles was enhanced when fed a lithogenic diet in all strains but to a greater degree in the leptin-deficient mice. Diehl 26 has suggested that leptin deficiency alters the liver's immune system and thereby increases its sensitivity to TNF-α which can inhibit insulin signal transduction. Interestingly, the heterozygotes had similar numbers of fat vacuoles compared to lean controls, which to our knowledge has not been previously reported. In humans, obesity-associated steatosis may also be associated with nonalcoholic steatohepatitis (NASH), characterized by, not only hepatic steatosis, but also fibrosis and inflammation. However, published reports examining the association between serum leptin and hepatic steatosis have yielded conflicting results. In two of these studies, Uygun et al. 27 and Chitturi et al. 28 reported that serum leptin levels were significantly higher in patients with NASH compared with controls. In contrast, Chalasani et al. 29 found no differences in hepatic leptin mRNA, leptin receptor mRNA or serum leptin levels between patients with NASH and controls. Therefore, while NASH is believed to be a major contributor to the overall morbidity and mortality of obesity a direct role of leptin in the pathogenesis of human NASH has yet to be established.
In addition to abnormal fatty acid metabolism, leptin-deficient mice have altered glucose and lipoprotein metabolism leading to hyperglycemia and hypercholesterolemia. In the current study the lithogenic diet increased serum cholesterol and HDL levels in both the homozygous and heterozygous leptin-deficient mice compared to controls. In addition, serum glucose increased with the lithogenic diet in the heterozygous mice and was significantly elevated in the homozygous leptin-deficient mice regardless of diet. We have recently reported a negative correlation between serum glucose, insulin, cholesterol, and triglyceride levels as well as body weight with gallbladder contractility 16. Moreover, we have also shown that gallbladder myocytes from both leptin-deficient and leptin-resistant mice are forshortened and have diminished response to cholecystokinin 30. Thus, hyperglycemia, insulin-resistance and hyperlipidemia in obese mice are associated with impaired gallbladder contractility which may contribute to obesity-associated gallstone pathogenesis.
In summary, lithogenic diet and mouse strain influenced body and liver weights, gallbladder volume, cholesterol crystal formation, serum cholesterol, glucose and leptin levels as well as the number of hepatic fat vacuoles. However, only diet, but not strain, altered biliary cholesterol saturation. Therefore, these data and our studies of gallbladder motility and cholesterol crystallization suggest that the association between obesity and gallstone formation may not require increased cholesterol saturation. However, the increased incidence of gallstones observed in obesity may result from altered gallbladder emptying and enhanced crystal formation in patients who already have cholesterol supersaturated bile.
References
- 1.Flegul KM, Cunoll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults 1999–2000. JAMA. 2002;288(14):1728–32. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Nakeeb A, Comouzzie AG, Martin L, Sonnenberg GE, Swartz-Basile DA, Kissebah AH, Pitt HA. Gallstones: Genetics versus environment. Ann Surg. 2002;235(6):842–9. doi: 10.1097/00000658-200206000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mabee TM, Meyer P, Den Besten L, Mason EE. The mechanism of increased gallstone formation in obese human subjects. Surgery. 1976;79:460–465. [PubMed] [Google Scholar]
- 5.Stahlberg D, Rudling M, Angelin B, Bjorkhem I, Forsell P, Nilsell K, Einarsson K. Hepatic cholesterol metabolism in human obesity. Hepatology. 1997;25:1447–1450. doi: 10.1002/hep.510250623. [DOI] [PubMed] [Google Scholar]
- 6.Hendel HW, Hojgaard L, Andersen T, Pedersen BH, Paloheimo LI, Rehfeld JF, Gotfredsen A, et al. Fasting gallbladder volume and lithogenicity in relation to glucose tolerance, total and intra-abdominal fat masses in obese non-diabetic subjects. Int J Obes Relat Metab Disord. 1998;22(4):294–302. doi: 10.1038/sj.ijo.0800583. [DOI] [PubMed] [Google Scholar]
- 7.Vezina WC, Paradis RL, Grace DM, Zimmer RA, Lamont DD, Rycroft KM, King ME, et al. Increased volume and decreased emptying of the gallbladder in large (morbidly obese, tall normal, and muscular normal) people. Gastroenterology. 1990;98(4):1000–1007. doi: 10.1016/0016-5085(90)90025-v. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 9.Goldblatt MI, Swartz-Basile DA, Svatek CL, Nakeeb A, Pitt HA. Decreased gallbladder response in leptin-deficient obese mice. J Gastrointest Surg. 2002;6:438–442. doi: 10.1016/s1091-255x(01)00046-4. [DOI] [PubMed] [Google Scholar]
- 10.Goldblatt MI, Swartz-Basile DA, Choi S-H, Blaser C, Sarna SK, Nakeeb A, Pitt HA. Cholesterol crystal pronucleators in genetically obese mice. Surg Forum. 2000;51:1. [Google Scholar]
- 11.Goldblatt MI, Swartz-Basile DA, Svatek CL, Nakeeb A, Pitt HA. Increased 46, 61, and 84 kDa gallbladder bile nonmucin proteins in genetically obese mice. Surg Forum. 2001;52:36. [Google Scholar]
- 12.Phillips J, Tran KQ, Goldblatt MI, Swartz-Basile DA, Nakeeb A, Pitt HA. Leptin ameliorates the gallbladder's response to neurotransmitters in congenitally obese mice. [Abstract] Gastroenterology. 2002;123:9A. [Google Scholar]
- 13.Swartz-Basile DA, Goldblatt MI, Blaser C, Decker PA, Ahrendt SA, Sarna SK, Pitt HA. Iron deficiency diminishes gallbladder neuronal nitric oxide synthase. J Surg Res. 2000;90 :26–31. doi: 10.1006/jsre.2000.5827. [DOI] [PubMed] [Google Scholar]
- 14.Carey MC. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res. 1978;19:945–955. [PubMed] [Google Scholar]
- 15.Wang DQ, Carey MC. Complete mapping of crystallization pathways during cholesterol precipitation from model bile: Influence of physical-chemical variables of pathophysiologic relevance and identification of a stable liquid crystalline state in cold, dilute and hydrophilic bile salt-containing systems. J Lipid Res. 1996;37:606–630. [PubMed] [Google Scholar]
- 16.Tran KQ, Goldblatt MI, Swartz-Basile DA, Svatek CL, Nakeeb A, Pitt HA. Diabetes and hyperlipidemia correlate with gallbladder contractility in leptin-related murine obesity. J Gastrointest Surg. 2003;7(7):857–62. doi: 10.1007/s11605-003-0030-z. [DOI] [PubMed] [Google Scholar]
- 17.Bouchard G, Johnson D, Carver T, Paigen B, Carey MC. Cholesterol gallstone formation in overweight mice establishes that obesity per se is not linked directly to cholelithiasis risk. J Lipid Res. 2002;43:1105–13. doi: 10.1194/jlr.m200102-jlr200. [DOI] [PubMed] [Google Scholar]
- 18.Palasciano G, Portincasa P, Belfiore A, Baldassarre G, Cignarelli M, Paternostro A, Albano O, Giorgino R. Gallbladder volume and emptying in diabetics: The role of neuropathy and obesity. J Intern Med. 1992;231(2):123–7. doi: 10.1111/j.1365-2796.1992.tb00512.x. [DOI] [PubMed] [Google Scholar]
- 19.Stone BG, Ansel HJ, Peterson FJ, Gebhard RL. Gallbladder emptying stimuli in obese and normal-weight subjects. Hepatology. 1992;15(5):795–8. doi: 10.1002/hep.1840150508. [DOI] [PubMed] [Google Scholar]
- 20.Acalovschi M, Badea R. Ultrasonographic study of gallbladder emptying in obese patients. Int J Obes Relat Metab Disord. 1992;16(4):313–5. [PubMed] [Google Scholar]
- 21.Tran KQ, Swartz-Basile DA, Nakeeb A, Pitt HA. Gallbladder motility in Agouti-yellow and leptin-resistant obese mice. J Surg Res. 2003;113:56–61. doi: 10.1016/s0022-4804(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 22.Tran KQ, Graewin SJ, Swartz-Basile DA, Nakeeb A, Svatek CL, Pitt HA. Leptin-resistant obese mice have paradoxically low biliary cholesterol saturation. Surgery. 2003;134:372–377. doi: 10.1067/msy.2003.234. [DOI] [PubMed] [Google Scholar]
- 23.Hyogo H., Roy S, Cohen SE. Restoration of gallstone susceptibility by leptin in C57BL/6J ob/ob mice. J Lipid Res. 2003;44:1232–1240. doi: 10.1194/jlr.M300029-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Tran KQ, Goldblatt MI, Swartz-Basile DA, Svatek CL, Nakeeb A, Pitt HA. Carboxylesterase is a cholesterol pronucleator in leptin deficient obese mice. [Abstract] J Am Coll Surg. 2002;195:S13. [Google Scholar]
- 25.den Boer M, Voshol PJ, Kuipers F, Havekes LM, Romijn JA. Hepatic Steatosis: A mediator of the metabolic syndrome lessons from animal models. Arterioscler Thromb Vasc Biol. 2004;24:644–649. doi: 10.1161/01.ATV.0000116217.57583.6e. [DOI] [PubMed] [Google Scholar]
- 26.Diehl AM. Nonalcoholic fatty liver disease: Implications for alcoholic liver disease pathogenesis. Alcohol Clin Exp Res. 2001;25:8S–14S. doi: 10.1097/00000374-200105051-00004. [DOI] [PubMed] [Google Scholar]
- 27.Uygun A, Kadayifici A, Yesilova Z, Erdil A, Yaman H, Saka M, Deveci S, et al. Serum leptin levels in patients with nonalcoholic steatohepatitis. Am J Gastroenterology. 2000;95:3584–3589. doi: 10.1111/j.1572-0241.2000.03297.x. [DOI] [PubMed] [Google Scholar]
- 28.Chitturi S, Farrell G, Frost L, Kriketos A, Lin R, Fung C, Liddle C, et al. Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: A manifestation of lipotoxicity? Hepatology. 2002;36:403–409. doi: 10.1053/jhep.2002.34738. [DOI] [PubMed] [Google Scholar]
- 29.Chalasani N, Crabb DW, Cummings OW, Kwo PY, Asghar A, Pandya PK, Considine RV. Does leptin play a role in the pathogenesis of human nonalcoholic steatohepatitis? Am J Gastroenterology. 2003;98:2771–2776. doi: 10.1111/j.1572-0241.2003.08767.x. [DOI] [PubMed] [Google Scholar]
- 30.Graewin SJ, Lee K, Kiely JM, Svatek CL, Nakeeb A, Pitt HA. Gallbladder myocytes are short and CCK-resistant in obese diabetic mice. Surgery 136(2):431–436. [DOI] [PubMed] [Google Scholar]