Abstract
Background: Orthotopic liver transplantation (OLT) may be associated with massive blood loss and the need for allogenic blood product transfusions. Cell salvage autotransfusion (CS) is an attractive alternative to allogenic red blood cell (RBC) transfusion. However, controversy surrounds its usefulness during OLT; some studies stated that CS decreased transfusions of allogenic blood products and others stated that blood loss was increased. The aim of this study was to evaluate the efficiency of the CS during OLT. Patients and methods: After approval by the institutional ethics committee, a prospective survey was undertaken. A total of 150 consecutive OLTs were included in the study. Two groups of patients were formed. Period 1 included patients 1–75 with no CS use. Period 2 comprised patients 76–150 with systematic CS use. Results: Patients from both periods were comparable. CS was used in all cases in period 2, and there was enough salvaged blood to retransfuse 65% of these OLTs. The mean volume of retransfused blood was 338±339 ml. The transfusion rate did not change from period 1 to period 2. The mean number of RBC units transfused per patient was 0.4±0.9 vs 0.4±1.2 with 78.7% vs 81.3% of cases not receiving transfusion of any blood product. The threshold for RBC transfusions was the same. The length of surgery and blood loss were greater in period 2 than in period 1 (associated with the arrival of two junior surgeons), but the hemoglobin (Hb) value was also higher at the end of surgery (93.8±19.3 g/L vs 85.2±17.8 g/L, p<0.0001). Conclusion: Despite increased blood loss in period 2, CS saved 21 g/L of Hb per patient or two RBC unit transfusions. As long as we cannot predict with accuracy which patients will bleed, we will continue to use the CS for all OLTs.
Keywords: liver transplantation, transfusion, cell salvage autotransfusion
Introduction
Orthotopic liver transplantation (OLT) could be associated with major blood loss and the need for allogenic blood product transfusion. During the last 20 years, a large and constant decrease in blood loss and blood product requirements has been observed, reducing from as much as 43 red blood cell (RBC) units 1 to only 0.3 RBC units per patient 2. Improvements in surgical technique, the use of anti-fibrinolytic agents 3,4,5, and the development of new anesthetic strategies (low central venous pressure (CVP), phlebotomy) 2,6 have contributed to steady reductions in blood loss and in transfusion needs during OLT.
Cell salvage autotransfusion (CS) is an attractive alternative to allogenic RBC transfusions. Nevertheless, controversy has arisen over its use during OLT because some studies have stated that the CS has reduced allogenic RBC transfusion requirements, and that its use is safe 7,8,9,10,11. Others, however, have reported higher blood losses through fibrinolysis with the CS or found that it was not cost-effective 12,13,14. The controversy could persist because these studies were retrospective or observational and there was some confusion between OLT from living donors and cadavers.
In view of the arrival of two junior surgeons in our center, we hypothesized that the length of surgery would increase, as would blood loss. The purpose of this survey was to assess CS efficiency to reduce or limit allogenic RBC requirements during OLT.
Patients and methods
After approval from the Ethics Committee of the CHUM-Hôpital St-Luc, a prospective survey (study with historical controls) 15 was undertaken. A total of 150 consecutive OLTs from cadaver donors, starting in 2002, were included. Two groups of patients were formed: period 1, patients 1–75 without CS use; and period 2, patients 76–150 where CS was used systematically for every OLT. The only contraindications were intra-abdominal infection or abscess.
Surgical protocol
Four surgeons were involved in the historic period and six in the prospective one, with two of them participating in each procedure. Complete classical cross-clamping of the suprahepatic and infrahepatic vena cava and the portal vein were used without any veno-venous bypass nor piggyback technique. All liver procurements were from cadaver donors and were ABO-Rh compatible. Regular steroid induction followed by maintenance therapy with tacrolimus was used.
Anesthetic protocol
Thirteen anesthesiologists were involved in OLTs during the study period. Monitoring and anesthesia were standardized 6. Coagulation defects were not corrected upon laboratory data in the absence of uncontrollable bleeding. The threshold for RBC transfusions was set at a hemoglobin (Hb) value of 68 g/L 6, and an effort was made not to start any transfusion until the blood losses were controlled. Aprotinin was given to every patient according to the Hammersmith protocol 16. Patients underwent serial arterial blood gas analysis which included Hb, potassium, and ionized calcium measurements. Coagulation was monitored as needed intraoperatively (INR, platelet count). No plasma (fresh frozen plasma) or platelets were to be transfused, unless there was uncontrollable bleeding (diffuse oozing without any clinical coagulation) when the American Society of Anesthesiology (ASA) guidelines 17 were met (on starting biochemical values).
Each anesthesiologist lowered baseline CVP before the anhepatic phase by about 40% by restricting volume infusion, by phlebotomy without volume replacement, or by a combination of both techniques 6. Harvested blood was returned to the patient at the end of the surgery or before as needed.
Cell salvage autotransfusion
The Fresenius CATS (Continuous Autotransfusion System) device was used for all OLTs during period 2 (only in the operating room). After collecting blood lost in the reservoir from the operating field, the perfusionist calculated the quantity of blood to permit its retransfusion with hematocrit (Ht) above 0.7 after washing and concentration of the harvested blood. If the quantity calculated was higher than 40 ml, the collected blood was washed, concentrated, and retransfused to the patient.
In conclusion, surgical and anesthesiologic techniques were the same in both periods, except for systematic use of the CS, and the arrival of two young surgeons in period 2.
Statistical analysis
The data are expressed as mean±standard deviation (SD) of the mean, and as percentages or absolute numbers. Distributions were examined to ensure proper statistical evaluation. Statistical analysis was performed by Student's t test or Welch's t test as appropriate. The χ2 test was used to compare percentages. p values <0.05 were considered significant. SPSS 10 statistical programs were implemented.
Results
In all, 150 OLTs were performed on 135 patients. Nine patients had two OLTs, three patients had three, and five patients had already undergone an OLT before the study period.
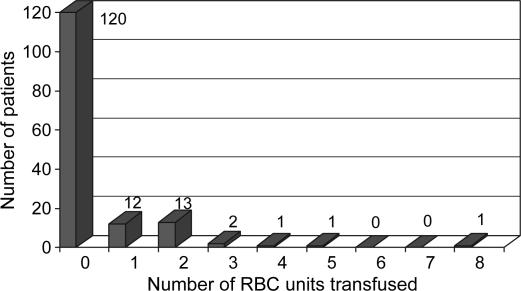
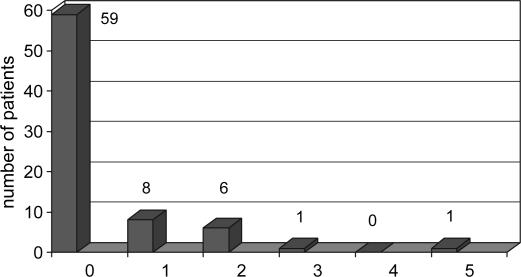
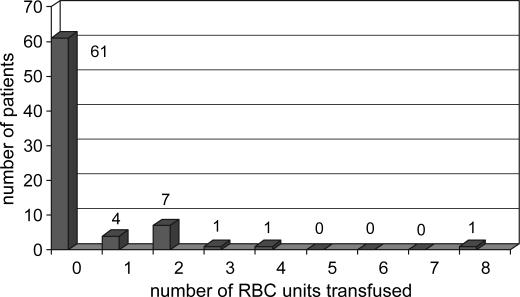
The mean number of intraoperative allogenic RBC units transfused per patient was 0.4±1.0 (median 0, maximum 8) (Figure 1); five units of plasma were transfused to two patients, and five units of platelets to one patient. No albumin or cryoprecipitate was transfused. One hundred and twenty patients (80%) received no allogenic blood products during their OLT, and the final Hb was 89.5±19.0 g/L. Table I presents the demographic characteristics and health status of patients for each period. Both groups were virtually the same. Table II enumerates the perioperative data for each group. During period 2, the CS was used for each OLT, but there was enough blood salvage to retransfuse 65% of the cases. The mean volume of retransfused blood was 338±339 ml with a minimum of 40 ml, a maximum of 2000 ml, and a median of 256 ml. For both periods, phlebotomy was undertaken in 42% of the cases and 616±227 ml of blood were withdrawn (minimum 300 ml, maximum 1200 ml) and the blood was retransfused at the end of the case. The length of surgery was greater in period 2 than in period 1 (266±68 min vs 225±57 min, p<0.0001), as was the amount of blood lost (1410±603 ml vs 818±302 ml, p < 0.0001). CVP values before clamping the vena cava were the same in both periods (6.9±4.2 mmHg vs 5.8±2.5 mmHg). The transfusion rate was the same for each period: the number of RBC units transfused per patient (0.4±0.9 vs 0.4±1.2), the percentage of cases without transfusion of any blood products (78.7% vs 81.3%) (Figures 2 and 3), and the threshold for RBC transfusion (57.0±7.5 g/L vs 55.7±8.6 g/L) (Table II). The final Hb value was higher in period 2 (93.8 ±19.3 g/L vs 85.2±17.8 g/L, p<0.0001).
Figure 1. .
Patient stratification by number of intraoperative RBC units transfused for all patients.
Table I. Groups comparison according to variables that could influence the blood loss or RBC transfusion rate.
| Variables | All patients (patients 1–150) | Period 1 (patients 1–75) | Period 2 (patients 76–150) | p value |
|---|---|---|---|---|
| Gender (male) | 66.7% | 64.7% | 68.7% | NS |
| Age (years) | 52±12 | 51±12 | 52±12 | NS |
| Weight (kg) | 74±12 | 71±18 | 77±18 | NS |
| Height (cm) | 168±10 | 168±10 | 169±9 | NS |
| Starting Hb value (g/L) | 107.0±23.4 | 105.7±22.5 | 108.5±24.3 | NS |
| Starting INR value | 1.8±0.9 | 1.7±0.7 | 1.8±1.1 | NS |
| Starting platelet count (109 platelets/L) | 99±58 | 102±64 | 95±54 | NS |
| Pugh's score | 9.7±2.5 | 9.5±2.2 | 9.9±2.7 | NS |
| MELD score | 17±9 | 17±8 | 17±9 | NS |
| Starting creatinine value (µmol/L) | 101±58 | 97±56 | 106±59 | NS |
| % of use of phlebotomy | 42.0% | 45.3% | 38.7% | NS |
| % of use of cell salvage | 32.6% | 0 | 65.3%* | <0.0001 |
Values are given as mean±SD or percentage. Hb, hemoglobin; INR, international normalized ratio; MELD, model of end-stage liver disease; creatinine, serum creatinine.
*CS was used for each OLT, but there was enough blood to retransfuse in 65.3% of the cases in period 2.
Table II. Surgical characteristics of all patients.
| Variables | All patients (patients 1–150) | Period 1 (patients 1–75) | Period 2 (patients 76–150) | p value |
|---|---|---|---|---|
| RBC transfused (units per patient) | 0.4±1.0 | 0.4±0.9 | 0.4±1.2 | NS |
| Threshold for RBC transfusion (g/L) | 56.5±8.3 | 57.0±7.5 | 55.7±8.6 | NS |
| Final Hb value (g/L) | 89.5±19.0 | 85.2±17.8 | 93.8±19.3 | <0.0001 |
| CVP before clamping (mmHg) | 6.4±3.8 | 6.9±4.2 | 5.8±2.5 | NS |
| Blood loss (ml) | 1114±556 | 818±302 | 1410±603 | < 0.0001 |
| Crystalloids before clamping (ml) | 1058±329 | 986±251 | 1134±383 | NS |
| Diuresis (ml/kg/h) | 1.9±1.7 | 1.8±1.3 | 1.9±1.7 | NS |
| Length of surgery (min) | 244±65 | 225±57 | 266±68 | <0.0001 |
| % of cases without any blood products | 80.0% | 78.7% | 81.3% | NS |
Values are given as mean±SD. RBC, red blood cells; Hb, hemoglobin; CVP, central venous pressure.
Figure 2. .
Patient stratification by number of intraoperative RBC units transfused for period 1 (patients 1–75).
Figure 3. .
Patient stratification by number of intraoperative RBC units transfused for period 2 (patients 76–150).
Surgeons 5 and 6 (two junior surgeons returning from their fellowship) joined the team of four surgeons during period 2. They performed 20 and 10 OLTs, respectively. Table III gives some of the variables for two groups of surgeons: seniors vs juniors. Length of surgery and blood loss were greater with the two junior surgeons compared with the senior surgeons but the transfusion rates were the same (0.3±0.7 RBC units per patient vs 0.4±1.3 RBC units per patient). The diagnostic classification of patients in both periods can be found in Table IV.
Table III. Surgical characteristics detailed for two groups of surgeons: seniors vs juniors.
| Variables | Four senior surgeons (120 patients) | Two junior surgeons (30 patients) | p value |
|---|---|---|---|
| Starting Hb value (g/L) | 110±21 | 103±30 | NS |
| Length of surgery (min) | 231±54 | 291±73 | <0.0001 |
| % of utilization of cell salvage | 36% | 57% | 0.03 |
| CVP before clamping (mmHg) | 5.7±3.7 | 4.7±2.4 | NS |
| Blood loss (ml) | 978±719 | 1382±868 | <0.0001 |
| RBC transfused (units per patient) | 0.3±0.7 | 0.4±1.3 | NS |
| Final Hb value (g/L) | 89±19 | 85±16 | NS |
Table IV. Number of patients according to diagnoses.
| Diagnosis | Period 1 | Period 2 |
|---|---|---|
| Alcoholic cirrhosis | 16 | 18 |
| Sclerosing cirrhosis | 12 | 4 |
| Cryptogenic cirrhosis | 12 | 4 |
| Chronic hepatitis C virus | 10 | 10 |
| NASH | 9 | 7 |
| Hepatocarcinoma secondary to cirrhosis B or C | 5 | 13 |
| Primary and secondary biliary cirrhosis | 3 | 5 |
| Chronic hepatitis B virus | 3 | 4 |
| Fulminant hepatitis A or B | 2 | 1 |
| Autoimmune hepatitis | 1 | 3 |
| Fulminant hepatitis secondary to acetaminophen | 1 | 3 |
| Miscellaneous | 1 | 3 |
| Total | 75 | 75 |
NASH, non-alcoholic steato-hepatitis; miscellaneous, hepatic artery stenosis or thrombosis, biliary duct stenosis. Period 1, patients 1–75; Period 2, patients 76–150.
Discussion
This prospective survey does not pretend to be randomized with a control group where CS was evaluated in two groups with the same blood loss. In view of the established low rate of blood product transfusions in our center, we thought that it was unethical to create a control group that would have been at increased risk of receiving blood products. If we were allowed to conduct a prospective randomized study with a control group, to determine if the CS or another technique could significantly reduce the transfusion rate (0.3±0.7) 2 by 25% with an alpha error of 0.05 and a power of 80%, we would have needed more than 100 000 patients in each group. Therefore, we are limited to observational studies or historical controls to investigate transfusion strategies in our center. This survey is a study with historical controls 15, where 2 groups of 75 patients were compared. We evaluated CS to limit allogenic RBC transfusion at a time that corresponded with the arrival of two junior surgeons and eventually an increase in blood loss.
Maturity 15 or experience (surgeon skill) was not a bias with potential impact on external validity, as two new surgeons joined the transplant team. Furthermore, the transfusion rate did not change with time.
The overall transfusion rate was 0.4±1.0 RBC units per patients. It could be argued that our patients were not very sick according to the model of end-stage liver disease (MELD) score. The MELD score is a combination of the starting INR value, serum creatinine, and bilirubin 18. It has been created to evaluate liver insufficiency more objectively than the Pugh's score that evaluates the severity of cirrhosis. MELD score is used mostly in USA to prioritize OLTs but not in Canada. So, it is not surprising that USA recipients for OLT might have an increased MELD score.
The MELD scores have been the same since 1998 for more than 450 patients in our center and the transfusion rate went from 2.8±3.5 RBC per patient with 4.1±4.0 units of plasma per patient 19 to 0.3±0.7 RBC units per patient without any plasma 2.
The purpose of our study was not to compare the level of morbidity between our patients and patients from other centers. We wanted to evaluate CS in patients with a comparable disease severity. Our patients, when compared to other series, seem to be as sick if not sicker. In the study by Frasco et al. 18, patients with a RBC transfusion rate of 2.9±2.7 RBC units per patient (total of 18 units of allogenic blood products per patient) had the same severity of disease as our patients. In the study by Ramos et al. 19, patients with the same transfusion rate of 2.9±2.9 RBC units per patient had a starting INR value of 1.2±0.2. In our series, 86 patients had a starting INR value of 1.5 or more and just 1 of these patients received plasma (1 unit). Moreover, 28 patients had a starting platelet count lower than 50×109 platelets/L and none received any platelets.
Patients from the two study periods were identical in all respects. There was no change in the transfusion rate from period 1 to 2: 0.4±0.9 vs 0.4±1.2 RBC units per patient (Table II), the threshold for transfusion, and the percentage of cases without transfusion of any blood products was also similar, 78.7% vs 81.3%. The length of surgery was higher in period 2, after the arrival of surgeons 5 and 6 (Tables II and III). Previously, we noted that when surgeons were compared, the length of surgery was an independent factor for RBC transfusions 20. This suggests that blood loss increases with the length of surgery. That was the case in our prospective survey. Blood loss, which was difficult to assess with accuracy, was higher in period 2 (the amount of blood was determined by adding the blood loss suctioned from the CS and sponges). As two surgeons always operated as a team and a junior surgeon was always teamed with a senior, blood loss could have been higher.
The arrival of two surgeons and the increase in blood loss did not modify the transfusion rate with the use of the CS and the final Hb value was higher in period 2 than in period 1 (Table II). In period 1, with a mean blood loss of 818 ml, the starting Hb value dropped from 105.7 g/L to 85.2 g/L, a decrease of 20.5 g/L. In period 2, we should have expected from a blood loss of 1410 ml and the same transfusion rate as for period 1, a drop in the starting Hb value of 35.3 g/L (1410 ml×20.5 g/L/818 ml). The final Hb value should have been 73 g/L, and we obtained 94 g/L. The CS saved a mean of 21 g/L of Hb per patient. The mean blood volume of patients was around 5.8 L (77 kg×75 ml/kg), so the CS has saved 122 g of Hb per patient (5.8 L×21 g/L). One unit of 300 ml of allogenic RBC contains around 70 g of Hb. To obtain a final Hb of 94 g/L without the use of the CS, we should have transfused 1.7 RBC units (122 g/70 g/RBC unit); therefore, the CS saved about 1.7 RBC units per patient. In the province of Québec, one unit of RBC costs around C$500. Therefore, a mean of C$1000 was saved for each OLT. If we look at the CS: the perfusionist's cost was C$125 per case with C$175 for equipment (tubing for suction, $30; reservoir, $53 for all cases; and washing: $92 ($142 for 65% of the cases), and the company (Ryan Medical) provided us with the CS device). The CS saved around C$700 for each OLT.
Kemper et al. 14 in 1997 encountered different expenses that would include the cost of the CS device and allogenic RBC units at other given prices. So, they stated about the break-even point of 12.6 RBC units transfused to be cost-effective. In our center the break-even point is 0.67 RBC units: prices for 2006 have changed and were applied to this study. We doubt the statement by de Boer et al. 13 that ‘the low amount of blood loss encountered in recent years no longer allows cost-effective use of the CS’. It should be pertinent to reassess cost-effectiveness of the series already studied according to today's prices.
Before this study, we could guess that the CS would compensate for a possible increase of blood loss with the arrival of two new surgeons. That was the case and more. The starting Hb value was the same for both periods; blood loss was higher in period 2 with the same transfusion rate (mean RBC transfusion, percentage without blood products, threshold of RBC transfusions), yet the final Hb value was higher in period 2 (Table II). The threshold for RBC transfusions was lower in this study (56.5 g/L) than the one chosen in our protocol (68 g/L) because the anesthesiologists aimed at transfusing after the bleeding was controlled, but the thresholds were the same for both periods.
The CS is a tool that decreases the transfusion of allogenic RBC units. In our center, with a transfusion rate of 0.3±0.7 RBC units per patient 2, with 79% of OLTs without blood products, it is difficult to evaluate a strategy aimed at decreasing blood loss or the transfusion rate. To use the CS, we need significant blood loss to prime it. After suctioning blood from the operating field, the blood is washed, and concentrated for retransfusion at a Ht value between 0.7 and 0.8. If the starting Ht value is 0.4, for each blood volume lost, we should retransfuse with an Ht of 0.8, at best, half of the blood volume suctioned. If the starting Ht is 0.2, for each blood volume lost, we will be able to retransfuse one-quarter of the blood loss. To be useful, the CS needs significant blood loss and a high starting Ht value. Often, in our center, the higher the starting Hb value, the smaller the blood loss. Nevertheless, the increase in blood loss in period 2 permitted us to demonstrate the utility of the CS in OLT in our center despite a low transfusion rate. It should be easier for liver transplantation centers with higher transfusion rates to evaluate it prospectively.
Despite the conclusions reached by de Boer et al. 13, in our series, the increase in blood loss was not secondary to the CS during period 2 but its usefulness was established in large blood loss. Moreover, the study by de Boer et al. was retrospective, and a direct causal relationship could not be verified between the CS and increased blood loss. When we retransfuse a large quantity of blood from the CS, it is like a massive transfusion of allogenic RBC (transfusion of RBC without coagulation factors and platelets). In both cases, we transfuse blood without coagulation factors and platelets. So we might encounter coagulation disturbances.
Unlike many liver transplantation centers, we do not control coagulation defects during surgery. In spite of what Frasco et al. 17 stated and what we found previously 19, baseline coagulation status is no more a predictor of OLT without RBC transfusion 6. Furthermore, we did not find any advantage in correcting coagulation defects before or during OLT. In addition, some authors have found a significant link between transfusion of plasma peroperatively and decreased 1-year survival rate 21.
We administered aprotinin according to the Hammersmith protocol 16, a high-dose protocol. Some randomized, controlled studies 5,22 have shown the efficacy of aprotinin in reducing blood loss. Aprotinin probably successfully limits fibrinolysis. We have used it for more than 600 OLTs without any major thromboembolic event 23,24. It is difficult to evaluate the utility of aprotinin in our center because we have been using it since 1993 for all OLTs.
In conclusion, for 150 OLTs, despite increases in the length of surgery and in blood loss, the transfusion rate did not change after the introduction of the Cell Saver after the 75th OLT. In all, 0.4 RBC units were transfused per patient, 80% of patients did not receive any blood product, and the final Hb value was found to be higher in period 2 with use of the CS. We think that the CS is helpful in cases with large blood loss, and in our center with a low transfusion rate, it saved a mean of 21 g/L of Hb per patient or two RBC unit transfusions. Better yet, it was cost-effective. As long as we cannot predict with accuracy which patients will bleed, we will continue to use the CS for all OLTs.
Acknowledgements
We thank Mrs Denise Bois for her clerical work, Mr Ovid DaSilva for revision of this manuscript, and all the anesthesiologists, surgeons, and perfusionists who participated in this survey.
References
- 1.Butler P, Israel L, Nusbacher J, Jenkins DE, Jr, Starzl TE. Blood transfusion in liver transplantation. Transfusion. 1985;25:120–3. doi: 10.1046/j.1537-2995.1985.25285169201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massicotte L, Lenis S, Thibeault L, Sassine M-P, Seal RF, Roy A. Reduction of blood product transfusions during liver transplantation. Can J Anaesth. 2005;52:545–6. doi: 10.1007/BF03016538. [DOI] [PubMed] [Google Scholar]
- 3.Dalmau A, Sabate A, Koo M, Bartolome C, Rafecas A, Figueras J, et al. The prophylactic use of tranexamic acid and aprotinin in orthotopic liver transplantation: a comparative study. Liver Transpl. 2004;10:285–8. doi: 10.1002/lt.20075. [DOI] [PubMed] [Google Scholar]
- 4.Rentoul TM, Harrison VL, Shun A. The effect of aprotinin on transfusion requirements in pediatric orthotopic liver transplantation. Pediatr Transpl. 2003;7:142–8. doi: 10.1034/j.1399-3046.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 5.Porte RJ, Molenaar IQ, Begliomini B, Groenland , Januszkierwicz A, Lindgren L, et al. Aprotinin and transfusion requirements in orthotopic liver transplantation: a multicentre randomised double-blind study. Lancet. 2000;355:1303–9. doi: 10.1016/s0140-6736(00)02111-5. [DOI] [PubMed] [Google Scholar]
- 6.Massicotte L, Lenis S, Thibeault L, Sassine M-P, Seal RF, Roy A. Effect of low central venous pressure and phlebotomy on blood product transfusion requirements during liver transplantations. Liver Transpl. 2006;12:117–23. doi: 10.1002/lt.20559. [DOI] [PubMed] [Google Scholar]
- 7.Jabbour N, Gagandeep S, Mateo R, Sher L, Genyk Y, Selby R. Transfusion free surgery: single institution experience of 27 consecutive liver transplants in Jehovah's Witnesses. J Am Coll Surg. 2005;201:412–17. doi: 10.1016/j.jamcollsurg.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Lutz JT, Valentin-Gamazo C, Gorlinger K, Malago M, Peters J. Blood-transfusion requirements and blood salvage in donors undergoing right hepatectomy for living related transplantation. Anesth Analg. 2003;96:351–5. doi: 10.1097/00000539-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Forro M, Mandli T. Continuous autotranfusion during liver transplantation. Magy Seb. 2002;55:3–8. [PubMed] [Google Scholar]
- 10.Kang Y, Aggarwal S, Virji M, Pasculle AW, Lewis JH, Freeman JA, et al. Clinical evaluation of autotransfusion during liver transplantation. Anesth Analg. 1991;72:94–100. doi: 10.1213/00000539-199101000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Houvenaeghel M, Lefevre P, Samson D, Dyen J, Limet , Manelli JC. Autologous transfusion by preoperative salvage in orthotopic transplantation of the liver. Ann Fr Anesth Reanim. 1989;8:326–33. doi: 10.1016/s0750-7658(89)80074-7. [DOI] [PubMed] [Google Scholar]
- 12.Hendriks HG, van der Meer J, Klompmaker IJ, Choudhury N, Hagenaars JA, Porte RJ, et al. Blood loss in orthotopic liver transplantation: a retrospective analysis of transfusion requirements and effects of autotransfusion of cell saver blood in 164 consecutive patients. Blood Coagul Fibrinolysis. 2000;11:87–93. doi: 10.1097/00001721-200004001-00017. [DOI] [PubMed] [Google Scholar]
- 13.De Boer MT, Molenaar IQ, Hendriks HG, Slooff MJ, Porte RJ. Minimizing blood loss in liver transplantation: progress through research and evolution of techniques. Dig Surg. 2005;22:265–75. doi: 10.1159/000088056. [DOI] [PubMed] [Google Scholar]
- 14.Kemper RR, Menitove JE, Hanto DW. Cost analysis of intraoperative blood salvage during orthotopic liver transplantation. Liver Transpl Surg. 1997;3:513–17. doi: 10.1002/lt.500030506. [DOI] [PubMed] [Google Scholar]
- 15.Contandriopoulos A-P, Champagne F, Potvin L, Denis J-L, Boyle P.Savoir préparer une recherche: la définir, la structurer, la financer. Les Presses de l'Université de Montréal; 1990;chapter 2:49. [Google Scholar]
- 16.Royston D, Bidstrup BP, Taylor KM. Effect of aprotinin on need for blood transfusion after repeat open-heart surgery. Lancet. 1987;2:1289–91. doi: 10.1016/s0140-6736(87)91190-1. [DOI] [PubMed] [Google Scholar]
- 17. ASA guidelines: http://www.asahq.org/publicationsandservices/transfusion.pdf. [Google Scholar]
- 18.Frasco PE, Poterack KA, Hentz JG, Mulligan DC. A comparison of transfusion requirements between living donation and cadaveric donation liver transplantation: relationship to model of end-stage liver disease score and baseline coagulation status. Anesth Analg. 2005;101:30–7. doi: 10.1213/01.ANE.0000155288.57914.0D. [DOI] [PubMed] [Google Scholar]
- 19.Ramos E, Dalmau A, Sabaté A, Lama C, Llado L, Figueras J, et al. Intraoperative red blood cell transfusion in liver transplantation: influence on patient outcome, prediction of requirements, and measures to reduce them. Liver Transpl. 2003;9:1320–7. doi: 10.1016/jlts.2003.50204. [DOI] [PubMed] [Google Scholar]
- 20.Massicotte L, Sassine M-P, Lenis S, Roy A. Transfusion predictors in liver transplant. Anesth Analg. 2004;98:1245–51. doi: 10.1213/01.ane.0000111184.21278.07. [DOI] [PubMed] [Google Scholar]
- 21.Massicotte L, Sassine M-P, Lenis S, Seal RF, Roy A. Survival changes with transfusion of blood products during liver transplantation. Can J Anesth. 2005;52:148–55. doi: 10.1007/BF03027720. [DOI] [PubMed] [Google Scholar]
- 22.Findlay JY, Rettke SR, Ereth MH, Plevak DJ, Krom RA, Kufner RP. Aprotinin reduces red blood cell transfusion in orthotopic liver transplantation: a prospective, randomized, double-blind study. Liver Transpl. 2001;7:808–10. doi: 10.1053/jlts.2001.27086. [DOI] [PubMed] [Google Scholar]
- 23.Baubillier E, Cherqui D, Dominique C, Khalil M, Bonnet F, Fagniez PL, et al. A fatal thrombotic complication during liver transplantation after aprotinin administration. Transplantation. 1994;57:1664–6. [PubMed] [Google Scholar]
- 24.Molennar IQ, Porte RJ. Aprotinin and thromboembolism in liver transplantation: is there really a causal effect? Anesth Analg. 2002;94:137767–8. doi: 10.1097/00000539-200205000-00065. [DOI] [PubMed] [Google Scholar]