Abstract
Background: Although liver resection has become an established procedure in western countries and South-east Asia it is still not performed frequently in most centres in India. In the last 10 years newly created specialized units have been performing more liver resections but no major series have been reported. Patients and methods: We analysed the results of 241 hepatic resections in the Gyan Burman Liver Unit, Sir Ganga Ram Hospital to compare our results with those published from established centres and to identify the factors relating to morbidity and mortality. To examine the effect of a greater experience with the procedure we compared the outcome of our operations from 1996–2000 (first period) and those from 2001–2005 (second period). Results: The overall mortality and morbidity rates were 6.6% and 44.8%, respectively, which are comparable with those of most recently published Western series. Life-threatening complications occurred in 12.4% patients. Multivariate analysis showed that the presence of comorbid conditions, intraoperative blood transfusions of >3 units, hepatocellular carcinoma with underlying cirrhosis and gall bladder carcinoma with jaundice were the independent risk factors for morbidity, whereas the presence of comorbid illness and underlying liver cirrhosis were the risk factors for mortality. During the second period there was an increase in the number of operations performed (66 vs 175; first vs second period), but the mortality rates remained essentially unchanged (6.1% vs 6.8%). Discussion: Hepatic resections can be performed safely in India with results comparable to those achieved in the West. Increasing experience did not reduce overall mortality. Perhaps more careful patient selection and better perioperative management of comorbid illnesses may reduce the morbidity and mortality further.
Keywords: liver, hepatic resection, Indian experience
Introduction
The results of liver surgery have improved dramatically in the last 20 years. There has been a major decrease in perioperative mortality from 20% to <5% in high volume centres 1,2 and this improvement has probably been due to a better understanding of hepatic anatomy, the use of hypotensive anaesthesia, better patient selection and the emergence of hepatobiliary surgery as a subspeciality 2,3. All this has occurred in spite of the extension of the indications for hepatic resection to include conditions like recurrent hepatocellular carcinoma and metastatic disease, hilar bile duct carcinoma and living related donor transplantion 4.
However, although there has been an improvement in overall survival, recent reports indicate that morbidity and mortality rates are still high after hepatic resection in patients with liver cirrhosis and jaundice, emphasizing the need for judicious patient selection 3,5,6. Reports of large numbers of patients have been mainly from Western countries and the Far East but there have been none from South Asia. We analysed 241 consecutive cases of hepatic resections undertaken in a tertiary referral centre in India to define the risk factors associated with postoperative morbidity and mortality. We also compared our results with those of other centres and examined whether experience with the procedure made a difference by contrasting our results obtained between 1996–2000 and 2001–2005.
Patients and methods
Between 1996 and 2005, we performed 241 hepatic resections in our department and recorded the details in a prospectively maintained database. All the patients underwent a thorough history and physical examination and any associated comorbid conditions were managed appropriately. The operation was done through an extended right subcostal incision or a bilateral subcostal incision with a vertical midline extension. Details of the technique of hepatectomy have been described elsewhere 2. In the first half we frequently used the Pringle manoeuvre (clamping of the portal triad for 15 min alternating with a 5 min release) and in the second half this was rarely employed. For parenchymal transection, the Kelly fracture technique was used during the first half of the study and the Cavitron Ultrasonic Surgical Aspirator (CUSA) was mainly used during the second half. In donors who were undergoing partial hepatectomy for liver transplantation, only CUSA was used for dissection so that the vessels and bile channels were not traumatized. Haemostasis was obtained during parenchymal transection using a combination of diathermy, argon beam coagulation and suturing.
The intraoperative variables we analysed included whether the procedure was an emergency or done electively, whether major (two or more segments removed) or minor hepatic resection was done, whether associated procedures were performed and whether the Pringle manoeuvre was used, and we recorded the resection technique, the volume of blood transfusion and the duration of surgery. The associated procedures performed with hepatic resection were considered only if they were likely to affect the outcome of surgery, e.g. organ resection, bile duct excision and reconstruction, portal vein or vena cava resection and lymphadenectomy at the porta hepatis. Such cases were labelled complex hepatectomies. Cholecystectomy and common bile duct exploration with T-tube drainage were not considered to be ‘associated’ procedures.
The postoperative variables analysed included minor or major complications, the duration of hospital stay and mortality (within 30 days of operation or during the same hospital admission). Complications were considered to be major only when they resulted in organ failure or required another operation or radiological intervention. Wound infection, transient ascites, minor bile leaks, jaundice and liver dysfunction responding to conservative treatment were considered to be minor complications. Pathological details on the resected specimens were obtained from the laboratory reports. Apart from the size and extent of the primary lesion in the liver, any underlying parenchymal abnormality (e.g. cirrhosis, fibrosis or steatosis) was recorded.
Statistical analysis
Continuous variables were depicted as the median value (range) and compared using the independent samples t test. Categorical variables were compared using the χ2 test with Yates’ correction or Fisher's exact test. Multivariate analysis was performed using a stepwise logistic regression analysis. Statistical significance was defined as p<0.05. All statistical analyses were performed using the statistical software package Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA).
Results
There were 119 men and 122 women who underwent hepatic resection for benign or malignant hepatobiliary disease. The preoperative characteristics of the patients are provided in Table I. The most common comorbid condition was hypertension (13.7%), followed by diabetes mellitus (10.4%). The patients operated in the first and second half of the study period were comparable with respect to comorbid factors.
Table I. Patient demographic and comorbid conditions (n=241).
| Parameter | Value |
|---|---|
| Male/female (n) | 144/97 |
| Age (years) | 47.83±15.15 (1–82) |
| Patients with ≥1 comorbid conditions, n (%) | 77 (2%) |
| Patients with ≥2 comorbid conditions, n (%) | 17 (7.1%) |
| Hypertension | 33 (13.69%) |
| Diabetes mellitus | 25 (10.37%) |
| Cardiac disease | 13 (5.39%) |
| COPD | 10 (4.15%) |
| Chronic renal failure | 6 (2.45%) |
| Others* | 8 (3.32%) |
| HBsAg positivity†, n (%) | 15 (6.2%) |
| Haemoglobin <10 g/dl | 25 (11.2%) |
| Serum bilirubin >2 mg/dl | 52 (21.6%) |
| Serum bilirubin >10 mg/dl | 25 (10.4%) |
| Serum albumin <3.5 g/dl | 67 (28.8%) |
| Platelet count <150 000/µl | 24 (10%) |
| Serum creatinine >1.3 mg/dl | 12 (5%) |
| Abnormal serum alkaline phosphatase, n (%) | 65 (27%) |
| Abnormal serum transaminases, n (%) | 33 (14.2%) |
| Abnormal prothrombin time, n (%) | 22 (9.1%) |
COPD, chronic obstructive pulmonary disease.
*Obesity (2), peripheral vascular disease (2), hypothyroidism (1), thyroiditis (1), major depression (1), transient ischaemic attack – TIA (1).
†Not all patients were routinely tested.
Operative variables
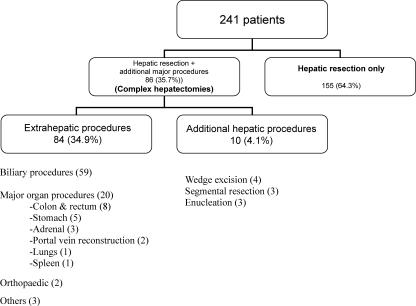
Table II and Figure 1 depict the indications for hepatectomies and the types of procedures performed. The most common indication for hepatic resection was carcinoma of the gall bladder, which accounted for 36.1% of all cases. In all, 111 (46.1%) patients underwent major hepatic resections, whereas the other 130 (53.9%) underwent minor hepatic resection of two or fewer segments (Table III). Complex hepatectomies were done in 86 patients (35.7%). The average operation time was 330 (range 110–840) min (Table IV). The Pringle manoeuvre was used in 42.7% of cases and CUSA was used in 42.3% of cases for parenchymal transection. The average amount of blood transfusion required during surgery was 2.4 (0–8) units (one unit equaling 450 ml). Twenty-one major and 27 complex hepatectomies were performed during the first period and 90 major and 59 complex hepatectomies were performed during the second period. The average duration of surgery was significantly higher in the second period (354 min) compared with the first (266 min) (p<0.001). The average blood transfusion requirement during operation was 2.5 units during the first half and 2.3 units during the second half of the study.
Table II. Indications for hepatic resection (n=241).
| Indication | n (%) |
|---|---|
| Benign | 84 (34.9%) |
| Malignant | 157 (65.1%) |
| Biliary malignancy | |
| Gall bladder carcinoma | 87 (36.1%) |
| Hilar cholangiocarcinoma | 12 (5%) |
| Primary liver tumor | 37 (15.4%) |
| Hepatocellular carcinoma (33) | |
| Hepatoblastoma (3) | |
| Embryonal carcinoma (1) | |
| Non-Hodgkins lymphoma (1) | |
| Metastatic liver tumours | 19 (7.9%) |
| Colorectal metastasis (11) | |
| From other site* (8) | |
| Haemangioma | 17 (7.1%) |
| Hydatid cyst | 11 (4.6%) |
| Liver trauma | 10 (4.1%) |
| Living donor liver transplant | 31 (12.9%) |
| Other benign diseases† | 16 (6.6%) |
| Other malignancy‡ | 1 (0.4%) |
*Stomach (3), adrenal gland (1), neuroendocrine tumor (1), pancreas (1), unknown (2).
†Biliary stricture (6), liver abscess (3), simple liver cysts ( 2), Caroli's disease (2), hepatolithiasis with left lobe atrophy (1), haemobilia with right hepatic artery pseudoaneurysm (1), chronic cholecystitis mimicking carcinoma of gall bladder (1).
‡Recurrent liposarcoma infiltrating into the liver (1).
Figure 1. .
Details of operative procedures in patients undergoing hepatic resection.
Table III. Extent of hepatic resection (n=241).
| Resection | n (%) |
|---|---|
| Major resections (>2 segments, n=111) | |
| Extended right hepatectomy (5 segments±1) | 8 (3.3) |
| Right hepatectomy (4 segments) | 54 (22.4) |
| Left hepatectomy (3 segments) | 34 (14.1) |
| Left extended hepatectomy (4±1 segment) | 1 (0.4) |
| Non-anatomical 3 segment resection | 8 (3.3) |
| < Bisegmentectomy (non-anatomical resection) | 4 (1.7) |
| Central hepatectomy (3±1 segment) | 2 (0.8) |
| Minor resections (≤2 segments, n=130) | |
| < Bisegmentectomy (non-anatomical resection) | 99 (41.1) |
| Left lateral segmentectomy (2 segments) | 8 (3.3) |
| Wedge resection (1 segment) | 5 (2.1) |
| Wide local excision/metastatectomy (1 segment) | 6 (2.4) |
| Enucleation/cystopericystectomy | 12 (5) |
Table IV. Operative and perioperative results (n=241).
| A Operation time (minutes) | 330±141.2 | (110–840) |
| Pringle manoeuvre (yes) | 103 | (42.7%) |
| Techniques of resection | ||
| Kellyclysis | 139 | (57.7%) |
| CUSA | 102 | (42.3%) |
| Blood transfusion (units) | 2.4±1.6 (0–8) | |
| Hospital stay (days) | 10.1±4.82 (3–41) | |
| B | ||
| Patient suffering ≥1 complication | 108 | (44.8%) |
| ≥ 2 complications | 38 | (15.8%) |
| Major complications | 30 | (12.4%) |
| Surgical intervention needed | 11 | (4.6%) |
| Mortality | 16 | (6.6%) |
| Complications | No. of patients | No. of survivors |
| Ascites (requiring diuretics or paracentesis) | 24 | 1 |
| Hepatic insufficiency/failure | 17 | 7 |
| Bile leak/biloma | 16 1 | 1 |
| Renal insufficiency/failure* | 15 | 8 |
| Perihepatic fluid collection (sterile) | 12 1 | 10 |
| Wound infection | ||
| Pleural effusion | 9 2 | 2 |
| Sepsis | 9 | 3 |
| Paralytic ileus | 5 | 7 |
| Chest infection | ||
| Postoperative intra-abdominal haemorrhage | 5 2 | 2 |
| Respiratory insufficiency/failure† | 4 | 2 |
| Arrhythmia | 4 | |
| Portal vein thrombosis | 3 2 | 2 |
| Perihepatic abscess | 3 1 | 1 |
| Wound dehiscence | 3 1 | 1 |
| Unexplained fever | 4 | |
| Pneumothorax | 4 1 | 1 |
| Gastrointestinal tract haemorrhage | 3 | 1 |
| Persistent hypotension | 3 | |
| Bronchospasm | 2 | |
| Congestive heart failure | 2 | |
| Pulmonary embolism | 1 | 1 |
| DIC (disseminated intravascular coagulation) | 1 | 1 |
| Hepatic artery embolism and infarction | 1 |
Note that some patients had more than one complication. Values in square brackets are numbers of patients who underwent surgical interventions.
*Prolonged bilirubin level >5 mg/dl unrelated to biliary obstruction or leak, prolonged coagulopathy requiring fresh frozen plasma (FFP) and/or hepatic encephalopathy.
†Prolonged ventilatory requirement or re-intubation, hypoxia with prolonged supplemental oxygen requirement.
Morbidity
The overall morbidity rate was 44.8% (108) and major complications were seen in 30 (12.4%) cases (Table IV). Thirty-eight (15.8%) patients developed two or more complications in the postoperative period. The most common complications were ascites, hepatic insufficiency and bile leaks, which usually resolved with conservative treatment. The major complications requiring re-exploration and/or image-guided intervention were intra-abdominal haemorrhage, pleural effusion and portal vein thrombosis. The morbidity rate of patients with underlying cirrhosis was 85% (17/20) and in patients without underlying cirrhosis was 41.2% (91/221) and this difference was statistically significant (p<0.001).
Postoperative hospital stay
The overall median length of postoperative hospital stay was 10.2 days (range 3–41 days); 63.5% (153) patients were discharged within 10 days of surgery and 36.5% (88) patients had a hospital stay of >10 days. The lengths of stay during the first and second halves of the study were 10.8 days and 9.9 days, respectively, and this difference was not significant (p=0.70).
Mortality
There were 16 postoperative deaths (6.6%). The mortality rate was higher after resection for malignant disease than after resection for benign disease (8.3% vs 3.6%) and the mortality rate in cirrhotic patients was higher than in non-cirrhotic patients (30% vs 4.5%, p=0.001). After major hepatectomy the mortality was 6.3% (7/111) and 9.3% (8/86) after complex hepatectomies. The complications leading to death included postoperative renal failure (n=8), hepatic failure (7), multi-organ failure (3), septicaemia (3), respiratory failure (2), portal vein thrombosis (2), intra-abdominal abcess (1), gastrointestinal haemorrhage (1), cardiac arrhythmia (1), acute myocardial infarction (1) and pulmonary embolism (1). The mortality rate was 6.1% (4/66) during the first half and 6.8% (12/175) during the second half of the study period and this difference was not statistically significant (p=1.000). None of the patients died intraoperatively.
Risk factor analysis
Univariate analysis of various preoperative and intraoperative variables that influenced the morbidity and mortality rates are shown in Tables V and VI, respectively.
Table V. Univariate analysis of risk factors for overall morbidity after hepatic resection.
| Variable | No. of patients with complications | Percentage (%) | p value | |
|---|---|---|---|---|
| Age (years) | >50 | 64/119 | 53.8 | 0.007* |
| <50 | 44/122 | 27.9 | ||
| Gender | Male | 64/144 | 44.4 | 0.896 |
| Female | 44/97 | 45.4 | ||
| Diagnosis | Benign | 22/84 | 26.2 | <0.001* |
| Malignant | 86/157 | 54.8 | ||
| Comorbid medical conditions | No | 60/164 | 36.6 | <0.001* |
| Yes | 48/77 | 62.3 | ||
| Clinical jaundice | No | 74/189 | 39.1 | 0.001* |
| Yes | 34/52 | 65.4 | ||
| Preoperative serum bilirubin (>10 mg) | No | 92/216 | 42.6 | 0.055 |
| Yes | 16/25 | 64 | ||
| Preoperative serum creatinine (>1.3 mg/dl) | No | 98/229 | 42.8 | 0.007* |
| Yes | 10/12 | 83.3 | ||
| Preoperative serum albumin | Normal (>3.5 g/dl | 68/172 | 39.5 | 0.010 |
| Subnormal (<3.5 g/dl) | 40/69 | 58 | ||
| Preoperative platelet count | >150 000 | 96/217 | 44.2 | 0.667 |
| <150 000 | 12/24 | 50 | ||
| Prothrombin time | Normal | 95/219 | 43.3 | 0.181 |
| Deranged | 13/22 | 59.1 | ||
| HBsAg status | No | 83/200 | 41.5 | 0.006* |
| Yes | 12/15 | 80 | ||
| Extent of hepatic resection | Minor (≤2 segments) | 52/130 | 40 | 0.119 |
| Major (>2 segments) | 56/111 | 50.5 | ||
| Complex hepatectomy | No | 62/155 | 40 | 0.058 |
| Yes | 46/86 | 53.5 | ||
| Bile duct excision | No | 76/181 | 42 | 0.136 |
| Yes | 32/60 | 53.3 | ||
| Elective/emergency | Elective | 99/229 | 43.2 | 0.038* |
| Emergency | 9/12 | 75 | ||
| Liver cancer with cirrhosis | No | 94/226 | 41.6 | <0.001* |
| Yes | 14/15 | 93.3 | ||
| Gall bladder cancer with jaundice | No | 88/213 | 41.3 | 0.004* |
| Yes | 20/28 | 71.4 | ||
| Pringle manoeuvre | No | 55/138 | 51.5 | 0.089 |
| Yes | 53/103 | 39.9 | ||
| Intraoperative blood transfusion | No | 8/36 | 22.2 | 0.003* |
| Yes | 100/205 | 48.8 | ||
| Intraoperative blood transfusion (units) | <3 units | 46/141 | 32.6 | <0.001* |
| >3 units | 62/100 | 62 | ||
| Techniques of resection | Kellyclysis | 59/139 | 42.8 | 0.513 |
| CUSA | 49/102 | 47.6 | ||
| Duration of surgery | >180 min | 97/200 | 43.9 | 0.015* |
| <180 min | 11/41 | 26.8 | ||
| Liver pathology | Non-cirrhotic | 91/221 | 41.2 | <0.001* |
| Cirrhotic | 17/20 | 85 | ||
| Year 1996–2000 | 36/66 | 54.5 | 0.081 | |
| Year 2001–2005 | 72/175 | 41.1 |
*Significant p value.
Table VI. Univariate analysis of risk factors for overall mortality after hepatic resections.
| Variable | No. of patients with complications | Percentage (%) | p value | |
|---|---|---|---|---|
| Age (years) | >50 | 11/119 | 9.24 | 0.126 |
| <50 | 5/122 | 4.10 | ||
| Gender | Male | 10/144 | 6.9 | 1.000 |
| Female | 5/97 | 5.1 | ||
| Diagnosis | Benign | 3/84 | 3.57 | 0.1870 |
| Malignant | 13/157 | 8.28 | ||
| Comorbid medical conditions | No | 5/164 | 3.05 | 0.004* |
| Yes | 11/77 | 14.28 | ||
| Clinical jaundice | No | 8/189 | 4.23 | 0.009* |
| Yes | 8/52 | 15.38 | ||
| Preoperative serum bilirubin (>10 mg) | No | 13/216 | 6.01 | 0.223 |
| Yes | 3/25 | 12 | ||
| Preoperative serum creatinine (>1.3 mg/dl) | No | 13/229 | 5.67 | 0.037* |
| Yes | 3/12 | 25 | ||
| Preoperative platelet count | >150 000 | 13/229 | 5.67 | 0.204 |
| <150 000 | 3/24 | 12.5 | ||
| Prothrombin time | Normal | 12/219 | 5.48 | 0.046* |
| Derranged | 4/22 | 18.18 | ||
| HBsAg status | No | 13/200 | 6.5 | 0.089 |
| Yes | 3/15 | 20 | ||
| Extent of hepatic resection | Minor (≤2 segments) | 9/130 | 6.92 | 1.000 |
| Major (>2 segments) | 7/111 | 6.30 | ||
| Complex hepatectomy | No | 8/155 | 5.16 | 0.280 |
| Yes | 8/86 | 9.30 | ||
| Bile duct excision | No | 8/181 | 4.42 | 0.031* |
| Yes | 8/60 | 13.33 | ||
| Elective/emergency | Elective | 15/229 | 6.55 | 0.570 |
| Emergency | 1/12 | 8.33 | ||
| Liver cancer with cirrhosis | No | 11/226 | 4.87 | 0.001* |
| Yes | 5/15 | 33.33 | ||
| Gall bladder cancer with jaundice | No | 12/213 | 5.63 | 0.099 |
| Yes | 4/28 | 14.28 | ||
| Pringle manoeuvre | No | 8/138 | 5.80 | 0.606 |
| Yes | 8/103 | 7.76 | ||
| Intraoperative blood transfusion | No | 2/36 | 5.55 | 1.00 |
| Yes | 14/205 | 6.82 | ||
| Intraoperative blood transfusion (units) | <3 units | 5/141 | 3.54 | 0.033 |
| >3 units | 11/100 | 11 | ||
| Techniques of resection | Kellyclysis | 9/139 | 6.47 | 1.000 |
| CUSA | 7/102 | 6.86 | ||
| Duration of surgery (min) | <180 | 3/41 | 7.38 | 0.740 |
| >180 | 13/200 | 6.5 | ||
| Liver pathology | Non-cirrhotic | 10/221 | 4,52 | 0.001* |
| Cirrhotic | 6/20 | 300 | ||
| Year 1996–2000 | 4/66 | 6.06 | 1.000 | |
| Year 2001–2005 | 12/175 | 6.86 |
*Significant p value.
The risk factors for morbidity were age (>50 years), malignant disease, the presence of comorbid conditions, hypoalbuminaemia, clinical jaundice, a raised serum creatinine level, hepatitis B surface antigen positivity, emergency surgery, major intraoperative blood transfusion, prolonged surgery (>3 hours), underlying liver cirrhosis, surgery in hepatocellular carcinoma with cirrhosis and surgery in gall bladder carcinoma with jaundice. The factors associated with increased mortality were the presence of comorbid conditions, clinical jaundice, raised serum creatinine levels, deranged prothrombin time, intraoperative blood transfusion (>3 units), underlying liver cirrhosis, common bile duct (CBD) excision and surgery in hepatocellular carcinoma with cirrhosis.
All risk factors with p values <0.1 by univariate analysis were subjected to stepwise multivariate logistic regression analysis (Table VII). Independent risk factors for overall morbidity were intraoperative blood transfusion (>3 units), presence of comorbid conditions, emergency hepatectomy, surgery for malignant hepatic lesions and severity of resection, while the independent risk factors for overall mortality were presence of comorbid conditions and underlying liver cirrhosis.
Table VII. Multivariate analysis of factors associated with postoperative morbidity and mortality.
| Variable | p value |
|---|---|
| Morbidity | |
| Primary liver cancer with cirrhosis | <0.001 |
| Elective or emergency | 0.001 |
| Comorbid illness | 0.002 |
| Gall bladder cancer with jaundice | 0.011 |
| Blood transfusion >3 units | 0.016 |
| Benign or malignant | 0.016 |
| Resection severity | 0.040 |
| Mortality | |
| Cirrhosis | 0.002 |
| Comorbid illness | 0.003 |
Discussion
Hepatic resection has evolved from being a high-risk, resource-intensive procedure to a mainstream operation with broad indications. It is now considered to be the most effective treatment for selected patients with primary and secondary hepatobiliary malignancies and is the only effective treatment for a number of benign hepatic diseases 2,7,8,9,10,11,12. This evolution has largely been due to the progressive improvement in the morbidity and mortality rates. In developing countries in South Asia, however, hepatic resection is still not performed routinely in most centres because it is viewed as being a difficult procedure associated with major blood loss and postoperative complications. Thus, to our knowledge, there have been no large reports on consecutive hepatic resections published from this part of the world.
At our centre we started performing liver resections in 1996 and have an active living donor liver transplant programme, so we felt that it might be worthwhile to analyse our prospectively maintained database to compare our results with those reported from other major centres, to determine which factors were associated with morbidity and mortality and to examine whether increasing experience had shown any improvement in results.
Our postoperative mortality rate for liver resection of 6.6% is comparable with recently published Western and Far Eastern figures of 5.8% to 8.4% 3,6.
We had a morbidity rate of 44.8% of cases and although this figure seems unduly high it is comparable with that of large series published previously 3,5,13. However, life-threatening complications occurred in only 12.4% of the cases. The main causes of postoperative morbidity were ascites, transient hepatic insufficiency and minor bile leaks. Although they prolonged the postoperative recovery period, these problems usually settled with conservative treatment and were rarely responsible for the mortality.
To assess the effect of experience in the procedure we analysed the results of hepatic resections during the first and second period of the study. We observed that despite a large increase in the number of major resections, as well as the complexity of the hepatic resections performed, the morbidity rates decreased, although not significantly, during the second period. However, mortality rates remained the same. Similar results despite adding more complex resections have been reported by other authors 3,11,14,15, and the lack of improvement in mortality rates may also be due to the extended indications for the procedure. Several reports have compared the morbidity and mortality rates among high volume (performing 10 or more hepatic resections per year) and low volume centres 6. These reports suggest that mortality rates are significantly lower (1.5–6.2%) in high volume centres compared with low volume centres (3.7–24.4%) 16,17,18. It is implied that because high volume centres have greater experience they provide care of a superior quality and therefore have a lower incidence of postoperative complications and death. However, as there are also reports showing a very low mortality from some low volume centres 19,20,21, we feel that other units should be encouraged to perform hepatic resections – especially in developing countries where the patient may not be able to reach or afford high volume centres. With judicious patient selection and meticulous surgical techniques they are likely now to achieve results comparable to those of large volume establishments.
The average median length of hospital stay after surgery for our patients was 10.2 days, which is comparable with that in other reported series which varied from 7 to 9.7 days 6,13,21. Perhaps the wider use of laparoscopic hepatic resections will shorten the stay further to a mean of 2.9 days (range 1–14 days) 22.
One of the most important outcomes of our study was the identification of the independent risk factors for overall morbidity and mortality. These were the presence of comorbid conditions, hepatic cirrhosis, carcinoma of the gall bladder with jaundice, extent of hepatic resection and large intraoperative blood transfusion (>3 units). This corroborates the findings in previous studies 3,6,13,14,23,24.
Our experience with hepatic resection in a specialized unit in India shows that with careful patient selection, scrupulous perioperative management of comorbid conditions and meticulous surgical dissection with either Kellyclasis or CUSA to minimize blood loss and blood transfusion requirements, major and complex hepatic resections can be performed safely in third world countries like India, with results comparable to those reported from the western world or South-east Asia.
References
- 1.Foster JH, Berman MM. Solid liver tumors. Major Probl Clin Surg. 1977;22:1–342. [PubMed] [Google Scholar]
- 2.Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–30. doi: 10.1097/00000658-199903000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei AC, Poon RT-P, Fan S-T, Wong J. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg. 2003;90:33–41. doi: 10.1002/bjs.4018. [DOI] [PubMed] [Google Scholar]
- 4.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvenet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 5.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 6.Dimick JB, Cowan JA, Jr, Knol JA, Upchurch GR., Jr Hepatic resection in the United States: indications, outcomes, and hospital procedural volumes from a nationally representative database. Arch Surg. 2003;138:185–91. doi: 10.1001/archsurg.138.2.185. [DOI] [PubMed] [Google Scholar]
- 7.Fong Y, Fortner JG, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–21. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gayowski TJ, Iwatsuki S, Madariaga JR, Selby R, Todo S, Irish W, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathological risk factors. Surgery. 1994;116:703–11. [PMC free article] [PubMed] [Google Scholar]
- 9.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–62. [PubMed] [Google Scholar]
- 10.Scheele J. Liver resection for colorectal metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 11.Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BSJ, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–17. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charny CK, Jarnagin WR, Schwartz LH, Frommeyer HS, De Matteo RP, Fong Y, et al. Management of 155 patients with benign liver tumours. Br J Surg. 2001;88:808–13. doi: 10.1046/j.0007-1323.2001.01771.x. [DOI] [PubMed] [Google Scholar]
- 13.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–710. doi: 10.1097/01.sla.0000141195.66155.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karanjia N, Quiney N, Fawcett W, Remington J. Hepatic resection: towards minimal blood loss and mortality. Br J Surg. 2005;92:60. [Google Scholar]
- 16.Glasgow RE, Showstack JA, Katz PP, Corvera CU, Warren RS, Mulvihill SJ. The relationship between hospital volume and outcomes of hepatic resection for hepatocellular carcinoma. Arch Surg. 1999;134:30–5. doi: 10.1001/archsurg.134.1.30. [DOI] [PubMed] [Google Scholar]
- 17.Choti MA, Bowman HM, Pitt HA, Sosa JA, Sitzmann JV, Cameron JL, et al. Should hepatic resection be performed at high volume referral centers? J Gastrointest Surg. 1998;2:11–20. doi: 10.1016/s1091-255x(98)80098-x. [DOI] [PubMed] [Google Scholar]
- 18.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–51. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 19.Shiu MH, Siu DLS, Hui WM, Yu HC, Lam KC. Hepatic resection for primary liver cancer at a private community hospital: retrospective study of 61 patients. Hong Kong Med J. 1999;5:353–9. [PubMed] [Google Scholar]
- 20.Ston ME, Jr, Rehman SU, Conaway G, Sardi A. Hepatic resection at a community hospital. Gastrointest Surg. 2000;4:349–53. doi: 10.1016/s1091-255x(00)80011-6. [DOI] [PubMed] [Google Scholar]
- 21.Metreveli RE, Sahm K, Denstman F, Abdel-Misih R, Petrelli NJ. Hepatic resection at a major community-based teaching hospital can result in good outcome. Ann Surg Oncol. 2005;12:133–7. doi: 10.1245/ASO.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Buell JF, Thomas MJ, Doty TC, Gersin KS, Merchen TD, Gupta M, et al. An initial experience and evolution of laparoscopic hepatic resectional surgery. Surgery. 2004;36:804–11. doi: 10.1016/j.surg.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Shimada M, Takenaka K, Fujiwara Y, Gion T, Shirabe K, Yanaga K, et al. Risk factors linked to postoperative morbidity in patients with hepatocellular carcinoma. Br J Surg. 1998;85:195–8. doi: 10.1046/j.1365-2168.1998.00567.x. [DOI] [PubMed] [Google Scholar]
- 24.Das BC, Isaji S, Kawarada Y. Analysis of 100 consecutive hepatectomies: risk factors in patients with liver cirrhosis or obstructive jaundice. World J Surg. 2001;25:266–72. doi: 10.1007/s002680020059. [DOI] [PubMed] [Google Scholar]