Abstract
Background: Thermal ablation techniques have become important treatment options for patients with unresectable hepatic malignancies. Microwave ablation (MWA) is a new thermal ablative technique that uses electromagnetic energy to produce coagulation necrosis. We report outcomes from the first clinical trial in the United States using MWA and a 915 MHz generator. Patients and methods: Patients with unresectable primary or metastatic liver cancer were enrolled in a multi-institutional trial from March 2004 through May 2006. Demographic information, diagnosis, treatment, and outcomes were documented. Results: Eighty-seven patients underwent 94 ablation procedures for 224 hepatic tumors. Forty-two ablations (45%) were performed open, 7 (7%) laparoscopically, and 45 (48%) percutaneously. The average tumor size was 3.6 cm (range 0.5–9.0 cm). Single antenna ablation volumes were 10.0 ml (range 7.8–14.0 ml), and clustered antennae ablation volumes were 50.5 ml (range 21.1–146.5 ml). Outcome variables were measured with a mean follow-up of 19 months. Local recurrence at the ablation site occurred in 6 (2.7%) tumors, and regional recurrence occurred in 37 (43%) patients. With a mean follow-up of 19 months, 41 (47%) patients were alive with no evidence of disease. There were no procedure-related deaths. The overall mortality rate was 2.3%. Conclusions: Microwave ablation is a safe and effective technology for hepatic tumor ablation. In our study, clustered antennae resulted in larger ablation volumes. Further studies with histological confirmation are needed to verify clinical results.
Introduction
Options for liver-directed therapy of unresectable hepatic tumors have expanded in the last decade. Up to 33% of patients with hepatocellular cancer (HCC) and up to 20% of patients with metastatic colon cancer are not candidates for surgical resection 1,2. Currently available ablative techniques include cryotherapy, radiofrequency ablation (RFA), microwave ablation (MWA), laser ablation, high frequency ultrasonic ablation (HIFU), and ethanol ablation. RFA is the most widely used technique due to its availability, efficacy, marketing, recent technical advances, and low complication rates 3. However, RFA can be time-consuming and associated with higher recurrence rates in larger lesions, particularly with impedance-based systems 4.
MWA is a new treatment option with several theoretical advantages over RFA. MWA has an improved convection profile, consistently higher intratumoral temperatures, larger ablation volumes, faster ablation times, and the option of using multiple antennae simultaneously 5. MWA uses electromagnetic energy to agitate adjacent water molecules. Thermal energy is released due to molecular friction, allowing nearby tumor cells to undergo coagulation necrosis. Recent technical advances in MWA include the reduction of feedback and an increase in energy delivery by tuning antennae to the dielectric properties of liver tumors 6. MWA antennae and generators are similar to RFA; however, they use a different part of the electromagnetic spectrum. Like RFA, MWA instruments have been developed for percutaneous, laparoscopic, and open procedures 7.
The use of microwave technology in Japan began in the late 1980s; however, it was not widely adopted due to small ablation diameters obtained with single 2.4 GHz needle antenna 8,9. With the discovery that larger coagulation diameters could be obtained by clustering the antennae, microwave technology became more clinically relevant and was reported to be useful for larger tumors 10,11. However, MWA is not widely available in the USA, and experience with this approach is limited. A recent Phase I clinical trial evaluated treatment with multiple microwave antennae in patients undergoing subsequent liver resection. The study concluded that clustering multiple needle antennae causes a synergistic effect and results in mean maximum ablation diameters of 5.5 cm and average ablation zone volumes of 50.8 cm37. The purpose of this trial was to evaluate the outcomes of patients with unresectable liver tumors after undergoing treatment with MWA.
Patients and methods
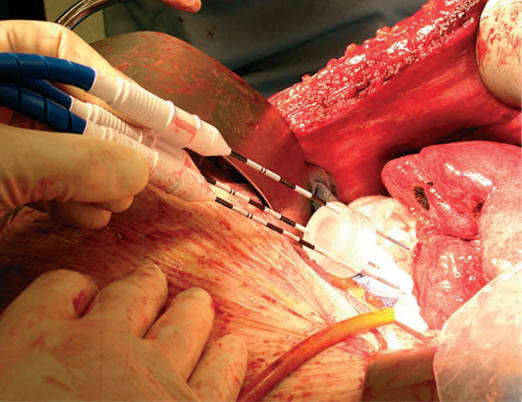
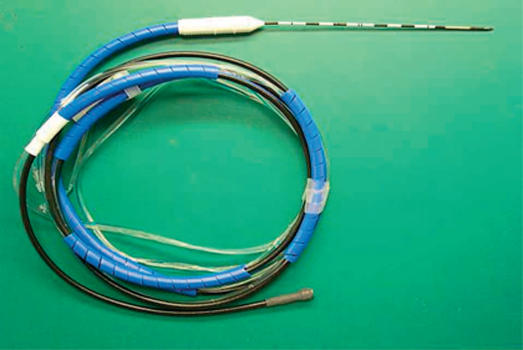
Institutional Review Board approval was obtained for a multi-institutional study from March 2004 through May 2006. Patients with unresectable primary or metastatic liver cancer were eligible for enrollment. Data regarding patient demographics, diagnosis, and type of procedure were collected. Percutaneous, laparoscopic, and open procedures (Figure 1) were included. All tumors were localized and measured using intraoperative ultrasound or CT fluoroscopy. Clustered and single microwave antennae were used for ablations at a power of 45 W for 10 min using a 915 MHz microwave generator (VivaWave™ System, Valleylab, Boulder, CO, USA) (Figure 2) for each needle antenna (Figure 3). Patients were imaged within 1 month after the procedure and every 4 months for 2 years thereafter. Outcome variables included morbidity, mortality, technical success, local tumor control, ablation size, and recurrence.
Figure 1. .
Open microwave liver ablation.
Figure 2. .
Microwave generators.
Figure 3. .
Microwave antenna.
Results
Eighty-seven patients were enrolled in the study; 47% male and 53% female. The average age was 67 years (range 37–92). Diagnoses are listed in Table I. The most common indications for liver-directed therapeutic ablation were colorectal metastases (38%) and HCC (26%).
Table I. Indications for ablation and diagnoses in 87 patients.
| Indication for ablation | No. of patients (%) |
|---|---|
| Colorectal metastasis | 33 (37.93) |
| Hepatocellular carcinoma | 23 (26.44) |
| Breast metastasis | 11 (12.64) |
| Carcinoid metastasis | 8 (9.19) |
| Renal metastasis | 3 (3.45) |
| Lung metastasis | 3 (3.45) |
| Adrenal metastasis | 1 (1.15) |
| Esophageal metastasis | 1 (1.15) |
| Gallbladder metastasis | 1 (1.15) |
| Gastric metastasis | 1 (1.15) |
| Melanoma metastasis | 1 (1.15) |
| Ovarian metastasis | 1 (1.15) |
There were 94 ablation procedures for 224 tumors. Forty-two ablations (45%) were performed open, 7 (7%) laparoscopically, and 45 (48%) percutaneously. The average tumor size was 3.6 cm (range 0.5–9.0 cm). Twenty-two lesions were > 4 cm. Mean single antenna ablation volumes were 10.0 ml (range 7.8–14.0 ml), and clustered antennae ablation volumes were 50.5 ml (range 21.1–146.5 ml). Outcome variables were measured with a mean follow-up of 19 months.
Unexpected residual disease was found in five patients. Expected residual disease was found in three patients. Local recurrence at ablation sites occurred in 6 (2.7%) tumors and regional recurrence was noted in 37 (43%) patients. Procedure-related complications are listed in Table II. Cancer status at follow-up is listed in Table III. With a mean follow-up of 19 months, 41 (47%) patients were alive with no evidence of disease. There were no procedure-related deaths. Two deaths unrelated to the procedure occurred several months after the ablations, resulting in an overall mortality rate of 2.3%. One death was due to a myocardial infarction and the other to a cerebrovascular ischemic event.
Table II. Procedure-related complications in 87 patients.
| Complication | No. of patients |
|---|---|
| Skin burns | 3 |
| Wound breakdown | 2 |
| Re-admission (nausea, over-sedation) | 2 |
| Pain requiring termination of procedure | 1 |
| Fluid collections | 2 |
| Persistent postoperative ileus | 2 |
| Subscapular hematoma | 1 |
| Fever/staphylococcal infection | 1 |
Table III. status at 19 months follow-up in 87 patients.
| Type of cancer | Total no. of patients | No evidence of disease (%) | Alive with disease (%) | Dead of disease (%) |
|---|---|---|---|---|
| Hepatocellular carcinoma | 23 | 14 (61) | 3 (13) | 6 (26) |
| Colorectal cancer metastasis | 33 | 19 (58) | 8 (24) | 6 (18) |
| Breast metastasis | 11 | 4 (36) | 1 (9) | 6 (55) |
| Carcinoid metastasis | 8 | 2 (25) | 6 (75) | 0 (0) |
| Renal metastasis | 3 | 0 (0) | 0 (0) | 3 (100) |
| Lung metastasis | 3 | 0 (0) | 1 (33) | 2 (67) |
| Adrenal metastasis | 1 | 1 (100) | 0 (0) | 0 (0) |
| Esophageal metastasis | 1 | 0 (0) | 0 (0) | 1 (100) |
| Gallbladder metastasis | 1 | 0 (0) | 0 (0) | 1 (100) |
| Gastric metastasis | 1 | 0 (0) | 0 (0) | 1 (100) |
| Melanoma metastasis | 1 | 1 (100) | 0 (0) | 0 (0) |
| Ovarian metastasis | 1 | 0 (0) | 1 (100) | 0 (0) |
Discussion
Surgical resection of HCC and colorectal cancer metastases has been shown to increase 5-year survival and disease-free survival for patients with resectable lesions 7,12,13,14. Unfortunately, some patients have unresectable disease due to inadequate hepatic reserve or extensive disease. Liver-directed therapy for patients with unresectable disease includes a wide range of ablation techniques including RFA, MWA, laser ablation, high frequency ultrasound ablation, cryoablation, and ethanol ablation. RFA is the most widely used and best studied technique worldwide. It is considered first-line treatment for small (<3 cm) unresectable HCC 15,16. Controversy exists as to which new technology offers the most advantages over RFA. MWA is one of the most recent and exciting advances in the field of thermoablative technology and, similar to RFA, can be performed open, laparoscopically, or percutaneously. The potential advantages of microwave technology compared with RFA include consistent production of higher intratumoral temperatures, faster ablation times, improved convection profiles, larger tumor ablation volumes, the use of multiple antennae, and less procedural pain 6,10,17,18. Another advantage of MWA is that it does not require the use of grounding pads, which decreases the time required for patient preparation.
Thermal ablative techniques for unresectable hepatic lesions have been described previously. Taura and colleagues 19 reported outcomes of more than 600 patients after hepatic resection for HCC. They observed, in a multivariate analysis, that application of local thermal ablative therapies was an independent favorable prognostic factor for this patient population 19. Recent studies have evaluated the use of MWA in HCC. Lu and colleagues 20 reviewed surgical resection versus radiofrequency and MWA for early stage HCC. They reported that RFA and MWA had equivalent 3-year survival outcomes and local effectiveness compared with surgical resection, and recommended that both thermoablative techniques be considered a first-line treatment modality for early stage HCC 20. Dong and colleagues 21 reported the efficacy of MWA in early HCC with complete tumor necrosis and a low complication rate in 96% of patients. Contrary to these results, Wakai and colleagues 22 retrospectively reviewed 149 patients with HCC tumors = 4 cm with a median follow-up of 69 months. They concluded that hepatectomy provided significantly better local control and improved long-term survival over various ablation techniques, including percutaneous ethanol ablation, RFA, and MWA 22.
MWA has also been evaluated previously as treatment for colorectal liver metastases. Liang and colleagues 23 examined the long-term effects of ultrasound-guided MWA for colorectal metastatic liver lesions. With an average follow-up of 29 months, they reported significantly improved long-term survival in patients with well-differentiated tumors measuring 3.0 cm or less and no local recurrence or new colorectal metastases after MWA therapy 23. Shimizu and colleagues 24 reviewed combined hepatic resection plus MWA in patients with multiple bilobar liver metastases from colon cancer and reported that hepatic resection plus MWA was equally effective to hepatic resection alone.
Most clinical trials examining the safety and efficacy of MWA have been done outside of the US with 2.4 GHz generators. In 2003, a new 915 MHz MWA system was engineered in the US 6. We report results from a clinical series of MWA in the US. Similar to previous studies, we found that HCC and metastatic colon cancer are the most common indications for MWA use. We also observed MWA to be a flexible thermoablative technique, with no increased difficulty with procedures performed open, laparoscopically, or percutaneously. We and other authors have reported that larger ablations can be obtained with multiple or clustered microwave antennae 7,10,25. Simon and colleagues 7 reported the feasibility of using multiple microwave antennae simultaneously in the treatment of liver tumors with an average ablation zone volume of 50.8 cm2. We also found that clustering antennae resulted in higher ablation volumes – 50.5 ml compared with 10.0 ml for a single antenna. The feasibility of using MWA for larger tumors was demonstrated in our study, as the average tumor size was 3.6 cm. MWA was also used in 23 lesions that were > 4 cm. While this technology can achieve higher ablation volumes with clustering of antennae, it is important to remember that increasing size of tumors may adversely affect the efficacy of MWA as well as other thermoablative techniques. This was evidenced by a study by Liang and colleagues 26, who reviewed prognostic factors of 288 patients who underwent MWA for HCC and showed improved survival in treatment of single lesions < 4 cm for patients with Childs-Pugh class A cirrhosis.
Another attractive characteristic of MWA and thermal ablation techniques in general is the low morbidity and mortality associated with the procedure. Dong and colleagues 21 reported three severe complications and one unrelated death from a pulmonary infection in 216 patients treated with MWA for early stage HCC. We also report a low morbidity rate with a total of 14 (16%) complications, the most common being skin burns, which occurred in 3 patients. In addition, hypothermia, disseminated intravascular coagulation (DIC), and hemolysis, which can occur in cryoablation, did not occur in our study and traditionally have not been reported in patients undergoing MWA.
In conclusion, MWA is a safe and effective technology for hepatic tumor ablation. At a mean follow-up of 19 months, 47% of patients were alive with no evidence of disease, and local recurrence rates and regional recurrence rates were 2.7% and 43%, respectively. Our study provides additional clinical data that clustered antennae result in larger ablation volumes. Histological confirmation and comparison to real-time 3-dimensional Doppler ultrasound are needed to verify clinical results. Further trials with long-term follow-up and controlled trials comparing various types of liver-directed thermoablative technologies in vivo are needed.
References
- 1.Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122:1609–19. doi: 10.1053/gast.2002.33411. [DOI] [PubMed] [Google Scholar]
- 2.Colorectal cancer facts & figures, special edition 2005. American Cancer Society, 2005. [Google Scholar]
- 3.Bilchik AJ, Wood TF, Allegra D, Tsioulias GJ, Chung M, Rose DM, et al. Cryosurgical ablation and radiofrequency ablation for unresectable hepatic malignant neoplasms: a proposed algorithm. Arch Surg 2000;135:657–62; discussion 662–4. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol. 1996;3:636–44. doi: 10.1016/s1076-6332(96)80188-7. [DOI] [PubMed] [Google Scholar]
- 5.Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25(Suppl 1):S69–S83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 6.Stauffer PR, Rossetto F, Prakash M, Neuman DG, Lee T. Phantom and animal tissues for modelling the electrical properties of human liver. Int J Hyperthermia. 2003;19:89–101. doi: 10.1080/0265673021000017064. [DOI] [PubMed] [Google Scholar]
- 7.Simon CJ, Dupuy DE, Iannitti DA, Lu DS, Yu NC, Aswad BI, et al. Intraoperative triple antenna hepatic microwave ablation. AJR Am J Roentgenol. 2006;187:W333–40. doi: 10.2214/AJR.05.0804. [DOI] [PubMed] [Google Scholar]
- 8.Seki S, Sakaguchi H, Kadoya H, Morikawa H, Habu D, Nishiguchi S, et al. Laparoscopic microwave coagulation therapy for hepatocellular carcinoma. Endoscopy. 2000;32:591–7. doi: 10.1055/s-2000-9014. [DOI] [PubMed] [Google Scholar]
- 9.Ohmoto K, Miyake I, Tsuduki M, Shibata N, Takesue M, Kunieda T, et al. Percutaneous microwave coagulation therapy for unresectable hepatocellular carcinoma. Hepatogastroenterology. 1999;46:2894–900. [PubMed] [Google Scholar]
- 10.Wright AS, Lee FT, Jr, Mahvi DM. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol. 2003;10:275–83. doi: 10.1245/aso.2003.03.045. [DOI] [PubMed] [Google Scholar]
- 11.Yu NC, Lu DS, Raman SS, Dupuy DE, Simon CJ, Lassman C, et al. Hepatocellular carcinoma: microwave ablation with multiple straight and loop antenna clusters – pilot comparison with pathologic findings. Radiology. 2006;239:269–75. doi: 10.1148/radiol.2383041592. [DOI] [PubMed] [Google Scholar]
- 12.Jamison RL, Donohue JH, Nagorney DM, Rosen CB, Harmsen WS, Ilstrup DM. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch Surg 1997;132:505–10; discussion 511. [DOI] [PubMed] [Google Scholar]
- 13.Cance WG, Stewart AK, Menck HR. The National Cancer Data Base Report on treatment patterns for hepatocellular carcinomas: improved survival of surgically resected patients, 1985–1996. Cancer. 2000;88:912–20. doi: 10.1002/(sici)1097-0142(20000215)88:4<912::aid-cncr23>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Weber SM, Jarnagin WR, DeMatteo RP, Blumgart LH, Fong Y. Survival after resection of multiple hepatic colorectal metastases. Ann Surg Oncol. 2000;7:643–50. doi: 10.1007/s10434-000-0643-3. [DOI] [PubMed] [Google Scholar]
- 15.Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954–60. doi: 10.1148/radiol.2343040153. [DOI] [PubMed] [Google Scholar]
- 16.Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961–7. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- 17.Skinner MG, Iizuka MN, Kolios MC, Sherar MD. A theoretical comparison of energy sources – microwave, ultrasound and laser – for interstitial thermal therapy. Phys Med Biol. 1998;43:3535–47. doi: 10.1088/0031-9155/43/12/011. [DOI] [PubMed] [Google Scholar]
- 18.Shock SA, Meredith K, Warner TF, Sampson LA, Wright AS, Winter TC 3rd, et al. Microwave ablation with loop antenna: in vivo porcine liver model. Radiology. 2004;231:143–9. doi: 10.1148/radiol.2311021342. [DOI] [PubMed] [Google Scholar]
- 19.Taura K, Ikai I, Hatano E, Fujii H, Uyama N, Shimahara Y. Implication of frequent local ablation therapy for intrahepatic recurrence in prolonged survival of patients with hepatocellular carcinoma undergoing hepatic resection: an analysis of 610 patients over 16 years old. Ann Surg. 2006;244:265–73. doi: 10.1097/01.sla.0000217921.28563.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu MD, Kuang M, Liang LJ, Xie XY, Peng BG, Liu GJ, et al. [Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial.] Zhonghua Yi Xue Za Zhi. 2006;86:801–5. [PubMed] [Google Scholar]
- 21.Dong BW, Liang P, Yu XL, Yu DJ, Zhang J, Feng L, et al. [Long-term results of percutaneous sonographically-guided microwave ablation therapy of early-stage hepatocellular carcinoma.] Zhonghua Yi Xue Za Zhi. 2006;86:797–800. [PubMed] [Google Scholar]
- 22.Wakai T, Shirai Y, Suda T, Yokoyama N, Sakata J, Cruz PV, et al. Long-term outcomes of hepatectomy vs percutaneous ablation for treatment of hepatocellular carcinoma < or = 4 cm. World J Gastroenterol. 2006;12:546–52. doi: 10.3748/wjg.v12.i4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang P, Dong BW, Yu XL, Yu DJ, Feng L, Gao YY, et al. [Evaluation of long-term therapeutic effects of ultrasound-guided percutaneous microwave ablation of liver metastases.] Zhonghua Yi Xue Za Zhi. 2006;86:806–10. [PubMed] [Google Scholar]
- 24.Shimizu T, Tanaka K, Makino H, Matsuo K, Ueda M, Nagano Y, et al. [Microwave ablation for multiple bilobar liver tumors from colorectal cancer.] Gan To Kagaku Ryoho. 2005;32:1646–8. [PubMed] [Google Scholar]
- 25.Meredith K, Lee F, Henry MB, Warner T, Mahvi D. Microwave ablation of hepatic tumors using dual-loop probes: results of a phase I clinical trial. J Gastrointest Surg. 2005;9:1354–60. doi: 10.1016/j.gassur.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 26.Liang P, Dong B, Yu X, Yu D, Wang Y, Feng L, et al. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology. 2005;235:299–307. doi: 10.1148/radiol.2351031944. [DOI] [PubMed] [Google Scholar]