Abstract
Background: Pentoxifylline (PTX) has been shown to reduce hepatic injury after normothermic ischemia and reperfusion (I-R) in rat liver. Aim: The aim of this study was to evaluate the effects of PTX on liver expression of tumor necrosis factor alpha (TNFα) mRNA following normothermic liver I-R. Materials and methods: A segmental normothermic ischemia of the liver was induced in male Lewis rats by occluding the blood vessels including the bile duct to the median and left lateral lobes for 90 min. At the end of ischemia the nonischemic liver lobes were resected. Rats were divided into three groups: group 1, control Ringer lactate administration; group 2, PTX treatment; group 3, sham-operated control rats. PTX (50 mg/kg) was injected intravenously 30 min before and 60 min after induction of ischemia. Survival rates were compared and the serum activities of TNFα, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) were measured. Histology of the liver was assessed 6 h after reperfusion. Liver TNFα mRNA was assessed by PCR amplification at 0, 60, 120, and 210 min after reperfusion. Results: PTX treatment significantly increased 7 day survival (93.3%) compared with nontreated control rats (46.6%, p<0.007). The extent of liver necrosis and the release of liver enzymes were significantly decreased after PTX treatment. Serum activities of TNFα were significantly decreased and liver expression of TNFα mRNA was inhibited after PTX treatment. Conclusion: PTX protects the liver from ischemic injury and inhibits liver expression of TNFα mRNA.
Keywords: normothermic liver ischemia, pentoxifylline, tumor necrosis factor, TNFα mRNA, rat
Introduction
Normothermic ischemia-reperfusion (I-R) injury occurs during liver surgery (resection and transplantation) as well as during trauma and hemorrhagic shock 1. Normothermic I-R is poorly tolerated and rapidly leads to death of hepatocytes, followed by liver dysfunction 1. In normothermic I-R, the activation of hepatic macrophages, the Kupffer cells (KCs), is responsible for a release of various cytokines including tumor necrosis factor alpha (TNFα) 2. TNFα plays an important role in the induction of hepatocyte and sinusoidal cell injury 3,4. Pentoxifylline (PTX) is a methylxanthine phosphodiesterase inhibitor commonly used to treat peripheral vascular diseases 5. PTX has been shown to improve survival rate after normothermic liver I-R 6,7,8 and hepatocellular function after hemorrhagic trauma and resuscitation 9. Blockage of TNFα release from KCs by PTX prevents up-regulation of TNFα expression after normothermic I-R 10. This inhibition of TNFα expression results in a significant decrease of liver injury and improves animal survival. PTX seems to antagonize the effects of TNFα by inhibiting the intracellular phosphodiesterase, resulting in a subsequent accumulation of intracellular c-AMP 11,12. The aim of this study was to evaluate the effects of PTX on TNFα mRNA liver expression following normothermic liver I-R.
Materials and methods
Animal preparation and hepatic ischemia procedure
A total of 146 male Lewis rats (LEW RTI1), weighing 250–300 g, were purchased from the CNRS-CNSEAL (Orléans La Source, France); in each experiment there was <25 g difference between the animals. The rats were housed individually in plexiglass cages and were allowed free access to food and water before, during, and after the ischemia. The animal rooms were windowless with a temperature of 22±2°C and lighting controls (light on at 07.00 h and off at 21.00 h; 14 h light/10 h dark). All animal experiments were conducted in accordance with local institutional guidelines for the care and use of laboratory animals. All experiments were started between 8 and 11 a.m. Before the operation an indwelling intravenous line (Biotrol, Paris, France) was placed in the right external jugular vein, with the catheter tip positioned in the suprahepatic vena cava just above the level of the hepatic dome to obtain immediate post-hepatic blood samples and to administer intravenous fluids; correct catheter position was confirmed at laparotomy. A segmental normothermic ischemia of the liver was induced as described previously 13. Briefly, the anterior abdominal wall was shaved and prepped with povidone-iodine solution (Betadine, Astra Medica, Merignac, France). The abdomen was entered through a midline incision under ether anesthesia and ischemia was induced by occluding the blood vessels including the bile duct to the median and left lateral lobes with an atraumatic vascular clamp. After 90 min of warm ischemia the vascular clamp was released and the nonischemic (right lateral and caudate) lobes were resected, leaving only the ischemic anterior lobes. This procedure was considered to render ischemia in 70% of liver tissue 14 and to avoid splanchnic stasis 15. The abdomen was closed in two layers with 2-0 silk and intravenous lactated Ringer solution at a dose of 0.75 ml was administered to replace operative fluid and blood losses. After the operation animals were kept in individual cages. Mortality rate and survival times were recorded every 6 h. Animals that were alive 7 days after the operation were considered permanent survivors. Necropsy was performed in all animals to ensure absence of surgically related complications.
PTX (Torental®, Hoechst, Paris, France) was suspended in Ringer lactate solution to a concentration of 50 mg/kg (0.75 ml) as described previously in other studies 8,16,17.
Experimental groups
For survival analysis, 35 rats were prepared as described above and divided into three groups. In group 1 (control group, n=15), animals were injected intravenously via the dorsal penile vein with 0.75 ml Ringer lactate solution 30 min before induction of ischemia and 30 min before reperfusion and resection of nonischemic liver lobes. Animals in group 2 (n=15), were injected intravenously via the dorsal penile vein with 0.75 ml of PTX 30 min before induction of ischemia and 30 min before reperfusion and resection of nonischemic liver lobes. Group 3 animals were sham-operated control animals (n=5), in which the hilum of the median and left lateral liver lobes was isolated without applying a vascular clamp and the right lateral and caudate lobes were resected 90 min after the first operation. They received an intravenous injection of 0.75 ml Ringer lactate solution via the dorsal penile vein 30 min before the incision of the abdominal wall and 30 min before resection of right lateral and caudate lobes.
Measurement of liver enzymes
Another set of 23 animals was prepared in the same manner as decribed above for measurement of liver enzymes. In groups 1 and 2 (10 rats for each group), 6 h after the end of ischemia, blood samples for measurement of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) were obtained via the indwelling venous line and quantified using standard clinical automated analysis. Blood sampling at identical times was performed in three sham-operated control animals.
Liver histology
For the histological study a total of 35 rats were subjected to 90 min of normothermic hepatic ischemia. Liver tissue was taken 6 h after the end of ischemia from groups 1 and 2 (10 rats in each group) and from 3 sham-operated control rats. Specimens were fixed in 10% formalin and stained with hematoxylin and eosin (4 µm sections) for light microscopic examination. The degree of damage to the liver was determined as percentage of total area that exhibited hepatocellular necrosis. To avoid potential error in statistical sampling, 15 fields were randomly selected under microscopy from each of 3 sections prepared from a liver. The area of hepatic necrosis was determined in each field and the average value calculated from 45 fields was used. Congestion, infiltration of polymorphonuclear cells (PMNs), hyperplasia of sinusoidal cells, and steatosis were evaluated according to the following criteria: no evidence (0), low intensity (1), mild intensity (2), or high intensity (3). Tissue from a further set of 12 rats (5 from group 1, 5 from group 2, and 2 from the sham-operated control group), was examined with a transmission electron microscope (TEM). At 6 h after reperfusion, rats were re-anesthetized and the liver was fixed in vivo. Briefly, after isotonic saline administration by direct puncture to the left ventricle and drainage by an incision in the right atrium, a perfusate of 2% glutaraldehyde (buffered to pH 7.4) was administered. Liver was excised immediately after cardiac arrest and cut into 1 mm3 pieces that were post-fixed by immersion in 2% glutaraldehyde for 2 h, then in 1% osmic acid for 1 h. Each sample was dehydrated in increasing alcohol concentrations and embedded in epoxy resin. Ultra-thin sections were obtained by means of an Ultratom. The samples were contrasted in 0.2% uranyl acetate and lead citrate solution and subsequently checked in a Jeol 1200 TEM.
Blood sampling for TNFα
Blood sampling was done in an additional group of 30 animals, which did not undergo the sampling for liver enzyme measurement. To study the kinetics of TNFα serum production following normothermic liver I-R, in seven rats from the control group 300 µl of blood were sampled from the indwelling suprahepatic catheter immediately after clamp removal and every 30 min up to 420 min of reperfusion. Blood sampling of 300 µl at identical times was performed in three sham-operated control animals.
To study the effects of PTX on TNFα serum production following normothermic liver I-R, a further set of 20 animals (10 rats each from groups 1 and 2) was prepared as described above for the hepatic ischemia procedure. Blood sampling (300 µl) was performed immediately after clamp removal and at 60, 270, and 360 min of reperfusion; these time points corresponded to the peaks in the kinetics of TNFα serum production in the control group.
Lost blood was replaced with 300 µl of lactated Ringer solution. Plasma was separated from the samples and stored at −20°C until assayed.
TNFα assay
Serum activity of TNFα was evaluated by measuring its cytotoxic effect on Wehi 164 (clone 13) cells (25 MDP). Fifty microliters of successive serial dilutions was distributed in 96-well microliter plates (Falcon, BD, Biosciences, Le Pont de Claix, France), then, 50 µl of cells (5–10×103 cells/well) was added. Sample treated with neutralizing anti-TNFα antibody (Genzyme, Cergy Saint Christophe, France) was used as a test control. A TNFα solution with known titer was the reference for the sample titer evaluation. After incubation for 8–10 h, the cytotoxic effect was evaluated by optic microscopy and could be followed by a spectrophotometric method using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, Saint Quentin Fallavier, France). TNFα titer corresponded to the reciprocal of the dilution factor that lysed 50% of the cells. Wehi 164 is an established cell line from murine fibrosarcoma. Clone 13 is highly TNFα sensitive. Cells were cultivated in RPMI 1640 culture medium enriched with 5–10% SVF (Flow), 0.1 mM glutamine, and 30 µg/ml gentamicin. In this assay, the lower limit of detectable TNFα was 10 pg/ml.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis for TNFα
To study the kinetics of TNFα at the transcription level, liver tissue was taken from 23 rats as follows: 0 (immediately after clamp removal), 60, 120, and 210 minutes after reperfusion. Total RNAs were isolated from samples of frozen liver (1 g) using a guanidine isothiocyanate-cesium chloride gradient procedure 18. Total RNAs (5 µg) were first reverse-transcribed into cDNA using oligo (dT) 12–18 as primer and AMV reverse transcriptase (Boehringer, Mannheim, Germany). Reverse transcripts (equivalent to 125 ng of total RNA) were used directly for each amplification reaction. Conditions for PCR were as follows. In a 50 µl reaction, 25 nmol of each primer, 125 µM each of dGTP, dATP, dCTP, and dTTP, 50 mM KCl, 10 mM Tris-HCl, pH 8.3, 1.5 mM MgCl2, 1 mg/ml gelatin, 100 µg/ml of nonacetylated BSA, and 1 unit of TAQ DNA polymerase (Boehringer). Primer sequences were as follows. TNFα-sense: 5′-CAG GGC AAT GAT CCC AAA GTA-3′ and TNFα-anti-sense 5′-GCA GTC AGA TCA TCT TCT CGA-3′. Reactions were incubated in a DNA thermal cycler for 35 cycles (denaturation 1 min at 94°C, annealing 90 s at 56°C, and extension 2 min at 72°C). PCR amplification of a constitutively expressed control mRNA encoding actin (β actin-sense: 5'-GTG GGG CGC CCC AGG CAC CA-3′, β actin-anti-sense: 5′-GTC CTT AAT GTC ACG CAC GAT TTC-3′) was used as a measure of the amount of input RNA. The reaction mixture was extracted with CHCl3, separated in a 1% agarose gel, and Southern blot analysis was performed with a 1.13 kb HindIII-EcoRI fragment from pTNFαAM5 plasmid as probe 19. Probe was labelled with [a 32P]dCTP (Amersham, Little Chalfont, UK) to high specific activity (>108 cpm/µg) by the nonamer random primed labeling technique (Megaprime kit, Amersham, Little Chalfont, UK). Filter was washed under stringent conditions (65°C, in 0.1×SSC/0.1% SDS) and exposed to Hyperfilm MP (Amersham).
A 1.13 kb HindIII-EcoRI fragment from pTNFαAM5 plasmid was used for the identification of TNFα in the rat liver. TNFαAM5 plasmid, which contains the cDNA of the murine TNFα 20, was generously provided by Dr J. Doly (Villejuif, France).
Statistical analysis
Results were expressed as mean±SEM. The proportions of surviving rats in the groups were compared using Fisher's exact test. The comparison for statistical significance was performed according to the Student-Newmann-Keuls test for serum activities of aminotransferases, LDH, TNFα, and histological parameters. Statistical significance was set at p<0.05. Error bars in figures represent standard errors.
Results
Survival rate
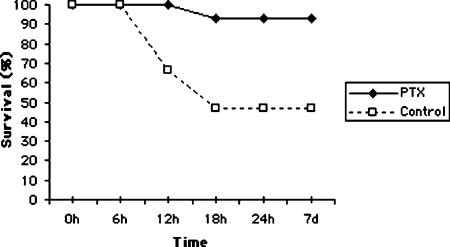
The 7 day survival of animals is illustrated in Figure 1. Treatment with PTX significantly improved the survival rate following 90 min normothermic liver ischemia (93.3%), compared with the control group (46.6%, p<0.007). In the control group seven rats died within 18 h of surgery, whereas one rat died in the PTX-treated group. Autopsy of the rats showed severe necrosis of the liver in all cases. In sham-operated control rats the 7 day survival was 100%.
Figure 1. .
Effect of pentoxifylline (PTX) on the 7 day survival following normothermic liver I-R. Control group, n=15; PTX group, n=15; h, hours; d, days.
AST, ALT, and LDH serum levels
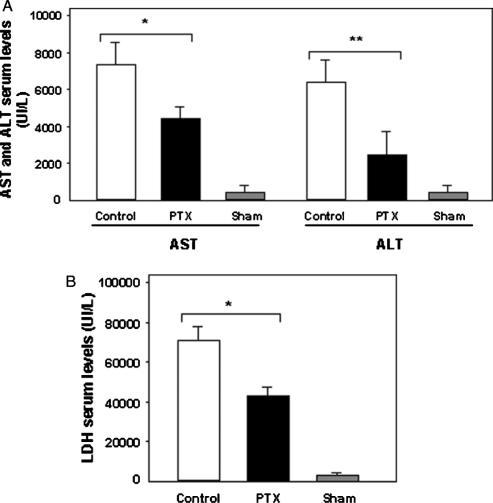
In groups 1 and 2, serum AST, ALT, and LDH levels were increased 6 h after the end of ischemia (Figure 2A and B). However, the release of liver enzymes was significantly lower in animals treated with PTX (AST, 4405±2519 UI/L; ALT, 3418±2874 UI/L; LDH, 32802±27768 UI/L) compared with those of the control group (AST, 7476±213 UI/L; ALT, 6501±304 UI/L; LDH, 74173±169 UI/L) (AST, p<0.008; ALT, p<0.03; LDH, p<0.001). In sham-operated control animals, serum AST, ALT, and LDH were 250±24 UI/L, 295±79 UI/L, and 590±55 UI/L, respectively.
Figure 2. .
(A) Effect of pentoxifylline (PTX) on AST and ALT serum levels 6 h after normothermic liver I-R. Group control (n=10), group PTX (n=10), and group sham-operated rats (n=3). Values are mean±SEM. Error bars represent standard errors. *p<0.008, **p<0.03. (B) Effect of PTX on LDH serum levels 6 h after normothermic liver I-R. Control group (n=10), PTX group (n=10), and sham-operated group (n=3). Values are mean±SEM. Error bars represent standard errors. *p<0.001.
Macroscopic appearance and histology
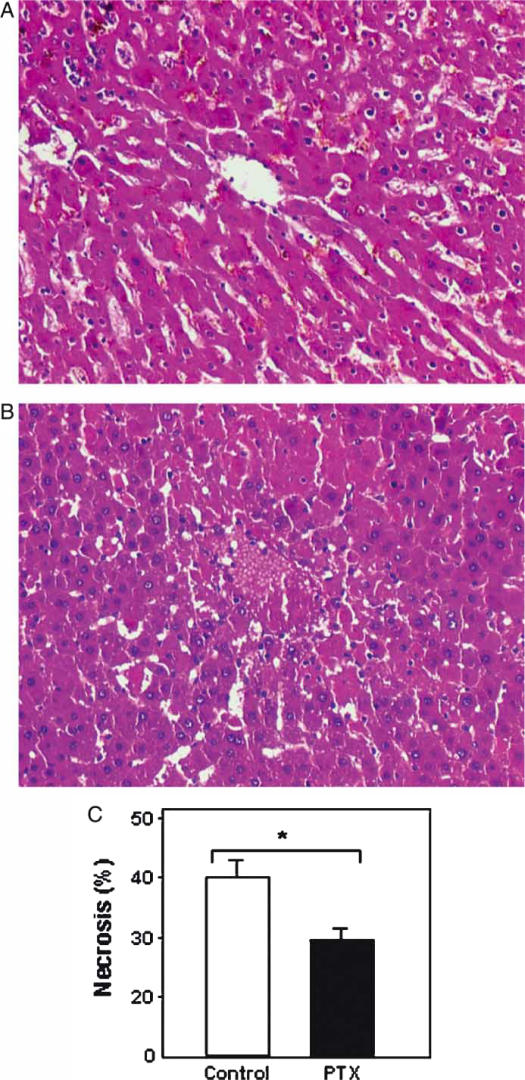
Immediately after occlusion of the hepatic vessels, the anterior lobes became pale. After releasing the clamp, the liver turned dark and rapidly regained its normal color. At 6 h after reperfusion the severity of liver necrosis was more evident in the control group (Figure 3A) than in the PTX-treated group (Figure 3B). As shown in Figure 3C, the percentage of necrotic area 6 h after reperfusion was significantly lower in the PTX-treated group than in the control group (31.1±11 vs 41.0±6.4, p<0.02). The localization of necrosis was heterogeneous, predominating in subcapsular and mediolobular areas and centered around the central vein. No significant difference was observed between groups 1 and 2 with respect to congestion (2.9±0.3 vs 2.8±0.6, p>0.05), infiltration of PMNs (1.6±0.9 vs 1.1±0.8, p>0.05), hyperplasia of sinusoidal cells (1.4±1.0 vs 1.2±0.9, p>0.05), and steatosis (0.6±0.6 vs 0.5±0.7, p>0.05). In the sham-operated control animals, necrotic area was <5%.
Figure 3. .
Effect of pentoxifylline (PTX) on liver necrosis following normothermic liver I-R. Representative hematoxylin and eosin images of liver sections 6 h after reperfusion (original magnification×100) from control group (A) and PTX-treated group (B). Quantitative analysis of liver necrosis (C). Ten animals per group were studied at each time interval. Values are mean±SEM. Error bars represent standard errors. *p<0.02.
At 6 h after reperfusion, TEM showed no significant difference between groups 1 and 2. Lesions were observed particularly near to the necrotic regions (results not shown). These lesions involved the hepatocytes (mitochondria and endoplasmic reticulum dilation, destruction of microvilli, vacuolization), the Disse space (destruction of basal membrane and accumulation of heterogeneous microfibrillar materials), and endothelial cells (accumulation of intracytoplasmic vacuoles and rupture of basal membrane). Kupffer cells showed various degrees of activation. No ultrastructural lesions were observed in sham-operated control animals.
TNFα serum levels following liver I-R
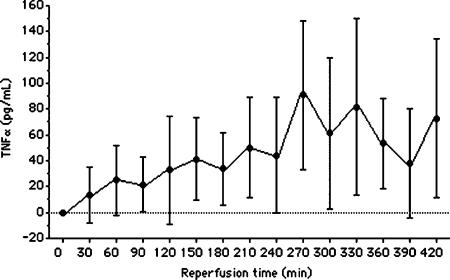
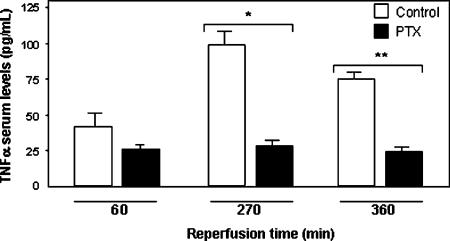
After 90 min normothermic liver I-R, TNFα was detectable in the blood of animals of the control group between 30 and 420 min of reperfusion (Figure 4), whereas it remained undetectable at any time point in sham-operated control animals (results not shown). The time point at which the TNFα serum level peaked was variable, occurring between 270 and 420 min after reperfusion. The mean peak of TNFα level was 90.7±57.4 pg/ml with a range of 5–160 pg/ml. As shown in Figure 5, treatment with PTX significantly decreased the TNFα serum levels 270 min (31.2±5.6 pg/ml in group 2 vs 103.5±7.3 pg/ml in group 1, p<0.001) and 360 min (19.5±3.2 in group 2 vs 66.5±4.8 pg/ml in group 1, p<0.02) after reperfusion. There was no significant difference between the groups 60 min after reperfusion (29.0±4.7 pg/ml in group 2 vs 38.7±2.9 pg/ml in group 1, p>0.59). In both groups, TNFα was undetectable in samples obtained immediately after clamp removal (0 min after reperfusion).
Figure 4. .
Kinetic of TNFα serum production following normothermic liver I-R. Seven animals were studied at each time interval. Values are mean±SEM. Error bars represent standard errors.
Figure 5. .
Effect of pentoxifylline (PTX) on TNFα serum levels during normothermic liver I-R. Ten animals per group were studied at each time interval. Values are mean±SEM. Error bars represent standard errors. *p<0.001, **p<0.02.
Detection of liver TNFα mRNA by PCR amplification
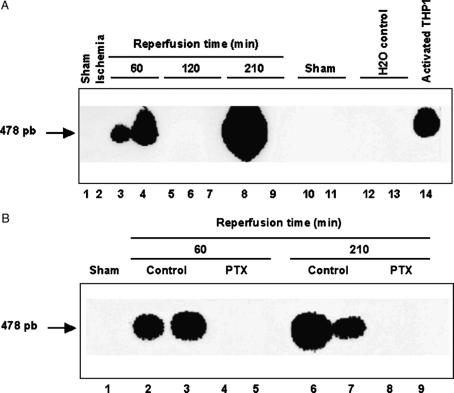
TNFα mRNA was not detectable by Northern blot analysis. Therefore, TNFα mRNA was estimated by PCR amplification from liver tissue harvested at different times after reperfusion. In the control group, TNFα transcript was detected at 60 and 210 min after reperfusion, but was not detectable at the intermediate time of 120 min after reperfusion (Figure 6A). In contrast, at each time interval studied, TNFα mRNA was not detected in the liver of PTX-treated animals (Figure 6B).
Figure 6. .
(A) Liver expression of TNFα mRNA following normothermic liver I-R. A total of 14 rats were studied, each number below the figure corresponds to one rat. Data are expressed as means±SEM of a representative experiment out of three comparable ones, each performed in triplicate. (B) Effects of pentoxifylline (PTX) on liver expression of TNFα mRNA following normothermic liver I-R. A total of nine rats were studied, each number below the figure corresponds to one rat. Data are expressed as means±SEM of a representative experiment out of three comparable ones, each performed in triplicate.
Discussion
This study showed that PTX treatment improves survival after normothermic liver I-R, and leads to a reduction of liver injury, a decrease in aminotransferases, LDH, and TNFα serum levels, and an inhibition of the TNFα mRNA liver expression.
There are two distinct phases of liver reperfusion injury, the early phase (up to 2 h post reperfusion) and the late phase (4–24 h) 2. The early phase is characterized mainly by activation of complement and KCs and the production of free radicals. Activation of KCs during reperfusion results in a massive release of cytokines such as TNFα 2. The main event during the late phase is the accumulation of activated neutrophils and the production of free radicals and proteases 2.
TNFα is responsible for lung and hepatic injury and leukocyte infiltration after hepatic I-R in rats 4. Treatment with anti-TNFα antibodies has reduced pulmonary and hepatic injury after normothermic and cold hepatic ischemia 3,4. It has been reported that TNFα stimulates neutrophil adhesion to hepatic sinusoidal endothelial cells in rats 21 and may activate endothelial cells to initiate coagulation 22.
In this study, in the control group, 90 min lobar normothermic liver ischemia followed by reperfusion and 30% hepatectomy (involving the nonischemic lobes) resulted in post-hepatic (suprahepatic vena cava) blood release of TNFα. This effect is in line with a previous study 13. PTX treatment decreased TNFα serum levels at each time interval studied. These findings are consistent with reports from other investigators 6,7.
Previous studies have reported that PTX improves the portal circulation and reduces the intrahepatic vascular resistance 23, reducing leukocyte–endothelial adherence 24,25. PTX has an inhibitory effect on the production of cell mediators like superoxide anions 26, IL-2 25, and TNFα [26].
In this study, in the control group, PCR amplification assay showed TNFα mRNA liver expression at 60 and 210 min after I-R. This biphasic kinetic corresponded to the time necessary for protein translation. TNFα mRNA liver expression at 60 and 210 min after I-R was inhibited by PTX treatment. This inhibitory effect in vivo of PTX on TNFα mRNA liver expression after normothermic liver I-R confirms the results of a previous study in vitro11.
Because of its inhibitory effect on TNFα mRNA liver expression at 60 and 210 min after I-R, it can also be assumed that PTX treatment was responsible for the decrease of TNFα serum levels at 270 and 360 min after I-R. Nevertheless, the inhibition of TNFα mRNA liver expression by PTX as the protective mechanism of liver I-R injury remains only associative in the present study.
In conclusion, our study showed that PTX treatment improves survival, reduces liver injury, and decreases TNFα serum levels with inhibition of TNFα mRNA liver expression in the liver after normothermic I-R.
Acknowledgements
We thank Dr J. Doly (Institut de Recherches Scientifiques sur le Cancer, UPR-37 CNRS, Villejuif, France) for the generous gift of TNFαAM5 plasmid, which contains the cDNA of the murine TNFα, and Mr Ch. Petit for excellent technical assistance.
References
- 1.Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–36. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 2.Jaeshke H, Farhood A. Neutrophils and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–62. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 3.Goto M, Takei Y, Kawano S, Tsuji S, Fukui H, Fushimi H, et al. Tumor necrosis factor and endotoxin in the pathogenesis of liver and pulmonary injuries after orthotopic liver transplantation in the rat. Hepatology. 1992;16:487–93. doi: 10.1002/hep.1840160230. [DOI] [PubMed] [Google Scholar]
- 4.Colletti LM, Remick DG, Burtch GD, Kunkel L, Strieter RM, Campbell DA. Role of tumor necrosis factor-alpha in the pathophysiologic alteration after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85:1936–43. doi: 10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward A, Clissold SP. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs. 1987;34:50–97. doi: 10.2165/00003495-198734010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Peng X-X, Currin RT, Thurman RG, Lemasters JJ. Protection by pentoxifylline against normothermic liver ischemia/reperfusion in rats. Transplantation. 1995;59:1537–41. [PubMed] [Google Scholar]
- 7.Fabia R, Travis DL, Levy MF, Husberg BS, Goldstein RM, Klintmalm GB. Effect of pentoxifylline on hepatic ischemia and reperfusion injury. Surgery. 1997;121:520–5. doi: 10.1016/s0039-6060(97)90106-9. [DOI] [PubMed] [Google Scholar]
- 8.Iwamoto H, Kozaki N, Nakamura K, Hama K, Narumi N, Matsuno N, et al. Beneficial effects of pentoxifylline and propentoxifylline on the warm ischemic injury of rat livers. Transplant Proc. 2002;34:2677–8. doi: 10.1016/s0041-1345(02)03372-9. [DOI] [PubMed] [Google Scholar]
- 9.Wang P, Zheng FB, Morrison MH, Ayala A, Chaudry IH. Mechanism of the beneficial effects of pentoxifylline on hepatocellular function after trauma hemorrhage and resuscitation. Surgery. 1992;112:451–8. [PubMed] [Google Scholar]
- 10.Rudiger HA, Clavien PA. Tumor necrosis factor-alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology. 2002;122:202–10. doi: 10.1053/gast.2002.30304. [DOI] [PubMed] [Google Scholar]
- 11.Doherty GM, Jensen JC, Alexander HR, Buresh CM, Norton JA. Pentoxifylline suppression of tumor necrosis factor gene transcription. Surgery. 1991;110:192–8. [PubMed] [Google Scholar]
- 12.Strieter RM, Remick DG, Ward PA, Spengler RN, Lynch JP 3rd, Larrick J, et al. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun. 1988;155:1230–6. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- 13.Cursio R, Gugenheim J, Panata-Ferrari P, Lasfar A, Tovey M, Chastanet S, et al. Improvement of normothermic rat liver ischemia/reperfusion by muramyl dipeptide. J Surg Res. 1998;80:339–44. doi: 10.1006/jsre.1998.5445. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura T, Yoshida Y, Watanabe F, Koseki M, Nishida T, Tagawa K. Blood level of mitochondrial aspartate aminotransferases as an indicator of the extent of ischemic necrosis of the rat liver. Hepatology. 1986;6:701–7. doi: 10.1002/hep.1840060427. [DOI] [PubMed] [Google Scholar]
- 15.Asakawa H, Jeppsson B, Mack P, Hultberg B, Hägerstrand I, Bengmark S. Acute ischemic liver failure in the rat: a reproducible model not requiring portal decompression. Eur Surg Res. 1989;21:42–8. doi: 10.1159/000129002. [DOI] [PubMed] [Google Scholar]
- 16.Nishizawa H, Egawa H, Inomata Y, Uemoto S, Asonuma K, Kiuchi T, et al. Efficiency of pentoxifylline in donor pretreatment in rat liver transplantation. J Surg Res. 1997;72:170–6. doi: 10.1006/jsre.1997.5169. [DOI] [PubMed] [Google Scholar]
- 17.Aslan A, Karaguzel G, Celik M, Uysal N, Yucel G, Melikoglu M. Pentoxifylline contributes to the hepatic cytoprotective process in rats undergoing hepatic ischemia and reperfusion injury. Eur Surg Res. 2001;33:285–90. doi: 10.1159/000049719. [DOI] [PubMed] [Google Scholar]
- 18.Chirgwin JM, Przybyla AE, Macdonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–9. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch EF, Maniatis T. Cold Spring Harbor Laboratory; Cold Spring Harbor: 1989. Molecular cloning: a laboratory manual2nd edn. [Google Scholar]
- 20.Caput D, Beutler B, Thayer R, Brown-Shimer S, Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci USA. 1986;83:1670–4. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlayer HJ, Laaff H, Peters T, Woort-Menker M, Estler HC, Karck U, et al. Involvement of tumor necrosis factor in endotoxin-triggered neutrophil adherence to sinusoidal cells of mouse liver and its modulation in acute phase. J Hepatol. 1988;7:239–49. doi: 10.1016/s0168-8278(88)80488-4. [DOI] [PubMed] [Google Scholar]
- 22.Bevilacqua MP, Pober JS, Majeau GR, Fiers W, Cotran RS, Gimbrone MA. Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium. Proc Natl Acad Sci USA. 1988;83:4533–7. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chazouilleres O, Ballet F, Chretien Y, Marteau P, Rey C, Maillard D, et al. Protective effect of vasodilators on liver function after long hypothermic preservation: a study in the isolated perfused rat liver. Hepatology. 1989;9:824–9. doi: 10.1002/hep.1840090606. [DOI] [PubMed] [Google Scholar]
- 24.Takei Y, Marzi I, Gao W, Gores GJ, Lemasters JJ, Thurman RG. Leukocyte adhesion and cell death following orthotopic liver transplantation in the rat. Transplantation. 1991;51:959–65. doi: 10.1097/00007890-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Edwards MJ, Abney DL, Miller FN. Pentoxifylline inhibits interleukin-2-induced leukocyte-endothelial adherence and reduces systemic toxicity. Surgery. 1991;10:199–204. [PubMed] [Google Scholar]
- 26.Kozaki K, Egawa H, Bermudes H, Feduska NJ, Esquivel CO. Pentoxifylline inhibits production of superoxide anion and tumor necrosis factor by Kupffer cells in rat liver preservation. Transplant Proc. 1993;25:3025–6. [PubMed] [Google Scholar]