Abstract
Background. Currently, there is no consensus regarding the pancreaticoduodenectomy (PD) margins examined intraoperatively or the technical protocol for frozen section examination. The aim of this work was to summarize our experience regarding the intraoperative examination of the uncinate margin and to compare it with the published literature. Materials and methods. Our local protocol for the intraoperative assessment of the uncinate margin of the PD specimen is described in this article. A PubMed® search limited to English language publications using terms along the theme of pancreaticoduodenectomy and margin was performed. Retrieved articles were categorized according to whether they discussed frozen section margin examination. Results. Ten articles published between 1981 and 2005 were retrieved which discussed the intraoperative examination of PD specimens. Of the 10 articles, 5 discussed the intraoperative consultation for diagnostic purposes only, 2 discussed the consultation for both diagnostic purposes and assessment of margins, and 3 discussed intraoperative assessment of margins only. Of the total of five articles that discussed the intraoperative assessment of margins, none detailed the technical protocol for examining the uncinate margin. Discussion. Our proposed protocol for the intraoperative assessment of the uncinate margin of PD specimens allows for its accurate evaluation and has not been described previously in the English literature.
Keywords: uncinate margin, pancreaticoduodenectomy, Whipple, frozen section
Introduction
There is increasing evidence to emphasize the importance of the proper handling of the pancreaticoduodenectomy (PD) specimen, also known as Whipple specimen, to improve patient survival and prediction of prognosis 1,2,3,4. This is true both intraoperatively, as the surgeon tries to make critical technical decisions, and postoperatively as the oncologists optimize their categorization of patients for adjuvant therapy and follow-up. Intraoperatively, surgeons may request a pathology consultation for a variety of reasons. In this context, the intraoperative consultation (IOC) represents the first encounter of the pathologist with the resected specimen, which is also the optimal opportunity to start its proper handling. Assessment of the surgical margins is one of the most common indications for IOC during the PD procedure. As critical as this step is, there is no consensus regarding the margins examined intraoperatively or a standard technical protocol for frozen section examination of the selected margins.
We find that the intraoperative assessment of the uncinate margin is extremely important in cases of adenocarcinoma involving the head or the uncinate process of the pancreas. Achieving a negative resection margin in the uncinate process is complicated by its proximity to the superior mesenteric artery (SMA) and the need to preserve it as well as the nerve plexus around it. This margin is technically difficult to assess by frozen section, since in the resected specimen the uncinate process is covered by a rim of adipose tissue that is a few millimeters thick. Preparing an adequate frozen section from adipose tissue is technically challenging. As a result, over the past year we have changed our local protocol for the intraoperative processing of the uncinate margin. The aim of this work was to summarize our experience in this area and to compare it with the published literature. We also hope that through discussing our protocol we can raise awareness of the issues surrounding assessment of the uncinate margin.
Materials and methods
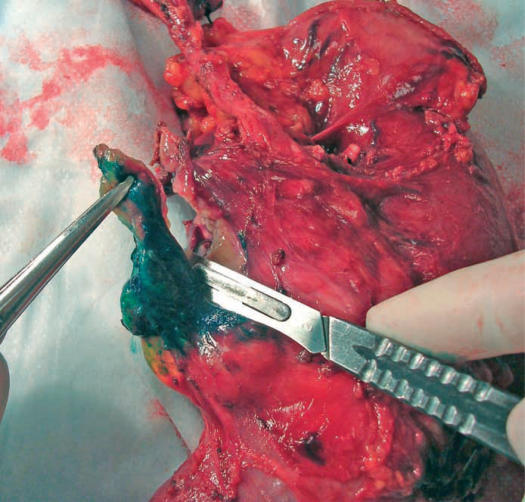
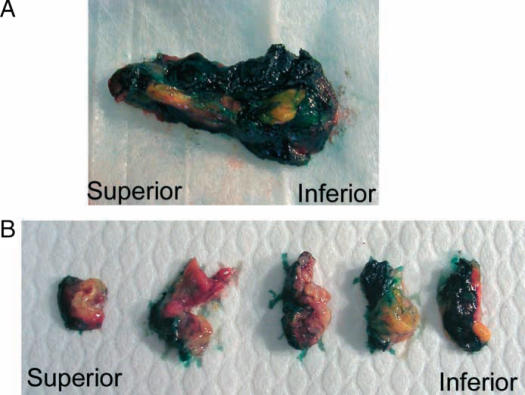
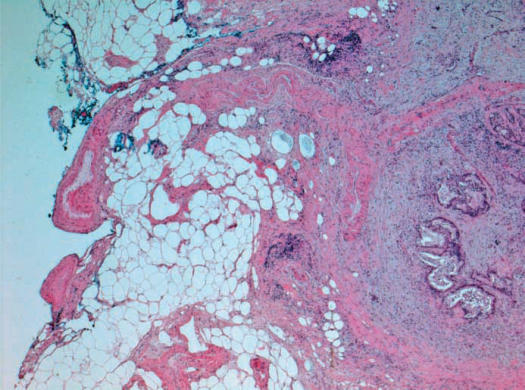
In 2005, our local surgical pathology protocol for intraoperative assessment of the PD specimen with carcinomas in the head or the uncinate process of the pancreas was modified to require inking of the posterior surface of the pancreas including the uncinate process. The tip of the uncinate process, including its distal 1 cm, is then cut off the rest of the specimen (Figure 1) and is serially sectioned at 3 mm intervals perpendicular to its longitudinal axis. This produces five to eight pieces of tissue that are submitted for frozen section examination (Figure 2). All pieces are submitted for frozen section examination, ensuring that the entire length of tissue where the uncinate process comes in proximity with the SMA is examined. The produced frozen section will show the inked margin with an underlying zone of adipose tissue overlying the native pancreatic tissue of the uncinate process. Figure 3 depicts an example of a case with a negative uncinate margin where malignant glands involved the uncinate process. One can see how if this margin was shaved off, submitted en face and sections trimmed of fat the produced frozen section would have probably been assessed as ‘positive’. This new protocol was also followed for assessment of the uncinate margin on permanent sections in cases where no IOC was requested. The institutional Research Ethics Board approved our request to carry out a retrospectively study, audit, and publish our IOC experience for the PD procedure.
Figure 1. .
The tip of the uncinate process, including its distal 1 cm is being cut off the rest of the specimen after it has been inked.
Figure 2. .
Further processing of the uncinate margin. (A) The tip of the uncinate process including its margin is laid down, inked, and well oriented. (B) It is then serially sectioned at 3 mm intervals perpendicular to its longitudinal axis producing 6–10 pieces of tissue that are submitted for frozen section examination.
Figure 3. .
A frozen section of the uncinate margin showing the inked true margin, an underlying rim of adipose tissue, and a portion of native pancreatic parenchyma invaded by adenocarcinoma. Note that the solid tissue on the right side of the section provided support and helped prevent curling of the adipose tissue on the left side.
The National Library of Medicine and the National Institutes of Health databases were searched for related English language publications. This was conducted through the PubMed® search engine using a combination of two groups of terms. The first group included the terms pancreas, pancreaticoduodenectomy, pancreatoduodenectomy and Whipple. The second group included the terms margin, frozen section and uncinate. All possible combinations including one term selected from each group were used. Retrieved articles were categorized according to whether they discussed the IOC of pancreatic margins. Unrelated articles, which appeared in the search but did not address the topic of IOC, were excluded.
Results
Since the implementation of our new protocol, the uncinate margin has been assessed 23 times, providing us with the opportunity to master the technique. In 11 cases, the uncinate process was involved by adenocarcinoma but the new protocol allowed us to provide an accurate and confident IOC of the uncinate margin. Figure 3 depicts one of these cases, illustrating how it probably could have been over-called as ‘positive’, had it been submitted by the traditional en face method. To date, the uncinate margin has confidently been deemed positive in three cases.
Ten articles published between 1981 and 2005 were retrieved which discussed the intraoperative examination of PD specimens 5,6,7,8,9,10,11,12,13,14. Of the 10 articles, 5 discussed the IOC for diagnostic purposes only 5,6,7,8,12, 2 discussed the consultation for both diagnostic purposes and assessment of margins 11,14, and 3 discussed intraoperative assessment of margins only 9,10,13. Of the total of five articles that discussed the intraoperative assessment of margins, none went into detail to describe the technique or procedure for submitting and examining the uncinate margin.
Discussion
The standard Whipple procedure involves resecting the antrum of the stomach, the entire four parts of the duodenum, and a small segment of proximal jejunum, as well as the distal common bile duct and head of the pancreas. The neck of the pancreas is transected, thus exposing the portal vein (PV). The final step in removing the Whipple specimen from the patient is transecting the uncinate process. The uncinate process of the head of the pancreas extends posterior to the PV and has a tongue-like projection that touches the right lateral aspect of the SMA. In order to deliver the specimen, the uncinate process needs to be divided as close to the SMA as possible, yet without damaging the SMA or the autonomic nerve plexus surrounding it; since in our experience denuding this peri-arterial nerve plexus often results in a prolonged postoperative gastric ileus. Technically, this is a very challenging step in the Whipple procedure since the uncinate process is very well vascularized and tends to bleed. Also, the SMA is very closely adherent to the most distal aspect of the uncinate process. In view of this, there is a natural tendency on the part of the surgeon to leave behind, in the patient, the bit of uncinate tissue closest to the SMA. This can sometimes lead to a positive uncinate margin of resection.
We define the uncinate margin as the cut surface produced by the surgeon's knife while dissecting the uncinate process from the SMA, which tends to be granular and irregular. This is different from the smooth and regular retroperitoneal surface to the right of the proximal 3–4 cm of the SMA, which some surgeons may refer to as the ‘uncinate margin’ 15. In cases of carcinoma involving the head or the uncinate process of the pancreas, dissecting away from the SMA and closer to the pancreatic tissue may run the risk of producing a positive margin, leaving behind extra-pancreatic malignant cells in the adipose tissue around the SMA or even intra-pancreatic malignancy if a portion of the uncinate process was left adherent to the SMA. Therefore, it is important to enable the pathologist to provide an accurate IOC on the uncinate margin. In cases where excision of additional tissue from the immediate vicinity of the SMA is still technically feasible, the intraoperative reporting of a positive uncinate margin may signal its revision to ensure complete excision of all cancerous tissue by completely stripping the SMA off any adjacent adipose tissue.
A microscopically negative (R0) uncinate margin is not only an important determinant of a good prognosis but is also a prerequisite for enrollment in many, if not all, adjuvant postoperative clinical trials of chemotherapy or radiation. On the other hand, a microscopically positive (R1) resection denotes a poorer prognosis and unfortunately condemns the patient to almost certain local recurrence. A macroscopically positive (R2) resection is an even worse prognostic marker.
Traditionally, examining the uncinate margin in our institute was done by shaving a 3–5 mm thick rim of tissue from the uncinate margin and submitting it en face for frozen sectioning. The true margin with the tissue that was closest to the SMA will be facing the knife to be cut first. Although still followed in many other medical centers, this procedure suffers several technical problems. According to this procedure, the first layer of tissue to be cut is almost entirely lipomatous, which is very difficult to cut. This tissue tends to curl, fold, and fragment, leading the operator to continue trimming. As trimming of tissue continues, the true margin gets lost, an inherent problem with any frozen section where the submitted tissue is embedded en face. By the time the operator is able to get an intact section adequate for staining and histologic examination, the cryostat blade will most likely be cutting through native pancreatic tissue. The pathologist examining the produced section may run the risk of over-calling the margin as ‘positive’, since they will typically be looking at deeper pancreatic tissue rather than the true margin. Our proposed protocol technically allows for the production of better sections, as the solid pancreatic tissue on one side of the frozen section acts as a scaffold supporting the softer adipose tissue and decreasing its folding (Figure 3). When malignant cells are present on the section, this protocol enables the pathologist to measure the distance between the deepest point of invasion and the inked margin (Figure 3). These advantages are lost when the uncinate margin is shaved and submitted en face.
To overcome the difficulties with the en face submission, some pathologists would rather cut a portion of the uncinate portion and then serially section the produced tissue. Although somewhat similar to our procedure, this latter protocol does not call for assessment of the entire length of the uncinate margin since only a portion of it is examined. In addition, our proposed protocol allows the operator to keep track of whether produced sections were obtained from the superior, middle, or inferior portions of the uncinate margin, which can provide the surgeon with a more precise localization of a positive margin. We have also noticed in most cases that submitting the entire length of the uncinate margin captures the additional superior mesenteric lymph nodes, boosting the number of lymph nodes examined in the specimen.
Our literature search shows that the significance of an accurate intraoperative assessment of the uncinate margin and a detailed description of the technique needed to provide this assessment were not documented in the English literature. One particular article provided a thorough description of the IOC for pancreatic surgery, without detailing the technical procedure 14.
Our proposed protocol for the intraoperative assessment of the uncinate margin of PD specimens allows for its accurate evaluation and has not been previously described in the English literature.
Footnotes
This work was presented in part at the 7th World Congress of the International Hepato-Pancreato-Biliary Association, Edinburgh, UK, September 3–7, 2006.
References
- 1.Luttges J, Zamboni G, Kloppel G. Recommendation for the examination of pancreaticoduodenectomy specimens removed from patients with carcinoma of the exocrine pancreas. A proposal for a standardized pathological staging of pancreaticoduodenectomy specimens including a checklist. Dig Surg. 1999;16:291–6. doi: 10.1159/000018738. [DOI] [PubMed] [Google Scholar]
- 2.Verbeke CS, Menon KV, Guthrie A, Toogood G, McMahon M, Leitch D, et al. Handling of pancreaticoduodenectomy specimens influences reporting of prognostically relevant findings. HPB. 2005;7(Supp 1):87. [Google Scholar]
- 3.Noto M, Miwa K, Kitagawa H, Kayahara M, Takamura H, Shimizu K, et al. Pancreas head carcinoma: frequency of invasion to soft tissue adherent to the superior mesenteric artery. Am J Surg Pathol. 2005;29:1056–61. [PubMed] [Google Scholar]
- 4.Hong S-M, Cho H, Lee O-J, Rao J. The number of metastatic lymph nodes in extrahepatic bile duct carcinoma as a prognostic factor. Am J Surg Pathol. 2005;29:1177–83. doi: 10.1097/01.pas.0000160978.77833.d7. [DOI] [PubMed] [Google Scholar]
- 5.Hyland C, Kheir SM, Kashlan MB. Frozen section diagnosis of pancreatic carcinoma: a prospective study of 64 biopsies. Am J Surg Pathol. 1981;5:179–91. doi: 10.1097/00000478-198103000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Weiland LH. Frozen section diagnosis in tumors of the pancreas. Semin Diagn Pathol. 1984;1:54–8. [PubMed] [Google Scholar]
- 7.O'Brien PH, Mincey KH. Analysis of pancreatoduodenectomy. J Surg Oncol. 1985;28:50–8. doi: 10.1002/jso.2930280113. [DOI] [PubMed] [Google Scholar]
- 8.Witz M, Shkolnik Z, Dinbar A. Intraoperative pancreatic biopsy: a diagnostic dilemma. J Surg Oncol. 1989;42:117–19. doi: 10.1002/jso.2930420210. [DOI] [PubMed] [Google Scholar]
- 9.Staley CA, Cleary KR, Abbruzzese JL, Lee JE, Ames FC, Fenoglio CJ, et al. The need for standardized pathologic staging of pancreaticoduodenectomy specimens. Pancreas. 1996;12:373–80. doi: 10.1097/00006676-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Pingpank JF, Hoffman JP, Ross EA, Cooper HS, Meropol NJ, Freedman G, et al. Effect of preoperative chemotherapy on surgical margin status of resected adenocarcinoma of the head of the pancreas. J Gastrointest Surg. 2001;5:121–30. doi: 10.1016/s1091-255x(01)80023-8. [DOI] [PubMed] [Google Scholar]
- 11.Cioc AM, Ellison EC, Proca DM, Lucas JG, Frankel WL. Frozen section diagnosis of pancreatic lesions. Arch Pathol Lab Med. 2002;126:1169–73. doi: 10.5858/2002-126-1169-FSDOPL. [DOI] [PubMed] [Google Scholar]
- 12.Shyr YM, Su CH, Wu CW, Lui WY. Is pancreaticoduodenectomy justified for chronic pancreatitis masquerading as periampullary tumor? Hepatogastroenterology. 2003;50:1163–6. [PubMed] [Google Scholar]
- 13.Sakamoto Y, Kosuge T, Shimada K, Sano T, Ojima H, Yamamoto J, et al. Prognostic factors of surgical resection in middle and distal bile duct cancer: an analysis of 55 patients concerning the significance of ductal and radial margins. Surgery. 2005;137:396–402. doi: 10.1016/j.surg.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Lechago J. Frozen section examination of liver, gallbladder, and pancreas. Arch Pathol Lab Med. 2005;129:1610–18. doi: 10.5858/2005-129-1610-FSEOLG. [DOI] [PubMed] [Google Scholar]
- 15.Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–46. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]