Abstract
Surgical intervention in patients with infected necrotizing pancreatitis generally consists of laparotomy and necrosectomy. This is an invasive procedure that is associated with high morbidity and mortality rates. In this report, we present an alternative minimally invasive technique: videoscopic assisted retroperitoneal debridement (VARD). This technique can be considered a hybrid between endoscopic and open retroperitoneal necrosectomy. A detailed technical description is provided and the advantages over various other minimally invasive retroperitoneal techniques are discussed.
Keywords: acute pancreatitis, minimal invasive surgery, necrotizing pancreatitis, necrosectomy, surgery
Introduction
In 2000 Carter et al. reported on minimally invasive retroperitoneal necrosectomy in the treatment of infected necrotizing pancreatitis (INP) 1. This technique, consisting of endoscopic necrosectomy over a dilated percutaneous drain tract, was later also described by Connor et al. 2. The first results were exciting but the authors stated that the technique might also be associated with drawbacks 1,2. The pure endoscopic character of the technique makes it a time-consuming effort that requires multiple repeated procedures to remove sufficient necrotic material.
In recent years, our groups have adopted videoscopic assisted retroperitoneal debridement (VARD) 3. This technique can be considered a hybrid between pure endoscopic retroperitoneal necrosectomy and the open (20 cm incision) translumbar approach, described by Fagniez et al. in 1989 4.
In this article, we describe the technical aspects of VARD because we feel that this minimally invasive technique carries advantages over other surgical strategies in INP and is not yet known to a wide audience.
Surgical technique
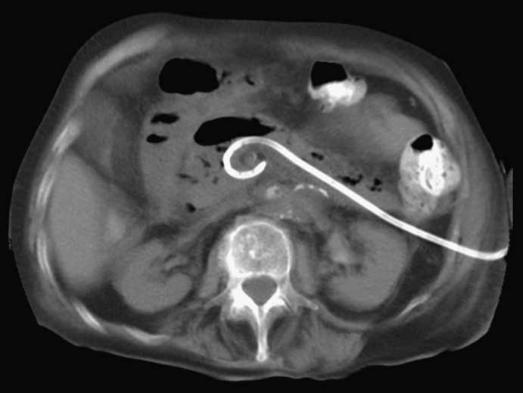
Once infection of (peri-)pancreatic necrosis is either suspected based on contrast-enhanced CT scan and clinical status or even confirmed by fine needle aspiration, a 12–14 French percutaneous drain is placed in the (peri-)pancreatic collection through the left retroperitoneum (Figure 1). If drainage does not lead to clinical improvement (subsidence of organ failure, reduction of temperature, white blood cell count and C-reactive protein), surgical intervention is deemed necessary and the patient is operated upon. Surgery is preferably postponed until after 4 weeks from the onset of the disease. This is considered essential as it allows for (peri-)pancreatic collections to sufficiently demarcate and the wall to mature, thus optimizing conditions for debridement.
Figure 1. .
A percutaneous catheter drain is positioned in the collection through a left retroperitoneal approach.
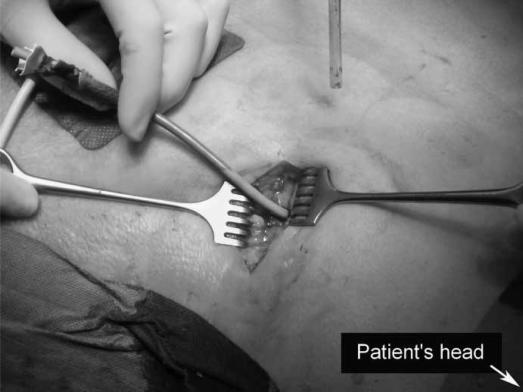
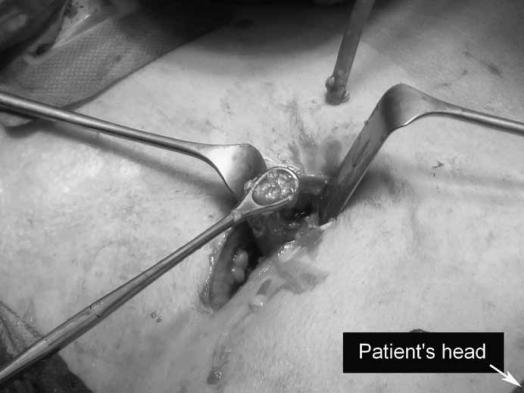
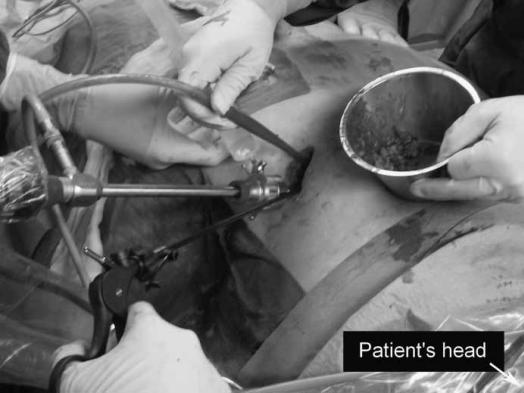
The patient is placed in supine position with the left side 30–40° elevated. A subcostal incision of 5 cm is placed in the left flank at the mid-axillary line, close to the exit point of the percutaneous drain (Figure 2). With the help of CT images and by using the in situ percutaneous drain as a guide into the (peri-)pancreatic collection, the fascia is dissected and the retroperitoneum is entered. The cavity is cleared of purulent material using a standard suction device. The first necrosis encountered is carefully removed with the use of long grasping forceps (Figure 3). Following the percutaneous drain deeper into the cavity, loose necrotic material is removed while periodic irrigation and consequent suction are performed to enhance vision. When debridement can no longer be performed under direct vision, a single extra-long laparoscopic port is placed into the incision and a 0° videoscope is introduced. At this stage CO2 gas (10 l/min) can be infused through the percutaneous drain, still in position, to inflate the cavity, thereby facilitating inspection. Under videoscopic assistance further debridement of retained necrotic tissue is performed with laparoscopic forceps (Figure 4).
Figure 2. .
A 5 cm subcostal incision is placed in the patient's left flank.
Figure 3. .
The first necrosis is removed with a grasping forceps.
Figure 4. .
A videoscope is inserted and residual necrosis is removed with a laparoscopic grasping forceps. A single trocar is used.
Complete necrosectomy is not the ultimate aim of this procedure. Only loosely adherent pieces of necrosis are removed, thereby keeping the risk of tearing underlying blood vessels to a minimum. In the rare case of extensive bleeding, packing of the retroperitoneal cavity should be performed, either as definite treatment or as a bridge to laparotomy or angiographic coiling in the situation of persistent haemorrhage.
When the bulk of necrosis is removed, the cavity is irrigated with saline until the fluid becomes clear. The percutaneous drain is removed and two large-bore single-lumen drains are positioned in the cavity extending through the edges of the incision. The first drain is placed at the deepest point of the cavity and is positioned more shallow. The fascia and skin are closed and the drains are sutured to the skin. Continuous postoperative lavage is performed with 10 litres of normal saline or dialysis fluid per 24 h until the effluent is clear. One week after the procedure repeat CT is performed to evaluate resolution of the collection and to assess whether necrosis is still present.
Discussion
A recent systematic review showed that mortality after necrosectomy by laparotomy for INP is 15–27% 5. In several series mortality rates after the open translumbar approach were not superior to laparotomy and major morbidity such as haemorrhage and fistulae occurred in 25–68% of patients 4,6,7 This high incidence of complications is attributed to the relative blindness of this technique 8. The concept of necrosectomy under direct vision by video-endoscopy might offer a partial solution to this problem.
Patients with INP are often severely ill and mortality is mainly due to septic multiple organ failure. Necrosectomy by minimally invasive techniques by inducing less preoperative and postoperative physiological stress as compared with laparotomy might be beneficial in these patients 1.
In recent years several relatively small series (range 6–46 patients) on necrosectomy by minimally invasive retroperitoneal approach have been reported 1,2,3,9,10,11,12. However, the described techniques show some variation and different nomenclature is used. We find this to be quite confusing. In 1998 Gambiez et al. described the results of the first patients in which they performed necrosectomy through a small (6 cm) left flank incision under visualization with a mediastinoscope 11. Castellanos et al. published a prospective series of 11 consecutive patients treated with a technique that involves a 15 cm translumbar incision which they call ‘retroperitoneal endoscopy’ 12. Although comparable to VARD, it is questionable whether the 15 cm incision should be considered ‘minimally invasive’.
The alternative method originally reported by Carter et al., which obviated the need for an incision, is known as ‘sinus tract endoscopy’ (STE) 1. In this technique a percutaneous catheter drain tract is serially dilated to a 30 French tract using fluoroscopic guidance in the operating room and necrosectomy is performed under continuous irrigation using a nephroscope and a long grasping forceps. Connor et al. applied the same technique as STE but use a different term: ‘minimally invasive retroperitoneal pancreatic necrosectomy’ (MIRPN) 2.
In 2001, the results of the first six patients who underwent VARD in one of our institutions were published 3. At that time the technique was still called ‘laparoscopic assisted percutaneous drainage’. Two minor complications occurred and all patients survived. Recently, an abstract was published on a second series of 13 VARD patients. Complications occurred in 54% of patients and 1 patient died (8%) 13. The various reports on different minimally invasive techniques by other authors show a mean morbidity of 44% (range 0–93%) and mortality of 23% (range 10–27%) 1,2,9,10,11,12. However, the type of complications and classification of severity of disease vary greatly, which makes comparison of these retrospective studies difficult.
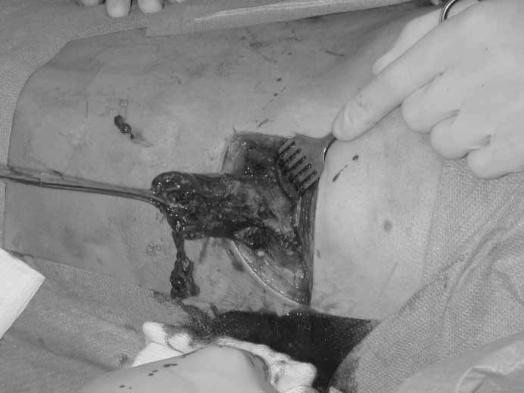
VARD is essentially a combination of the open translumbar approach and STE and we feel it contains ‘the best of both worlds’. Theoretically, it has the advantages of both an open approach and an endoscopic technique without many of the disadvantages. In the series of Connor et al. a median of 3–4 procedures was necessary to remove all infected material 2,10, which was reflected by a 2 weeks longer postoperative hospital stay 10. In VARD, the small incision enables the surgeon to remove larger pieces of necrosis (Figure 5), with a shorter operating time and less need for repetitive procedures. In our experience, the VARD technique is very simple and cost-effective. STE has the additional disadvantage of requiring a C-arm fluoroscopy in the operating room, which has the additional risks of radiation exposure to both the patient and the operating team, as well as possible increased costs. Finally, as opposed to the 15 cm incision for the translumbar approach 12, the 5 cm incision in VARD can still be considered minimally invasive. The use of a videoscope may reduce the risk of complications reported with the open translumbar approach in the past.
Figure 5. .
VARD allows for large pieces of necrosis to be removed.
In our experience, VARD is a relatively easy technique that is applicable in the majority of patients with INP 14 and provides an excellent alternative to necrosectomy by laparotomy. However, life-threatening complications are still possible, necessitating 24 h availability of experienced gastrointestinal surgeons, endoscopists and radiologists. In the absence of large prospective (randomized) studies, the true value of VARD in the treatment of INP obviously remains unclear. For this reason two multicentre studies have been initiated (one single arm 15 and one randomized 16).
References
- 1.Carter CR, McKay CJ, Imrie CW. Percutaneous necrosectomy and sinus tract endoscopy in the management of infected pancreatic necrosis: an initial experience. Ann Surg. 2000;232:175–80. doi: 10.1097/00000658-200008000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connor S, Ghaneh P, Raraty M, Sutton R, Rosso E, Garvey CJ, et al. Minimally invasive retroperitoneal pancreatic necrosectomy. Dig Surg. 2003;20:270–7. doi: 10.1159/000071184. [DOI] [PubMed] [Google Scholar]
- 3.Horvath KD, Kao LS, Ali A, Wherry KL, Pellegrini CA, Sinanan MN. Laparoscopic assisted percutaneous drainage of infected pancreatic necrosis. Surg Endosc. 2001;15:677–82. doi: 10.1007/s004640080010. [DOI] [PubMed] [Google Scholar]
- 4.Fagniez PL, Rotman N, Kracht M. Direct retroperitoneal approach to necrosis in severe acute pancreatitis. Br J Surg. 1989;76:264–7. doi: 10.1002/bjs.1800760316. [DOI] [PubMed] [Google Scholar]
- 5.Nieuwenhuijs VB, Besselink MG, van Minnen LP, Gooszen HG. Surgical management of acute necrotizing pancreatitis: a 13-year experience and a systematic review. Scand J Gastroenterol Suppl 2003;111–16. [DOI] [PubMed] [Google Scholar]
- 6.Nakasaki H, Tajima T, Fujii K, Makuuchi H. A surgical treatment of infected pancreatic necrosis: retroperitoneal laparotomy. Dig Surg. 1999;16:506–11. doi: 10.1159/000018777. [DOI] [PubMed] [Google Scholar]
- 7.Villazon A. Retroperitoneal drainage in the management of the septic phase of severe acute pancreatitis. World J Surg. 1991;15:408–9. doi: 10.1007/BF01658742. [DOI] [PubMed] [Google Scholar]
- 8.Werner J, Feuerbach S, Uhl W, Buchler MW. Management of acute pancreatitis: from surgery to interventional intensive care. Gut. 2005;54:426–36. doi: 10.1136/gut.2003.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castellanos G, Pinero A, Serrano A, Parrilla P. Infected pancreatic necrosis: translumbar approach and management with retroperitoneoscopy. Arch Surg. 2002;137:1060–3. doi: 10.1001/archsurg.137.9.1060. [DOI] [PubMed] [Google Scholar]
- 10.Connor S, Alexakis N, Raraty MG, Ghaneh P, Evans J, Hughes M, et al. Early and late complications after pancreatic necrosectomy. Surgery. 2005;137:499–505. doi: 10.1016/j.surg.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Gambiez LP, Denimal FA, Porte HL, Saudemont A, Chambon J-PM, Quandalle PA. Retroperitoneal approach and endoscopic management of peripancreatic necrosis collections. Arch Surg. 1998;133:66–72. doi: 10.1001/archsurg.133.1.66. [DOI] [PubMed] [Google Scholar]
- 12.Castellanos G, Pinero A, Serrano A, Llamas C, Fuster M, Fernandez JA, et al. Translumbar retroperitoneal endoscopy: an alternative in the follow-up and management of drained infected pancreatic necrosis. Arch Surg. 2005;140:952–5. doi: 10.1001/archsurg.140.10.952. [DOI] [PubMed] [Google Scholar]
- 13.Van Santvoort HC, Besselink MG, Bollen TL, van Leeuwen MS, van Ramshorst B, Gooszen HG. Videoscopic assisted retroperitoneal debridement in infected necrotising pancreatitis as a pilot study to introduce a randomised controlled trial. HPB. 2006;8(Suppl 2):39. doi: 10.1080/13651820701225688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Besselink MG, Van Santvoort HC, Bollen TL, Van Leeuwen MS, Hofker S, Boermeester MA, et al. Minimally invasive approach in acute necrotising pancreatitis: a strategy for a selected subgroup or a potential benefit for all? Dutch Acute Pancreatitis Study Group. Gastroenterology. 2005;128(Suppl 2):A171–2. [Google Scholar]
- 15.VARD trial, 2005. http://clinicaltrials.gov/ct/gui/show/NCT00061269?order=5 [Google Scholar]
- 16.Besselink MG, Van Santvoort HC, Nieuwenhuijs VB, Boermeester MA, Bollen TL, Buskens E, et al., Dutch Acute Pancreatitis Study Group. Minimally invasive ‘step-up approach’ versus maximal necrosectomy in patients with acute necrotising pancreatitis (PANTER trial): design and rationale of a randomised controlled multicenter trial [ISRCTN38327949]. BMC Surg 2006;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]