Abstract
Two natural variants of the lantibiotic nisin that are produced by Lactococcus lactis are known. They have a similar structure but differ in a single amino acid residue at position 27; histidine in nisin A and asparagine in nisin Z (J.W.M. Mulders, I.J. Boerrigter, H.S. Rollema, R.J. Siezen, and W.M. de Vos, Eur. J. Biochem, 201:581-584, 1991). The nisin variants were purified to apparent homogeneity, and their biological activities were compared. Identical MICs of nisin A and nisin Z were found with all tested indicator strains of six different species of gram-positive bacteria. However, at concentrations above the MICs, with nisin Z the inhibition zones obtained in agar diffusion assays were invariably larger than those obtained with nisin A. This was observed with all tested indicator strains. These results suggest that nisin Z has better diffusion properties than nisin A in agar. The distribution of the nisin variants in various lactococcal strains was determined by amplification of the nisin structural gene by polymerase chain reaction followed by direct sequencing of the amplification product. In this way, it was established that the nisZ gene for nisin Z production is widely distributed, having been found in 14 of the 26 L. lactis strains analyzed.
Full text
PDF
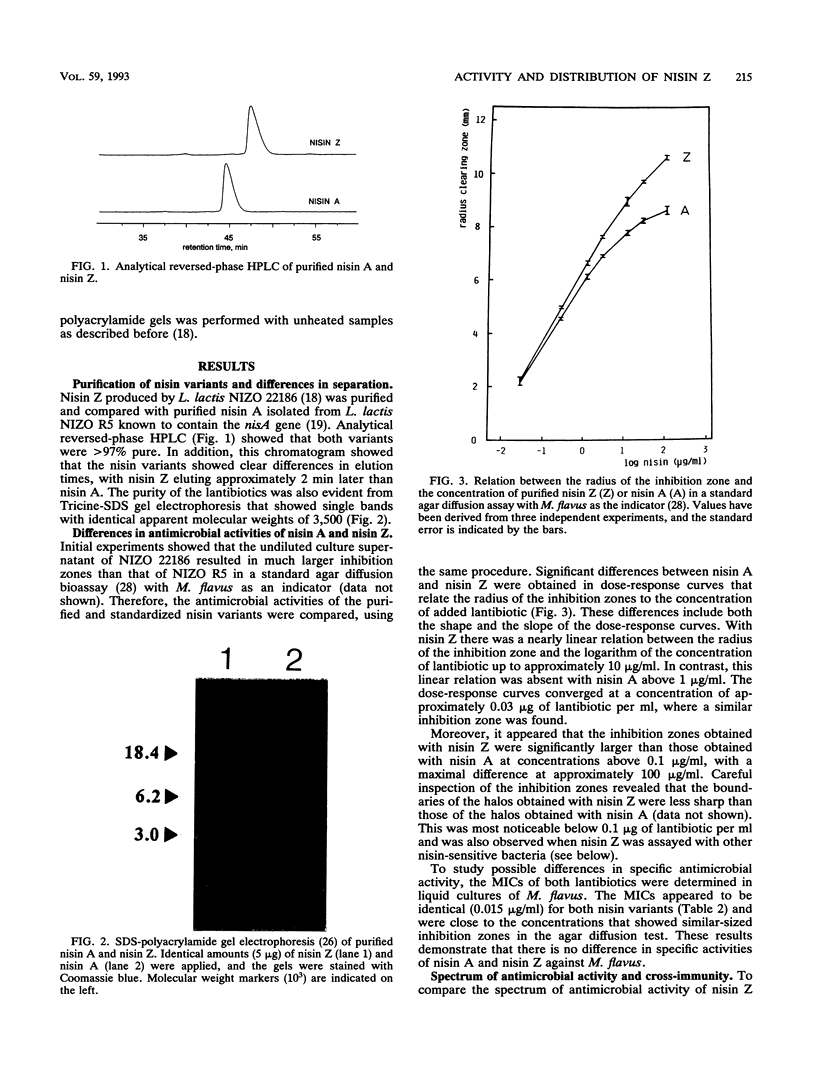



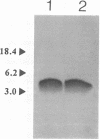
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERRIDGE N. J., NEWTON G. G. F., ABRAHAM E. P. Purification and nature of the antibiotic nisin. Biochem J. 1952 Dec;52(4):529–535. doi: 10.1042/bj0520529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S., Hansen J. N. Structure and expression of a gene encoding the precursor of subtilin, a small protein antibiotic. J Biol Chem. 1988 Jul 5;263(19):9508–9514. [PubMed] [Google Scholar]
- Buchman G. W., Banerjee S., Hansen J. N. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J Biol Chem. 1988 Nov 5;263(31):16260–16266. [PubMed] [Google Scholar]
- Casanova J. L., Pannetier C., Jaulin C., Kourilsky P. Optimal conditions for directly sequencing double-stranded PCR products with sequenase. Nucleic Acids Res. 1990 Jul 11;18(13):4028–4028. doi: 10.1093/nar/18.13.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd H. M., Horn N., Gasson M. J. Analysis of the genetic determinant for production of the peptide antibiotic nisin. J Gen Microbiol. 1990 Mar;136(3):555–566. doi: 10.1099/00221287-136-3-555. [DOI] [PubMed] [Google Scholar]
- Gao F. H., Abee T., Konings W. N. Mechanism of action of the peptide antibiotic nisin in liposomes and cytochrome c oxidase-containing proteoliposomes. Appl Environ Microbiol. 1991 Aug;57(8):2164–2170. doi: 10.1128/aem.57.8.2164-2170.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E., Morell J. L. The structure of nisin. J Am Chem Soc. 1971 Sep 8;93(18):4634–4635. doi: 10.1021/ja00747a073. [DOI] [PubMed] [Google Scholar]
- Harris L. J., Fleming H. P., Klaenhammer T. R. Characterization of two nisin-producing Lactococcus lactis subsp. lactis strains isolated from a commercial sauerkraut fermentation. Appl Environ Microbiol. 1992 May;58(5):1477–1483. doi: 10.1128/aem.58.5.1477-1483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn N., Swindell S., Dodd H., Gasson M. Nisin biosynthesis genes are encoded by a novel conjugative transposon. Mol Gen Genet. 1991 Aug;228(1-2):129–135. doi: 10.1007/BF00282457. [DOI] [PubMed] [Google Scholar]
- Kaletta C., Entian K. D., Kellner R., Jung G., Reis M., Sahl H. G. Pep5, a new lantibiotic: structural gene isolation and prepeptide sequence. Arch Microbiol. 1989;152(1):16–19. doi: 10.1007/BF00447005. [DOI] [PubMed] [Google Scholar]
- Kaletta C., Entian K. D. Nisin, a peptide antibiotic: cloning and sequencing of the nisA gene and posttranslational processing of its peptide product. J Bacteriol. 1989 Mar;171(3):1597–1601. doi: 10.1128/jb.171.3.1597-1601.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Hansen J. N. Some chemical and physical properties of nisin, a small-protein antibiotic produced by Lactococcus lactis. Appl Environ Microbiol. 1990 Aug;56(8):2551–2558. doi: 10.1128/aem.56.8.2551-2558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders J. W., Boerrigter I. J., Rollema H. S., Siezen R. J., de Vos W. M. Identification and characterization of the lantibiotic nisin Z, a natural nisin variant. Eur J Biochem. 1991 Nov 1;201(3):581–584. doi: 10.1111/j.1432-1033.1991.tb16317.x. [DOI] [PubMed] [Google Scholar]
- Rauch P. J., De Vos W. M. Characterization of the novel nisin-sucrose conjugative transposon Tn5276 and its insertion in Lactococcus lactis. J Bacteriol. 1992 Feb;174(4):1280–1287. doi: 10.1128/jb.174.4.1280-1287.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell N., Entian K. D., Schneider U., Götz F., Zähner H., Kellner R., Jung G. Prepeptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulphide-rings. Nature. 1988 May 19;333(6170):276–278. doi: 10.1038/333276a0. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Vos P., van Asseldonk M., van Jeveren F., Siezen R., Simons G., de Vos W. M. A maturation protein is essential for production of active forms of Lactococcus lactis SK11 serine proteinase located in or secreted from the cell envelope. J Bacteriol. 1989 May;171(5):2795–2802. doi: 10.1128/jb.171.5.2795-2802.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil H. P., Beck-Sickinger A. G., Metzger J., Stevanovic S., Jung G., Josten M., Sahl H. G. Biosynthesis of the lantibiotic Pep5. Isolation and characterization of a prepeptide containing dehydroamino acids. Eur J Biochem. 1990 Nov 26;194(1):217–223. doi: 10.1111/j.1432-1033.1990.tb19446.x. [DOI] [PubMed] [Google Scholar]