Abstract
Association between the nervous and immune system is well documented. Immune cells originate within the bone marrow that is innervated. Thermal injury induces adrenergic stimulation, augments monocytopoiesis and alters the β-adrenergic receptor (AR) profile of bone marrow monocyte committed progenitors. This provides an impetus to study AR expression in hematopoietic progenitors along myeloid lineage. Using FACS analysis and confocal microscopy, we report the expression of α1-, α2- and β2- AR in enriched populations of ER-MP20+ and ER-MP12+ myeloid progenitors, CD117+ and CD34+ multi-potential progenitors and more importantly pluripotent stem cells suggesting a plausible role for catecholamine in hematopoietic development.
Keywords: Alpha Adrenergic receptors, Beta Adrenergic receptors, Bone Marrow, Hematopoietic Stem cells, Myelopoiesis, Progenitors, Macrophage, TNF production
1. Introduction
Epinephrine from the adrenal medulla and norepinephrine from the peripheral sympathetic nerves mediate a cascade of cellular responses by binding to adrenergic receptors (ARs). Adrenergic receptors can be broadly classified into two distinct types namely α- and β-expressed in different tissues with differences in their affinity and response to various agonists and antagonists. α-ARs are further classified into α1 and α2 subtypes and β-ARs are sub typed as β1, β2 and β3 based on the structure and effectors (Bylund et al., 1994). Although AR regulation of heart and smooth muscle have been the most extensively studied tissues, AR expression and coupling mechanisms involving immune cells have been brought to light by accumulating evidence over the past two decades (Brogden et al., 2005; Eskandari and Sternberg, 2002; Kohm and Sanders, 2000; Steinman, 2004; Straub, 2004). AR mediated functions have been documented in several immune cell types including T and B-lymphocytes as well as antigen presenting cells (Kavelaars, 2002; Kohm and Sanders, 2001; Maestroni and Conti, 1994; Maestroni and Mazzola, 2003; Xu, 2001).
Bone marrow, with defined sympathetic innervations is the site of hematopoiesis and is a primary source of circulating and tissue leukocytes (Bruno et al., 2001; Kennedy and Abkowitz, 1998; Lawson et al., 1992; Merad et al., 2002; Takahashi et al., 1996). Our previous work has demonstrated the association of critical trauma with sympathetic activation leading to the dynamic release of norepinephrine within the bone marrow (Jones et al., 1986; Tang et al., 2001; Tang et al., 1999). Furthermore, we have previously shown that thermal injury and sepsis altered the β-AR expression and function on late monocyte committed progenitors (Muthu et al., 2005b). Moreover, sympathetic ablation of nerve terminals with 6-hydroxy dopamine was shown to reverse thermal injury and sepsis induced monocytopoiesis (Cohen et al., 2004; Tang et al., 2001). Such observations point to the importance of defining where ARs may appear during myeloid lineage development.
Bone marrow hematopoietic progenitor cells can be identified at different levels of commitment starting from monocyte committed progenitors and moving up through different levels of commitment until pluripotency through cell surface expression of specific cluster differentiation antigens (Bunting et al., 2004; Leenen et al., 1994; Leenen et al., 1990). Bone marrow cells with cell surface expression of ER-MP20+ are monocyte-committed cells whereas cells expressing ER-MP12+ are committed toward myeloid lineage with bi-potential capacity to form either a granulocyte or a monocyte (de Bruijn et al., 1994). Early progenitors with cell surface markers CD117+ and CD34+ are common lymphoid, myeloid or megakaryocytic progenitors with no self-renewing capacity. Uncommitted, lineage negative murine bone marrow cells that are Sca-1+ with high cell surface expression of CD117++ fall into the category of hematopoietic stem cells (HSCs). True pluripotent HSCs (Lin−, CD117high, Sca-1+) have the capacity to self-renew and are capable of providing multi-lineage progeny (Shizuru et al., 2005).
Here we have merged techniques for isolating cells at different stages of development with evidence for the expression of ARs. Our documentation that α1-, α2- and β2-ARs are expressed on enriched populations of ER-MP20+ and ER-MP12+ myeloid progenitors as well as CD117+ and CD34+ multi-potential progenitors and more importantly stem cells (HSCs) provides a platform for understanding mechanisms underlying the adrenergic regulation of hematopoiesis. To our knowledge this is the first report demonstrating adrenergic receptor expression on HSCs, multi-potential, bi-potential and monocyte committed bone marrow progenitor cells along the myeloid lineage.
2. Materials and Methods
2.1. Animals
Adult male B6D2F1 mice weighing 25 to 30 grams were purchased from Jackson Laboratories (Barr Harbor, ME). Prior to the start of the experiments, mice were allowed to acclimatize for seven days following arrival at our Comparative Medicine Facility under a controlled temperature (20–22 °C) and humidity (20–40%) environment with a 12hour light – dark cycle. All experimental protocols were approved by the IACUC (Institutional Animal Care and Use Committee) of Loyola University Medical Center.
2.2. Total bone marrow cells
Total bone marrow from each femur pair was eluted aseptically with 1-ml syringe and 25-gauge needle into 5 ml of McCoy’s medium supplemented with 10% fetal bovine serum (FBS), Penicillin (100U/ml) and Streptomycin (10 μg/ml). An aliquot of the cell suspension was diluted in 3% acetic acid to lyse the red blood cells. The total nucleated cells were quantified using Neubaur hemocytometer.
2.3. Isolation of hematopoietic progenitor cells
Total nucleated bone marrow cells were re-suspended in 100 μl PBS containing 2mM EDTA and 0.5% BSA. The cells were labeled by incubating with monoclonal antibody ER-MP20 or ER-MP12 (Accurate Scientific, Westbury, NY.), rat anti-mouse CD34 or CD117 antibody (BD Pharmingen, San Diego, CA) at 1:40 vol/vol for 10 min at 4° C. The cells were then washed and incubated in PBS (80 μl/107 cells) containing magnetic micro-bead conjugated goat anti-rat IgG (20 μl/107 cells) at 4° C for 15 min. The unlabeled cells were eluted through mini MACS separation columns (Miltenyi Biotech, Auburn, CA) while in a magnetic field. Positively labeled cells were collected in a tube by pushing 1 ml of PBS through the column removed from the magnetic field. The enriched populations of very early hematopoietic progenitors (CD117+ and CD34+), early (ER-MP12+), or late (ER-MP20+) monocyte progenitor cell fraction were diluted and counted in Neubaur hemocytometer. Purity of the separations was verified by flow cytometry and each of the progenitor isolation was found to be 95–98% pure (Data not shown).
2.4. Isolation of hematopoietic stem cells
Murine hematopoietic stem cells (HSC) have been shown to reside in the Sca1+/CD117High populations and these cells also have the capacity for self-renewal (Orlic et al., 1993; Uchida and Weissman, 1992). HSC were isolated employing a combination of magnetic separations followed by FACS. Total BM cells were depleted of lineage-committed cells by negative selection. Briefly total bone marrow cells pooled from 4 mice were incubated with a cocktail of lineage markers (biotinylated antibodies against CD11c, CD11b, B220, Gr1, Ter1, CD3e, CD19, CD86, CD8a, and Thy1.1) at 4° C for 10 min (BD Pharmingen, San Diego, CA). The cells were then washed and incubated in PBS (80 μl/107 cells) containing magnetic anti biotin micro-beads (20 μl/107 cells) at 4° C for 15 min. The unlabeled cells were collected through mini MACS separation columns (Miltenyi Biotech, Auburn, CA) while in a magnetic field. This lineage negative bone marrow fraction were dual stained with phycoerythrin conjugated anti Sca-1 and allopycocyanin conjugated anti CD117 antibodies. Subsequently, CD117high, Sca-1+ population were sorted by flow cytometry to yield HSCs (Lin−, CD117high, Sca-1+).
2.5. Immuno-staining and Flow cytometry of adrenergic receptors
Bone marrow HSCs and progenitor cell suspensions were fixed with ice-cold methanol prior to incubation with α1- (a mix of α1A, α1B and α1D [clones: C-19, C-18 and S-12]), α2- (a mix of α2A, αB and α2C [clones: C-19, C-19 and C-20]) or β2- adrenergic receptor antibody[clone: H-20] (all polyclonal Abs against a peptide mapping at the C-terminus; Santa Cruz Biotechnology, CA) at 4° C overnight. The cells were then pelleted and washed with PBS to remove excess unbound primary antibody prior to incubation with secondary antibody linked to FITC chromophore (Molecular Probes, Eugene, OR) for 2 hours at room temperature. After extensive washing, the cell suspension was stored on ice until quantitated by FACS analysis using CELLQuest program to determine the percentage of cells expressing adrenergic receptors and the mean fluorescent intensity of the adrenergic receptors. To examine specificity for the α1-, α2- and β2- adrenergic receptor antibodies, an aliquot of the cells was incubated with the specific Abs treated with the competing blocking peptide (Santa Cruz Biotechnology, CA) followed by the fluorescently labeled secondary antibody.
2.6. Confocal microscopy
For microscopy experiments, HSCs and the hematopoietic progenitor cells were labeled with adrenergic receptors following the same protocol as for FACS. An aliquot (104 cells/200μl) of the preparation was cytospun on to glass slides and cover slipped with DAPI containing vectashield (Vector Laboratories, Inc, Burlingame, CA). The slides were stored in the dark at 4° C until ready for visualization in a Zeiss LSM 510 confocal microscope. Since DAPI stains the nucleus blue, the cells were located in the field without any difficulty.
2.7. Differentiation into MØ and TNF-αmeasurements
Bone marrow ER-MP20+, ER-MP12+, CD34+, CD117+ progenitors and HSCs (5×104 – 5×105 cells) were seeded in 12 well cell culture plates and allowed to differentiate into mature MØ in vitro with specific growth factor cocktail (50ng of M-CSF for ER-MP20+; 20ng each of IL3, FLT3, GM-CSF and M-CSF for ER-MP12+ and CD34+ cells; all of the above growth factors plus 10ng/ml stem cell factor for CD117+ and HSCs) for 7–10 days with and without isoproterenol (1μM), phenylephrine (1μM) or clonidine (1μM) on day zero. One set of cells was given vehicle treatment only. At the end of culture period the adherent MØ were washed and treated with LPS (100ng) containing fresh medium (IMDM) for 18h and the conditioned medium was collected and stored at −80° C until cytokine measurement was made by ELISA. The protein content of the adherent MØ was determined and the TNF-α levels were normalized to the protein content.
All media and reagents were filtered through a polysulphonate filter to eliminate inadvertent lipopolysaccharide (LPS) contamination of the reagents.
3. Results
3.1. Adrenergic receptor expression in late monocyte committed progenitors
Adrenergic receptor distribution in ER-MP20+ monocyte committed progenitors is shown in Figure 1. Leenan et al have previously demonstrated that murine bone marrow progenitors expressing the cell surface marker ER-MP20 are monocyte committed progenitors and can be differentiated into monocyte/macrophages under the influence of the preeminent monocytopoietic growth factor M-CSF (Leenen et al., 1994). We have previously shown by ligand binding assay that ER-MP20+ progenitors express β adrenergic receptors and through further RT-PCR studies we revealed that murine ER-MP20+ monocyte committed cells express only functional β2- adrenergic receptors but not β1- or β3- adrenergic receptor subsets (Muthu et al., 2005b). Here we confirm our earlier observation that ER-MP20+ progenitors express β2- adrenergic receptors by an alternative feasible technique requiring only a fraction of the sample size in contrast to the traditional method. Furthermore, using confocal microscopic studies and flow cytometric (FACS) measurements we demonstrate that these progenitors also express α1- and α2- adrenergic receptors. Confocal images of α1-, α2- and β2- adrenergic receptor expression as determined by cell labeling with specific primary antibody followed by FITC conjugated secondary antibody are shown in Fig 1. Our FACS analyses and confocal images showed that the use of blocking peptides specific for each adrenergic receptor subtype significantly reduced the green fluorescence of the specific anti-adrenergic receptor antibodies in ER-MP20+ cells (Fig 1) and each of the hematopoietic progenitor cell tested (Table 1).
Figure 1.
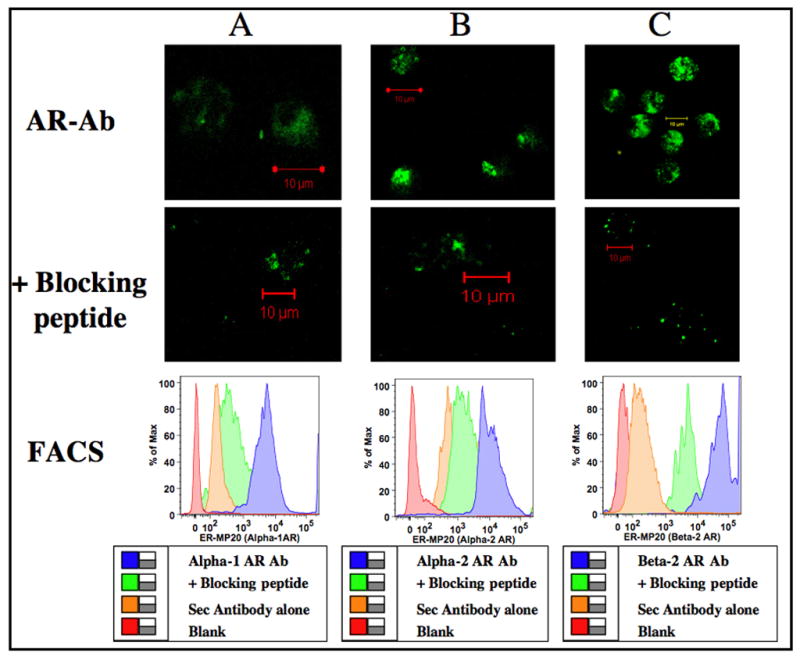
Adrenergic receptor distribution in ER-MP20+ progenitors. Murine bone marrow cells were enriched for ER-MP20+ using Ab coupled micro-bead technology and labeled with primary antibody specific for adrenergic receptor subtypes with and without specific blocking peptides followed by FITC conjugated secondary antibody. Confocal images were obtained in a Zeiss LSM 510 confocal microscope. Green color represents binding of the polyclonal Abs to the C-terminus of adrenergic receptor subtypes in ER-MP20+ cells. Image resolution=1024 × 1024; Scale bar=10μm. Histograms were generated by FACS. Logarithmic scale of mean fluorescent intensity of specific adrenergic receptor subtype is represented on the X-axis and the percentage of cells expressing α1-, α2- or β2- adrenergic receptors is represented on the Y-axis. Panels A, B and C represents α1-, α2- and β2- adrenergic receptors respectively.
Table 1.
Types of adrenergic receptors expressed by hematopoietic progenitors shown as MFI
| Hematopoietic Progenitors | α1- AR# | α1- AR+ Blocking peptide# | Mean Fold Difference | α2- AR# | α2- AR+ Blocking peptide# | Mean Fold Difference | β2- AR# | β2- AR+ Blocking peptide | Mean Fold Difference |
|---|---|---|---|---|---|---|---|---|---|
| ER-MP-20+ | 5,094±194 | 2,088±40 | 2.44 | 10,431±809 | 4,804±96 | 2.17 | 28,254±1,805 | 8,542±870 | 3.31 |
| ER-MP-12+ | 1,477±47 | 756±13 | 1.95 | 7,745±155 | 3,769±120 | 2.05 | 11,120±987 | 4,356±67 | 2.55 |
| CD34+ | 1,645±38 | 908±25 | 1.81 | 7,654±167 | 3,356±87 | 2.28 | 4,648±99 | 1,644±49 | 2.83 |
| CD117+ | 1,542±29 | 739±16 | 2.09 | 1,389±46 | 817±34 | 1.70 | 5,908±128 | 2,104±32 | 2.81 |
MFI (mean fluorescent intensity) expressed as Mean±SEM. Approximately a two-fold difference in MFI of the bound specific primary antibodies in the presence and absence of competing blocking peptides signifies the specificity of the α1-, α2- and β2- adrenergic receptor antibodies.
3.2. Early monocyte progenitor cells express adrenergic receptors
While ER-MP20 is a late monocyte marker, ER-MP12+ represents a bi-potential myeloid progenitor subset represented in the hierarchy of monocytic lineage (de Bruijn et al., 1994). Murine bone marrow cells enriched for ER-MP12+ were labeled with primary antibody specific for α1-, α2- and β2- adrenergic receptors followed by FITC conjugated secondary antibody and analyzed by confocal microscopy and FACS (Fig 2). Our results clearly show that ER-MP12+ myeloid progenitors that precede ER-MP20+ cells in monocyte differentiation sequence also express α1-, α2- and β2- adrenergic receptors.
Figure 2.
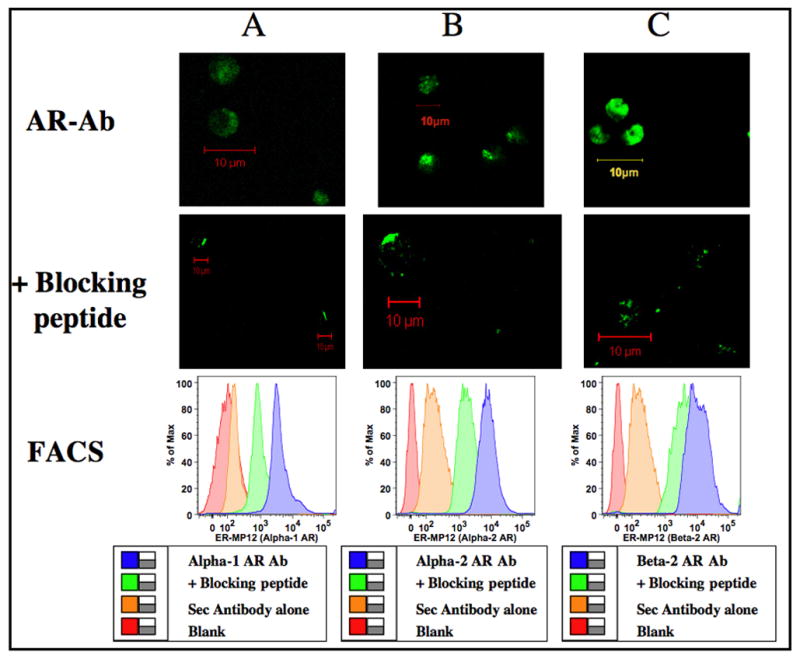
Adrenergic receptor distribution in ER-MP12+ progenitors. Murine bone marrow cells were enriched for ER-MP12+ using Ab coupled micro-bead technology and labeled with primary antibody specific for adrenergic receptor subtypes with and without specific blocking peptides followed by FITC conjugated secondary antibody. Confocal images were obtained in a Zeiss LSM 510 confocal microscope. Green color represents binding of the polyclonal Abs to the C-terminus of adrenergic receptor subtypes in ER-MP12+ cells. Image resolution=1024 × 1024; Scale bar=10μm. Histograms were generated by FACS. Logarithmic scale of mean fluorescent intensity of specific adrenergic receptor subtype is represented on the X-axis and the percentage of cells expressing α1-, α2- or β2- adrenergic receptors is represented on the Y-axis. Panels A, B and C represents α1-, α2- and β2- adrenergic receptors respectively.
3.3. Adrenergic receptors are present on very early multi-potential myeloid progenitors
Murine bone marrow cells expressing CD34+ and CD117+ are considered multi-potential hematopoietic progenitors representing lymphoid, erythroid or myeloid lineage. Our isolation technique using magnetic micro beads yield a blend of high, medium and low staining populations. Therefore, each of the CD34+ and CD117+ bone marrow isolates may represent a mix of multi-potential and lineage committed cells that lack self-renewal (Shizuru et al., 2005). Based on immuno-fluorescence images and flow cytometry, here we show that CD34+ and CD117+ progenitor subsets express adrenergic receptors. Fig 3 represents confocal images and FACS data on α1-, α2-and β2- adrenergic receptor expression by the CD34+ multi-potential progenitors. Similarly in Fig 4, Panels A, B and C represents confocal images and FACS data on CD117+ cells expressing α1-, α2-and β2- adrenergic receptors respectively.
Figure 3.
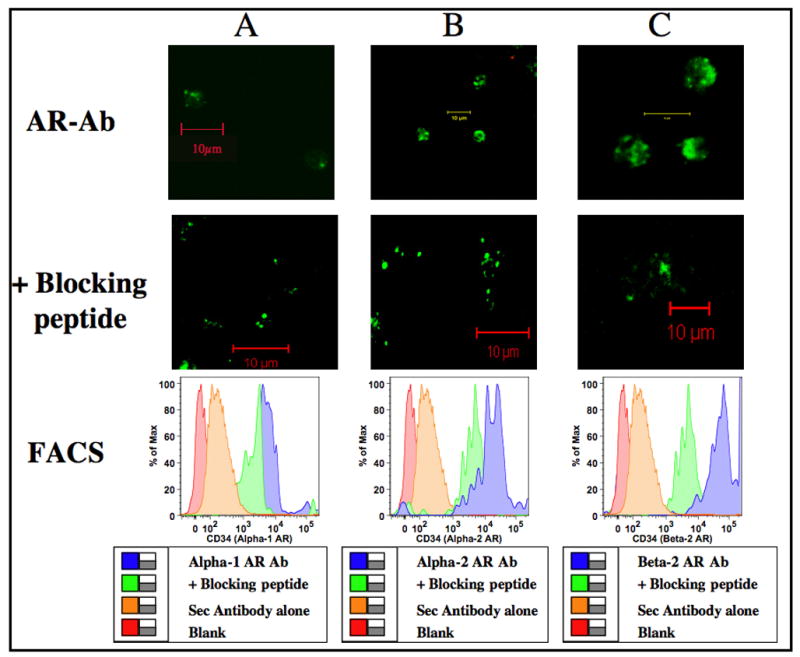
Adrenergic receptor distribution in CD34+ progenitors. Murine bone marrow cells were enriched for CD34+ using Ab coupled micro-bead technology and labeled with primary antibody specific for adrenergic receptor subtypes with and without specific blocking peptides followed by FITC conjugated secondary antibody. Confocal images were obtained in a Zeiss LSM 510 confocal microscope. Green color represents binding of the polyclonal Abs to the C-terminus of adrenergic receptor subtypes in CD34+ cells. Image resolution=1024 × 1024; Scale bar=10μm. Histograms were generated by FACS. Logarithmic scale of mean fluorescent intensity of specific adrenergic receptor subtype is represented on the X-axis and the percentage of cells expressing α1-, α2- or β2- adrenergic receptors is represented on the Y-axis. Panels A, B and C represents α1-, α2- and β2- adrenergic receptors respectively.
Figure 4.
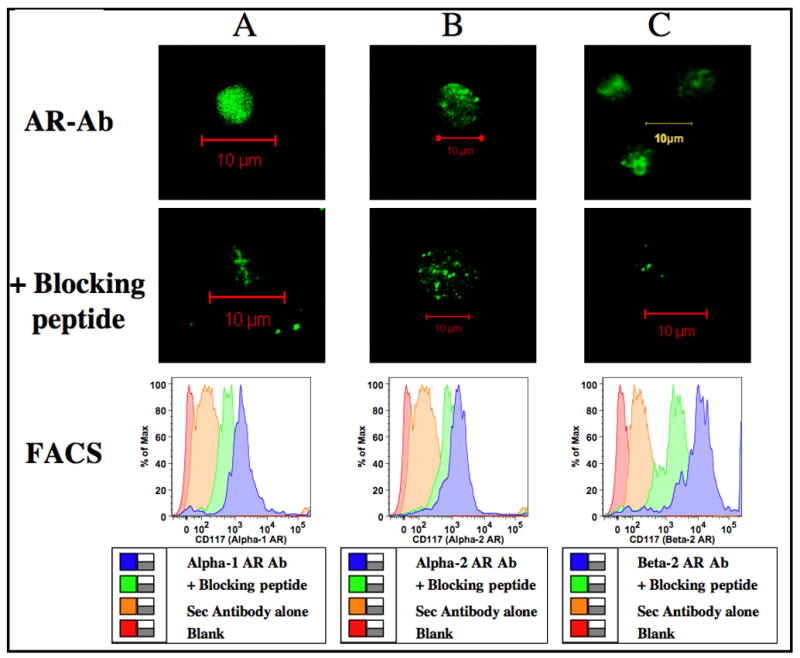
Adrenergic receptor distribution in CD117+ progenitors. Murine bone marrow cells were enriched for CD117+ using Ab coupled micro-bead technology and labeled with primary antibody specific for adrenergic receptor subtypes with and without specific blocking peptides followed by FITC conjugated secondary antibody. Confocal images were obtained in a Zeiss LSM 510 confocal microscope. Green color represents binding of the polyclonal Abs to the C-terminus of adrenergic receptor subtypes in CD117+ cells. Image resolution=1024 × 1024; Scale bar=10μm. Histograms were generated by FACS. Logarithmic scale of mean fluorescent intensity of specific adrenergic receptor subtype is represented on the X-axis and the percentage of cells expressing α1-, α2- or β2- adrenergic receptors is represented on the Y-axis. Panels A, B and C represents α1-, α2- and β2- adrenergic receptors respectively.
The results are representation of 4–6 individual samples analyzed for each progenitor subtype in the hierarchy of the hematopoietic paradigm. Since confocal images and FACS histograms are representative data, the average (n=4) of the mean fluorescent intensities (MFI) of the specific receptor antibodies with and without competing blocking peptides bound to the different hematopoietic progenitors are shown in Table 1. It is generally agreed that MFI represents the number of primary antibody molecule bound to the cell. Therefore a higher MFI is indicative of a greater number of surface receptors. Although all the hematopoietic progenitors tested expressed α1, α2 and β2 receptors, MFI data clearly indicate that the more differentiated monocyte committed ER-MP20+ progenitors express more adrenergic receptors compared to the less committed progenitors tested.
3.4. Murine Hematopoietic Stem cells (HSCs) also express adrenergic receptors
Hematopoietic stem cells (lineageneg Sca1+ CD117high) were isolated by a combination of antibody coupled magnetic micro-bead technique and positive selection by flow cytometric sorting. By eliminating lineage-committed cells and isolating Sca1+ CD117high population, we are selecting only CD117 high subset that is capable of self-renewal (Ito et al., 1996; Shizuru et al., 2005). Considering the limitation in the yield of HSCs, each of the subtypes of adrenergic receptors was analyzed by confocal imaging technique only. In Fig 5, boxed area represents the sorted Sca1+ CD117 high HSCs from the lineage neg fraction obtained by magnetic depletion. The red color in the confocal image right above the boxed area indicates HSCs (a combination of Sca1-APC and CD117 –PE) and the adrenergic receptor expressions are represented by green fluorescence (FITC). The scant green fluorescence in the presence of specific competing blocking peptides reveals the specificity of the adrenergic receptor antibodies used in these experiments.
Figure 5.
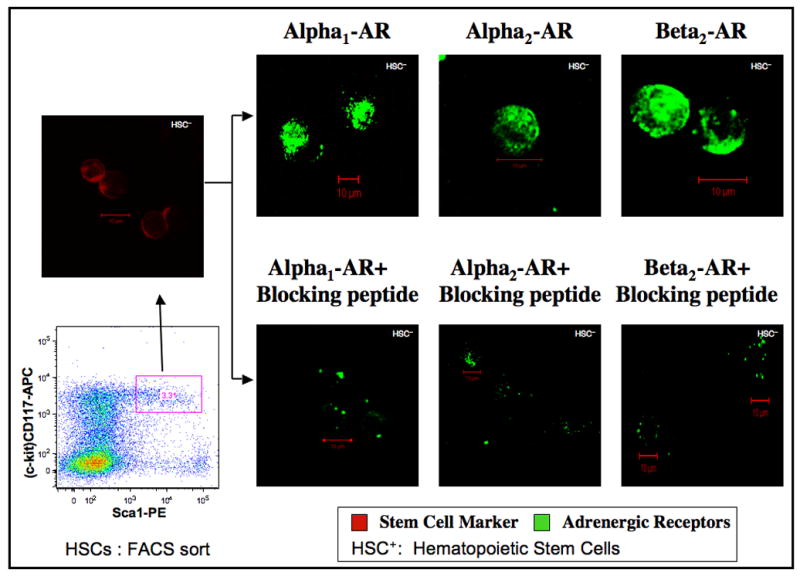
Adrenergic receptor distribution in murine hematopoietic stem cells (HSCs). Murine bone marrow cells were depleted of lineage-committed cells using Ab coupled micro-bead technology followed by dual staining with anti Sca-1- PE and anti CD117- APC antibodies. Subsequently, CD117high, Sca-1+ population were sorted by flow cytometry to yield HSCs (Lin−, CD117high, Sca-1+) represented in boxed area of the dot plot. 3.31% of Lin− cells are shown (red) in the confocal image right above the boxed area and representing HSCs. Aliquots of HSCs were labeled with primary antibody specific for adrenergic receptor subtypes with and without specific blocking peptides followed by FITC conjugated secondary antibody. Confocal images were obtained in a Zeiss LSM 510 confocal microscope. Green color represents binding of the polyclonal Abs to the C-terminus of adrenergic receptor subtypes. Image resolution=1024 × 1024; Scale bar=10μm.
3.5. Functionality of adrenergic receptors
One of the functional consequences of adrenergic stimulation in macrophages (MØ) is the alteration in endotoxin mediated cytokine expression through intracellular signaling mechanisms (Bergmann, 2002; Cohen et al., 2004; Deng et al., 2004; Muthu et al., 2005a). Since all FACS defined hematopoietic myeloid progenitors were shown to express adrenergic receptors, we tested the functionality of the receptors in terms of cytokine expression that would indirectly indicate the coupling of adrenergic receptors. Bone marrow progenitors isolated at different levels of commitment were subjected to adrenergic stimulation during MØ development. Subsequent to differentiation, MØ were stimulated with LPS. Fig 6 shows the LPS mediated TNF-α response of MØ differentiated from bone marrow myeloid progenitors with and without adrenergic stimulation during their development. LPS stimulation of bone marrow ER-MP20+, ER-MP12+, CD34+, CD117+ and HSC derived MØ differentiated in the presence of isoproterenol (β2 agonist) and phenylephrine (α1 agonist) augments TNF-α production. However, stimulation with clonidine (α2 agonist) did not affect TNF-α responses in comparison to vehicle treatment. In this experiment, TNF-α values were normalized to protein content and expressed as percentage in comparison to vehicle treatment taken as 100%.
Figure 6.
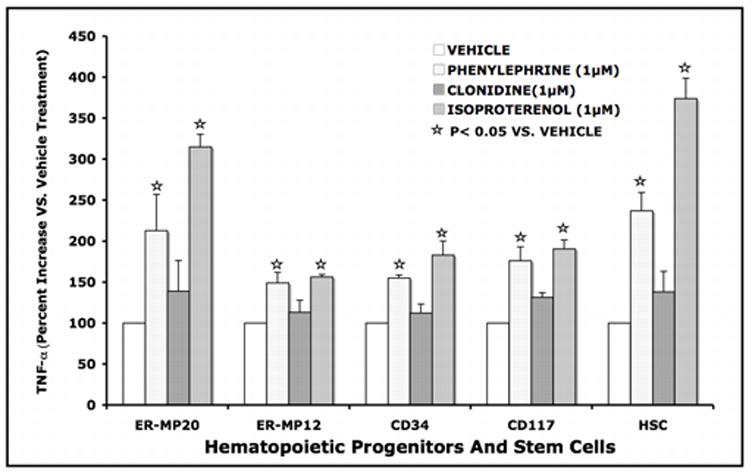
LPS mediated TNF-α response of MØ differentiated from bone marrow myeloid progenitors with and without adrenergic stimulation during their development. Bone marrow ER-MP20+, ER-MP12+, CD34+, CD117+ and HSCs were allowed to differentiate into mature MØ in vitro with specific growth factor cocktail with and without isoproterenol, phenylephrine and clonidine added on day 0. At the end of culture (7–10 days), the adherent MØ were washed, stimulated with LPS (100ng/ml for 18h) and the conditioned medium was collected for TNF-α measurements (ELISA). Data represents soluble TNF-α in the conditioned medium normalized to protein content and expressed as a percentage in comparison to vehicle treatment taken as 100%.
Monocyte committed ER-MP20+ cells responded to β2- AR stimulation during differentiation into MØ resulting in 315% increase in TNF-α release in response to LPS as compared to vehicle treatment (p<0.05). Similarly, α1- AR stimulation resulted in 212% increase in TNF-α release in response to LPS in comparison to vehicle treatment (p<0.05). Whereas, α2- AR stimulation led to a marginal increase in TNF-α response that is not significantly different than the vehicle treatment in ER-MP20+ cells. These results indicate that β2– and α1- AR are coupled in ER-MP20+ cells to induce significant changes in LPS mediated cytokine response as fully differentiated MØ.
Bi-potential ER- MP12+ cells showed an average of 156% and 148% increase in TNF-α release in response to β2- and α1- AR stimulation respectively (p<0.05). Multi-potential CD34+ and CD117+ cells demonstrated an average of 183% and 190% increase in TNF-α release in response to β2- AR stimulation (p<0.05) and a mean of 155% and 175% increase in TNF-α release in response to α1- AR stimulation (p<0.05) respectively.
Uncommitted murine hematopoietic stem cells (HSC) with self-renewing capacity responded to β2- AR stimulation during differentiation into MØ with a robust 374% increase in TNF-α release in response to LPS as compared to vehicle treatment (p<0.05). Similarly, α1- AR stimulus resulted in 236% increase in TNF-α release in response to LPS compared to vehicle treatment (p<0.05). Interestingly, none of the progenitors tested including HSCs responded to the α2 agonist Clonidine. This may perhaps suggest that α2 AR expressed on these cells are not functionally coupled to alter the differentiation and phenotype of MØ. Taken together our results indicate that murine hematopoietic stem cells (HSC) and bone marrow myeloid progenitors express α1-, α2- and β2- adrenergic receptors and only α1- and β2– receptors are coupled to adrenergic stimulus during MØ development that trigger an altered cytokine response to LPS in a fully differentiated MØ.
4. Discussion
Here we have documented that α- and β-ARs are expressed by the hematopoietic progenitor cells within the bone marrow compartment at various levels of lineage commitment. A schematic representation of the hematopoietic progenitors identified by the hierarchic expression of specific cell surface markers at different levels of monocyte commitment is shown in Fig 7.
Figure 7.
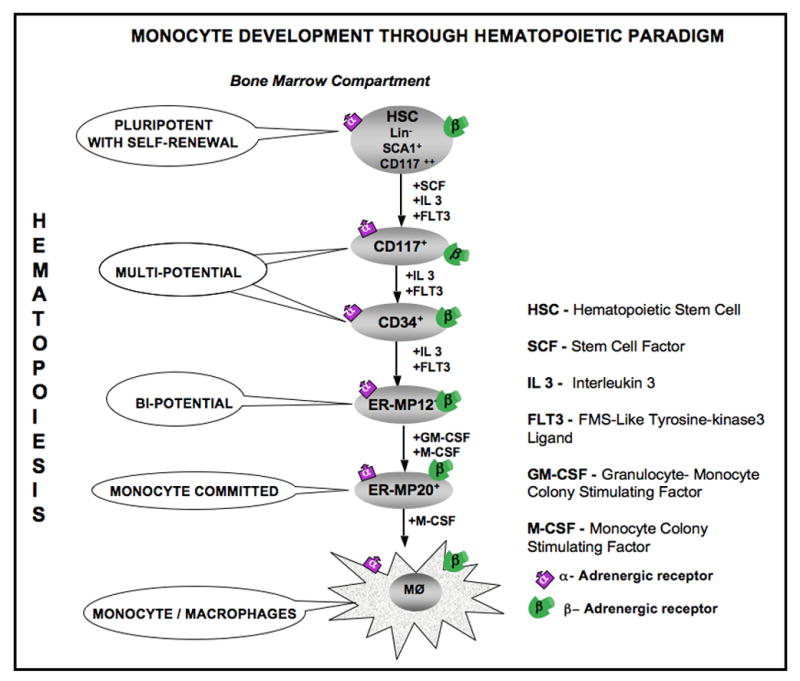
Hierarchical representation of progenitors of the hematopoietic paradigm down the monocytic lineage starting from pluripotent stem cells. α1-, α2- and β2-ARs are expressed by the hematopoietic progenitor cells enriched for ER-MP20+, ER-MP12+, CD34+ and CD117+ and pluripotent hematopoietic stem cells.
Since mature blood cells of different lineages originate from the bone marrow compartment, it is the site of proliferation and differentiation of pluripotent stem cells into T- and B-cells, monocytes, erythrocytes, megakaryocytes and other leukocytes in the adult (Shizuru et al., 2005). The bone marrow is innervated with both myelinated and non-myelinated nerve fibers that utilize various classes of neurotransmitters (Calvo and Forteza-Vila, 1969; Felten et al., 1985; Tabarowski et al., 1996; Yamazaki and Allen, 1990). Bone marrow has noradrenergic and cholinergic innervations providing a morphological basis for the neural modulation of hematopoiesis (Artico et al., 2002). Although there is direct evidence for the ligand-induced adrenergic responses by the immune cells, there are no reports on the expression of adrenergic receptors on the hematopoietic progenitor cells. However, there is indirect evidence that alpha-1 adrenergic receptor blockade or chemical sympathectomy increases granulocytes, platelets and granulocyte/macrophage colony-forming units in the peripheral blood (Maestroni and Conti, 1994) and that mice lacking α1B adrenergic receptors have poor hematopoietic recovery following irradiation (Maestroni, 2000). These observations clearly indicate adrenergic stimulation play a significant role in the development of the cells of the innate immune system and yet depiction of adrenergic receptors on the hematopoietic progenitor cells is lacking. Here, we have shown that hematopoietic progenitor cells inclusive of the pluripotent stem cells express α1-, α2- and β2-adrenergic receptors.
It is apparent from the work of Sanders and others that the cells of the adaptive immune system respond to adrenergic stimulus. B-cells, naïve T cells, Th 1 and Th 2 cells respond to β2– adrenergic stimulation; Th 1 but not Th 2 cells express β2–adrenergic receptors (Kin and Sanders, 2006; Kohm et al., 2000; Sanders et al., 2003). Bone marrow cells expressing CD34+ and CD117low are considered common lymphoid progenitor (CLP) that can differentiate into T cells, B cells, NK cells and dendritic cells (Shizuru et al., 2005). Here we have shown evidence that CD34+ and CD117+ bone marrow cells express α1-, α2- and β2- adrenergic receptors. However, the functional importance of adrenergic receptor expression during the development of the cells of adaptive immune system needs further investigation. While it is well documented that CD117 (c-kit) is expressed in a variety of hematopoietic tumor cells, it has recently been published that chronic stress promotes tumor growth in a β2– adrenergic receptor dependent manner (Thaker et al., 2006). Likewise, sympathetic stimulation also regulates hematopoietic stem cell egress from bone marrow (Katayama et al., 2006) intensifying the need for documentation of adrenergic receptor expression by the hematopoietic stem cells.
As a primordial response to stressful events such as critical injury, marked increases in endogenous release of catecholamines have been documented in patients resulting in a ten-fold increase in circulating levels (Angus and Wax, 2001; Benedict and Grahame-Smith, 1978; Frayn, 1986; Goodall et al., 1957; Wilmore and Aulick, 1978). Furthermore, critically injured patients are often administered exogenous catecholamines to maintain cardiovascular homeostasis. Experimentally, there is evidence for increased bone marrow norepinephrine turnover in response to physiologic stimuli and infectious challenge (Tang et al., 1999). Critical injury associated adrenergic stimulus is shown to modulate erythropoiesis and monocytopoiesis (Fonseca et al., 2004; Tang et al., 2001) with sympathetic ablation of nerve terminals reversing the thermal injury and sepsis induced monocytopoiesis (Cohen et al., 2004; Tang et al., 2001). Such findings imply the involvement of specific adrenergic receptor expression in hematopoietic progenitors and in the present work we indeed show evidence for the expression of α1-, α2- and progenitors. Moreover, patients with β2- adrenergic receptors on bone marrow hematopoietic complete spinal chord injury exhibit decreased NK cell activity and impaired proliferation of hematopoietic progenitor cells (Iversen, 1997; Iversen et al., 2000) also suggesting the functional influence of nerve stimulation in the hematopoietic compartment.
In this report, we substantiate that murine bone marrow hematopoietic progenitors and stem cells express functional α-and β-ARs. α1- and β2- adrenergic stimulus of the uncommitted, multi-lineage committed and monocyte committed myeloid progenitors lead to the differentiation of functionally altered MØ. In other words, bone marrow progenitors at different levels of commitment hierarchy not only express adrenergic receptors but also respond to adrenergic stimulus rendering phenotypic changes in MØ that are capable of producing more TNF-α in response to LPS stimulation. Immune cells express several toll-like receptors of which TLR4 is considered essential for LPS signaling (Blander and Medzhitov, 2004). CD14 is a co-receptor also critical in augmenting LPS mediated cytokine responses (Ebong et al., 2001; Wright et al., 1990). We have previously shown that monocyte committed cells subjected to adrenergic stimulation differentiate into MØ expressing higher levels of CD14 surface expression (Muthu et al., 2005b). This could partly explain why LPS mediated TNF-α levels are augmented in progenitor derived MØ exposed to adrenergic stimulation during differentiation.
It is important to note that the monocyte committed ER-MP20+ and the uncommitted hematopoietic stem cells are a homogenous population presenting a robust cytokine response to adrenergic stimulation. In contrast, the multi-potent CD34+ and CD117+ cells as well as the bi-potential ER-MP12+ cells represent heterogeneity in terms of lineage commitment with a moderate cytokine response to adrenergic stimulation.
Although α2-AR expression in bone marrow myeloid progenitors is evident through confocal and FACS analysis, α2-AR stimulus failed to induce similar cytokine responses to that of α1- and β2- adrenergic stimulation. This could be in part due to either a weak signal or a non-functional receptor expression. While either of the two reasoning is plausible, the data in Fig 6 shows a marginal increase in TNF-α expression in response to clonidine in the ER-MP20+, CD117+ and hematopoietic stem cells suggesting the probability of a week signal or a low affinity receptors. Increasing either the concentration of the agonist or the seeding density of the progenitor population or both may perhaps assess this phenomenon.
Radioligand binding is the widely accepted and powerful technique to determine receptor expression. Although we have used this method in our previous report (Muthu et al., 2005b), this technique requires a large sample size restricting the ability to apply this method for low yielding sample size such as the progenitor populations and stem cells. Therefore, we have adopted techniques such as flow cytometry and confocal microscopy to document the expression of adrenergic receptors on enriched populations of hematopoietic progenitors. Approximately, 15–20% of total bone marrow cells constitute late monocyte committed (ER-MP20+) progenitors and bi-potential (ER-MP12+) myeloid progenitors while 2.5–5% represent multi-potential (CD117+ and CD34+) progenitors and more importantly only about 0.1% of the total bone marrow cells are pluripotent hematopoietic stem cells (HSCs). Furthermore, it is important to recognize that these specific progenitors represent our present understanding of the developmental stages of hematopoiesis defined by the expression of lineage specific cell surface markers. In subjecting these different progenitors to our experimental techniques of immuno-staining followed by confocal microscopy and FACS analysis we have been able to define ARs that are present at each stage of development. Knowing the adrenergic receptor expression at distinct stages of hematopoiesis will aid in probing the mechanisms underpinning the consequences of adrenergic stimulus/block in hematopoietic development during physiology and pathology.
Acknowledgments
This work was supported by Dr. Ralph and Marion C. Falk Medical Research Trust and NIH grant RO1 GM 42577.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–116. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- Artico M, Bosco S, Cavallotti C, Agostinelli E, Giuliani-Piccari G, Sciorio S, Cocco L, Vitale M. Noradrenergic and cholinergic innervation of the bone marrow. Int J Mol Med. 2002;10:77–80. [PubMed] [Google Scholar]
- Benedict CR, Grahame-Smith DG. Plasma noradrenaline and adrenaline concentrations and dopamine-beta-hydroxylase activity in patients with shock due to septicaemia, trauma and haemorrhage. Q J Med. 1978;47:1–20. [PubMed] [Google Scholar]
- Bergmann MaST. Immunomodulatory effects of vasoactive catecholamines. Wiener Klinische Wochenschrift. 2002;114:752–761. [PubMed] [Google Scholar]
- Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- Brogden KA, Guthmiller JM, Salzet M, Zasloff M. The nervous system and innate immunity: the neuropeptide connection. Nat Immunol. 2005;6:558–564. doi: 10.1038/ni1209. [DOI] [PubMed] [Google Scholar]
- Bruno L, Seidl T, Lanzavecchia A. Mouse pre-immunocytes as non-proliferating multipotent precursors of macrophages, interferon-producing cells, CD8alpha(+) and CD8alpha(−) dendritic cells. Eur J Immunol. 2001;31:3403–3412. doi: 10.1002/1521-4141(200111)31:11<3403::aid-immu3403>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Bunting KD, Yu WM, Bradley HL, Haviernikova E, Kelly-Welch AE, Keegan AD, Qu CK. Increased numbers of committed myeloid progenitors but not primitive hematopoietic stem/progenitors in mice lacking STAT6 expression. J Leukoc Biol. 2004;76:484–490. doi: 10.1189/jlb.0903440. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR, Jr, Trendelenburg U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- Calvo W, Forteza-Vila J. On the development of bone marrow innervation in new-born rats as studied with silver impregnation and electron microscopy. Am J Anat. 1969;126:355–371. doi: 10.1002/aja.1001260308. [DOI] [PubMed] [Google Scholar]
- Cohen MJ, Shankar R, Stevenson J, Fernandez R, Gamelli RL, Jones SB. Bone marrow norepinephrine mediates development of functionally different macrophages after thermal injury and sepsis. Ann Surg. 2004;240:132–141. doi: 10.1097/01.sla.0000130724.84914.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn MF, Slieker WA, van der Loo JC, Voerman JS, van Ewijk W, Leenen PJ. Distinct mouse bone marrow macrophage precursors identified by differential expression of ER-MP12 and ER-MP20 antigens. Eur J Immunol. 1994;24:2279–2284. doi: 10.1002/eji.1830241003. [DOI] [PubMed] [Google Scholar]
- Deng J, Muthu K, Gamelli R, Shankar R, Jones SB. Adrenergic modulation of splenic macrophage cytokine release in polymicrobial sepsis. Am J Physiol Cell Physiol. 2004;287:C730–736. doi: 10.1152/ajpcell.00562.2003. [DOI] [PubMed] [Google Scholar]
- Ebong SJ, Goyert SM, Nemzek JA, Kim J, Bolgos GL, Remick DG. Critical role of CD14 for production of proinflammatory cytokines and cytokine inhibitors during sepsis with failure to alter morbidity or mortality. Infect Immun. 2001;69:2099–2106. doi: 10.1128/IAI.69.4.2099-2106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari F, Sternberg EM. Neural-immune interactions in health and disease. Ann N Y Acad Sci. 2002;966:20–27. doi: 10.1111/j.1749-6632.2002.tb04198.x. [DOI] [PubMed] [Google Scholar]
- Felten DL, Felten SY, Carlson SL, Olschowka JA, Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985;135:755s–765s. [PubMed] [Google Scholar]
- Fonseca RB, Mohr AM, Wang L, Clinton E, Sifri ZC, Rameshwar P, Livingston DH. Adrenergic modulation of erythropoiesis following severe injury is mediated through bone marrow stroma. Surg Infect (Larchmt) 2004;5:385–393. doi: 10.1089/sur.2004.5.385. [DOI] [PubMed] [Google Scholar]
- Frayn KN. Hormonal control of metabolism in trauma and sepsis. Clin Endocrinol (Oxf) 1986;24:577–599. doi: 10.1111/j.1365-2265.1986.tb03288.x. [DOI] [PubMed] [Google Scholar]
- Goodall M, Stone C, Haynes BW., Jr Urinary output of adrenaline and noradrenaline in severe thermal burns. Ann Surg. 1957;145:479–487. doi: 10.1097/00000658-195704000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Anan K, Misawa M, Kai S, Hara H. In vitro differentiation of murine Sca-1+Lin- cells into myeloid, B cell and T cell lineages. Stem Cells. 1996;14:412–418. doi: 10.1002/stem.140412. [DOI] [PubMed] [Google Scholar]
- Iversen PO. Blood flow to the haemopoietic bone marrow. Acta Physiol Scand. 1997;159:269–276. doi: 10.1046/j.1365-201X.1997.00107.x. [DOI] [PubMed] [Google Scholar]
- Iversen PO, Hjeltnes N, Holm B, Flatebo T, Strom-Gundersen I, Ronning W, Stanghelle J, Benestad HB. Depressed immunity and impaired proliferation of hematopoietic progenitor cells in patients with complete spinal cord injury. Blood. 2000;96:2081–2083. [PubMed] [Google Scholar]
- Jones SB, Kovarik MF, Romano FD. Cardiac and splenic norepinephrine turnover during septic peritonitis. Am J Physiol. 1986;250:R892–897. doi: 10.1152/ajpregu.1986.250.5.R892. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kavelaars A. Regulated expression of alpha-1 adrenergic receptors in the immune system. Brain Behav Immun. 2002;16:799–807. doi: 10.1016/s0889-1591(02)00033-8. [DOI] [PubMed] [Google Scholar]
- Kennedy DW, Abkowitz JL. Mature monocytic cells enter tissues and engraft. Proc Natl Acad Sci U S A. 1998;95:14944–14949. doi: 10.1073/pnas.95.25.14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. J Leukoc Biol. 2006;79:1093–1104. doi: 10.1189/jlb.1105625. [DOI] [PubMed] [Google Scholar]
- Kohm AP, Sanders VM. Norepinephrine: a messenger from the brain to the immune system. Immunol Today. 2000;21:539–542. doi: 10.1016/s0167-5699(00)01747-3. [DOI] [PubMed] [Google Scholar]
- Kohm AP, Sanders VM. Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev. 2001;53:487–525. [PubMed] [Google Scholar]
- Kohm AP, Tang Y, Sanders VM, Jones SB. Activation of antigen-specific CD4+ Th2 cells and B cells in vivo increases norepinephrine release in the spleen and bone marrow. J Immunol. 2000;165:725–733. doi: 10.4049/jimmunol.165.2.725. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48:405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- Leenen PJ, de Bruijn MF, Voerman JS, Campbell PA, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Leenen PJ, Melis M, Slieker WA, Van Ewijk W. Murine macrophage precursor characterization. II. Monoclonal antibodies against macrophage precursor antigens. Eur J Immunol. 1990;20:27–34. doi: 10.1002/eji.1830200105. [DOI] [PubMed] [Google Scholar]
- Maestroni GJ. Neurohormones and catecholamines as functional components of the bone marrow microenvironment. Ann N Y Acad Sci. 2000;917:29–37. doi: 10.1111/j.1749-6632.2000.tb05370.x. [DOI] [PubMed] [Google Scholar]
- Maestroni GJ, Conti A. Noradrenergic modulation of lymphohematopoiesis. Int J Immunopharmacol. 1994;16:117–122. doi: 10.1016/0192-0561(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Maestroni GJ, Mazzola P. Langerhans cells beta 2-adrenoceptors: role in migration, cytokine production, Th priming and contact hypersensitivity. J Neuroimmunol. 2003;144:91–99. doi: 10.1016/j.jneuroim.2003.08.039. [DOI] [PubMed] [Google Scholar]
- Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthu K, Deng J, Gamelli R, Shankar R, Jones SB. Adrenergic modulation of cytokine release in bone marrow progenitor-derived macrophage following polymicrobial sepsis. J Neuroimmunol. 2005a;158:50–57. doi: 10.1016/j.jneuroim.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Muthu K, Deng J, Romano F, He LK, Gamelli R, Shankar R, Jones SB. Thermal injury and sepsis modulates beta-adrenergic receptors and cAMP responses in monocyte-committed bone marrow cells. J Neuroimmunol. 2005b;165:129–138. doi: 10.1016/j.jneuroim.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Orlic D, Fischer R, Nishikawa S, Nienhuis AW, Bodine DM. Purification and characterization of heterogeneous pluripotent hematopoietic stem cell populations expressing high levels of c-kit receptor. Blood. 1993;82:762–770. [PubMed] [Google Scholar]
- Sanders VM, Kasprowicz DJ, Swanson-Mungerson MA, Podojil JR, Kohm AP. Adaptive immunity in mice lacking the beta(2)-adrenergic receptor. Brain Behav Immun. 2003;17:55–67. doi: 10.1016/s0889-1591(02)00056-9. [DOI] [PubMed] [Google Scholar]
- Shizuru JA, Negrin RS, Weissman IL. Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annu Rev Med. 2005;56:509–538. doi: 10.1146/annurev.med.54.101601.152334. [DOI] [PubMed] [Google Scholar]
- Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5:575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- Straub RH. Complexity of the bi-directional neuroimmune junction in the spleen. Trends Pharmacol Sci. 2004;25:640–646. doi: 10.1016/j.tips.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Tabarowski Z, Gibson-Berry K, Felten SY. Noradrenergic and peptidergic innervation of the mouse femur bone marrow. Acta Histochem. 1996;98:453–457. doi: 10.1016/S0065-1281(96)80013-4. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Naito M, Takeya M. Development and heterogeneity of macrophages and their related cells through their differentiation pathways. Pathol Int. 1996;46:473–485. doi: 10.1111/j.1440-1827.1996.tb03641.x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Shankar R, Gamboa M, Desai S, Gamelli RL, Jones SB. Norepinephrine modulates myelopoiesis after experimental thermal injury with sepsis. Ann Surg. 2001;233:266–275. doi: 10.1097/00000658-200102000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Shankar R, Gamelli R, Jones S. Dynamic norepinephrine alterations in bone marrow: evidence of functional innervation. J Neuroimmunol. 1999;96:182–189. doi: 10.1016/s0165-5728(99)00032-6. [DOI] [PubMed] [Google Scholar]
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- Uchida N, Weissman IL. Searching for hematopoietic stem cells: evidence that Thy-1.1lo Lin- Sca-1+ cells are the only stem cells in C57BL/Ka-Thy-1.1 bone marrow. J Exp Med. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore DW, Aulick LH. Metabolic changes in burned patients. Surg Clin North Am. 1978;58:1173–1187. doi: 10.1016/s0039-6109(16)41685-3. [DOI] [PubMed] [Google Scholar]
- Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Xu B. The importance of beta-adrenergic receptors in immune regulation: a link between neuroendocrine and immune system. Med Hypotheses. 2001;56:273–276. doi: 10.1054/mehy.2000.1127. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Allen TD. Ultrastructural morphometric study of efferent nerve terminals on murine bone marrow stromal cells, and the recognition of a novel anatomical unit: the “neuro-reticular complex”. Am J Anat. 1990;187:261–276. doi: 10.1002/aja.1001870306. [DOI] [PubMed] [Google Scholar]


