Abstract
Objectives
Bacterial vaginosis (BV) is the most common vaginal disorder worldwide. Certain lactobacilli produce H202 and lactic acid, which normally suppress growth of anaerobes, but in BV, G. vaginalis and other anaerobes proliferate, and the number of lactobacilli decreases. Gardnerella vaginalis colonizes the vaginal epithelium as a biofilm, which likely plays a role in colonization and relapsing infection.
Study Design
We developed an in vitro model for G. vaginalis biofilm formation, and compared susceptibilities of biofilms vs. planktonic cultures to H2O2 and lactic acid. The structure and composition of the biofilm matrix were studied in order to design a method for biofilm dissolution.
Results
Biofilms tolerated 5-fold and 4–8 fold higher concentrations of H2O2 and lactic acid (respectively) than planktonic cultures. Proteolytic dissolution of biofilms reduced sensitivity to H202 and lactic acid.
Conclusions
Increased tolerance to H2O2 and lactic acid suggests that biofilm formation contributes to survival of G. vaginalis in the presence of lactobacilli.
Keywords: Vaginosis, Gardnerella, Biofilm
INTRODUCTION
Bacterial vaginosis (BV) is the most common vaginal disorder worldwide, with a prevalence of around 10–20% in white, non-Hispanic women and 30–50% in African American women1. The major symptom of BV is a malodorous, thin vaginal discharge. Current therapeutic modalities to treat BV are unsatisfactory. BV generally responds to antibiotic therapy, but there is a 50% rate of relapse is within a year2. BV poses a significant health risk because it predisposes women to serious complications including preterm delivery3–4 increased risk of pelvic inflammatory disease1, and increased HIV transmission in both directions5–6.
In the healthy vagina, lactobacilli such as Lactobacillus crispatus predominate and produce H2O2 and lactic acid thereby reducing the vaginal pH to less than 4.5 and preventing colonization by pathogenic anaerobes from the gastrointestinal tract7–8. BV is characterized by an increase in vaginal pH to greater than 4.5, a decrease in the number of normal lactobacilli, and a considerable increase in anaerobic bacteria including Gardnerella vaginalis, Atopobium vaginae, and Mobiluncus spp.9. The etiology of BV is poorly understood and a single causative agent has not been identified. G. vaginalis has, however, been isolated from up to 95% of BV cases, and infection with 2×109 bacteria from pure culture produced BV in 5 of 9 women thus inocculated10–11. It is not presently known whether a drop in the number of lactobacilli and/or an increase in the vaginal pH must precede colonization by anaerobic species, or whether the anaerobes can somehow gain a foothold on their own and out-compete the lactobacilli.
Recent evidence suggests that in many infections, bacteria assume a biofilm, rather than a planktonic mode of growth. Biofilms are adherent communities of microorganisms held together by a polymeric matrix composed of polysaccharides, proteins and/or nucleic acids. The distinct gene expression pattern, as well as the physical structure of biofilms increases bacterial resistance to many negative stimuli including chemical disinfectants, pH extremes, host immune defenses and antibiotics. It was recently demonstrated that G. vaginalis forms a biofilm on the vaginal epithelium12. In addition, a previous study showed that G. vaginalis biofilms tolerate higher concentrations of certain antibiotics13. We hypothesized that the biofilm phenotype confers a survival advantage upon BV-associated anaerobes within the vagina and is an important component of the pathogenesis of BV. More specifically, elaboration of a biofilm could enable G. vaginalis to survive even in the presence of lactobacilli-derived H2O2 and lactic acid, and could explain the high rate of relapse. We compared sensitivities of biofilm versus planktonic cultures to H2O2 and lactic acid and found that the biofilms exhibited increased tolerance to the compounds. Susceptibility to H2O2 and lactic acid was restored upon enzymatic dissolution of the biofilms.
MATERIALS AND METHODS
Strains and Culture Conditions
Gardnerella vaginalis strain ATCC 49145 (American Type Culture Collection, Manassas, VA) and strains 5-1 and 465-5 (kindly provided by Dr. Robin Ross, Boston, MA) were grown under anaerobic conditions, in ~10% CO2 using the AnaeroPack system (MGC, Tokyo, Japan). 96-well tissue culture plate assays were performed essentially as previously described to determine optimal growth conditions for biofilm formation14. G. vaginalis was grown in Corning Cell-Bind Plates (Corning, Corning, NY) in various media (Becton-Dickinson Franklin Lakes, NJ) without or with 1% supplemental glucose (G) including Luria broth (LB, LBG), Tryptic Soy Broth (TSB, TSBG), Mueller-Hinton (MH, MHG), Brain Heart Infusion (BHI, BHIG), De Man Rogosa Sharpe (MRS, MRSG), and a chemically defined medium resembling vaginal secretions (CDM, CDMG)14.
For sensitivity assays, biofilms were grown in 0.2 ml BHIG or CDM in 96-well plates. Media was replaced every 24hr and biofilms were used after 48hr growth. Planktonic cultures were grown for 24hr in BHI or CDM and were agitated with a magnetic stirbar.
Luciferase assay for bacterial viability
The BacTiter luciferase assay for ATP (Promega) was used to determine bacterial viability. To correlate viability with relative light units, planktonic cultures and biofilm cultures that had been resuspended by pipetting up and down were serially diluted 10-fold in BHI. 10μL of each dilution was plated on BHI agar. The plates were incubated anaerobically for 72 hr and colony forming units (CFU’s) were determined. 200μL of each serial dilution was placed in CellBIND-coated 96 well clear bottom black plates (Corning). Cells were collected by centrifuging the plates and the media was replaced with 50 μL BacTiter reagent. After 10 min at 21 °C luminescence was analyzed using a plate-reader luminometer (Tropix – GE Healthcare Piscataway, NJ) for 1 sec.
Sensitivity assays
For sensitivity assays, 200 mM hydrogen peroxide and 2 M lactic acid (Sigma) were diluted 2-fold in BHI or CDM and added to biofilm and planktonic cultures. Plates were incubated anaerobically for 20 hours at 37°C, cells were collected by centrifugation, resuspended in 50 μL BacTiter reagent, transferred to black 96 well plates, and viability was assessed as above. The concentration of H2O2 or lactic acid required to bring about a 90% decrease in relative light units was recorded as the MBC90.
Biochemical characterization
For biofilms, strain ATCC 49145 was grown anaerobically in BHI, in T75 tissue culture flasks. Planktonic cultures were grown in BHI with shaking. Media was removed from biofilms, planktonic bacteria were collected by centrifugation, and they were washed once with ddH2O. Loosely associated surface material was extracted by vigorously resuspending bacteria in ddH2O, for a final cell density of 109 CFU/mL as estimated by OD650. Protein concentrations in the extracts were determined using Coomassie Protein Assay Reagent (Pierce, Rockford, IL). Nucleic acid content was estimated by measuring the OD260. Hexosamine contents were estimated by standard phenol-sulfuric acid assay15 using glucose dilutions as standards. Three separate experiments were performed and statistical significance was determined using Student’s t-test.
For lectin blots, 100μL of the cellular extracts were serially diluted 5-fold in TBS and dilutions were blotted onto nitrocellulose using a vacuum manifold, and probed with peroxidase-conjugated lectins: wheat germ agglutinin, concanavalin A, and Glycine Max (Sigma, St. Louis, MO) as described previously17. The blots were developed using the ECL-Plus Western blotting detection system (GE Healthcare, Piscataway, NJ).
Confocal Microscopy
Strain 49145 was grown in BHIG in 35mm collagen-coated dishes (MatTek, Ashland, MA) at 37°C anaerobically for 48hr. Biofilms were stained with 3μL SYTO® 9 and 3uL Alexa Fluor® 633-conjugated WGA (Invitrogen, Calrsbad, CA) in 3mL PBS 15min with rocking. Biofilms were washed 2X with PBS and confocal imaging was performed using a Zeiss LSM 510 multiphoton confocal scanning laser microscope (Microscopy Core Facility, Neuroscience Department, VCU, Richmond, VA). The objective was an Acroplan 63X/0.9W. The excitation wavelengths used were 488nm and 633nm and bandpass filters for emitted light were 500nm–530nm and 650nm.
Biofilm Dissolution
To analyze dissolution of established biofilms, 5-fold dilutions of various compounds were made in fresh BHIG (or in 50mM NaOAc (pH 4.2) for sodium metaperiodate). Stock solutions were: 20 mg proteinase K/mL, 20 mg trypsin/ml, 5U chitinase/mL, 30% H2O2, 10 M lactic acid, 20%SDS, 4mg sodium metaperiodate/mL 50mM sodium acetate pH 4.2, and 25U DNaseI/mL. Biofilms were incubated for 20hr, the media was removed, biofilms were washed with PBS, dried, and stained with safranin.
The effect of treating biofilms with 100μg proteinase K/mL for 24hr on viability was determined using the BacTiter assay as described above. For proteinase K-treated biofilm sensitivity assays the media in the wells was replaced with BHIG containing 100μg proteinase K/mL. The biofilms were pre-incubated with the protease for 1hr and sensitivity to H2O2 and lactic acid was determined as described above.
RESULTS
Biofilm formation by G. vaginalis in vitro
To determine optimal conditions for in vitro biofilm formation, G. vaginalis was cultured anaerobically in 96-well plates in LB, TSB, BHI, MH, MRS, or a chemically defined medium resembling vaginal secretions (CDM)16. Because glucose increases biofilm formation in other bacterial species18, G. vaginalis was also cultured in the presence of 1% glucose (LBG, TSBG, BHIG, MHG, MRSG, and CDMG). Biofilms were stained with safranin. Growth was poor in TSB and MRS (Figure 1). Growth was slow in CDM although a biofilm formed in this media with or without added glucose. A thin biofilm was formed in LB and MH and was heavier in the presence of glucose. Growth and biofilm formation were greatest in BHIG.
Fig. 1. G. vaginalis biofilm formation.
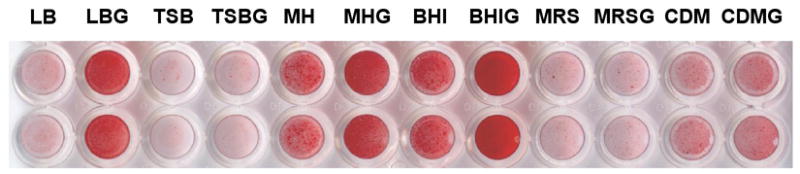
G. vaginalis strain ATCC 49145 was grown in various media with or without added glucose anaerobically at 37°C for 24hr. Non-adherent cells were removed from the wells and the adherent bacteria were stained with safranin. Strains 465-5 and 5-1 produced similar results.
Sensitivity of G. vaginalis biofilms versus planktonic cultures
Viability of G. vaginalis cultures was determined using a luciferase reagent. The results in Fig. 2A demonstrate that the relative light units (RLU) emitted were directly related to the number of colony forming units. To determine if biofilm bacteria were more resistant than planktonic bacteria to H2O2 and lactic acid, we exposed cultures to these agents and determined the viability by luciferase assay. Fig. 2A and 2C show the MBC90‘s (minimum concentration required to reduce viability by at least 90%) for H2O2 and lactic acid. Biofilms were more resistant and tolerated 4-fold higher concentrations of H202 and 8-fold higher concentrations of lactic acid than planktonic cultures.
Fig. 2. Biofilms exhibit increased tolerance to H2O2 and lactic acid.
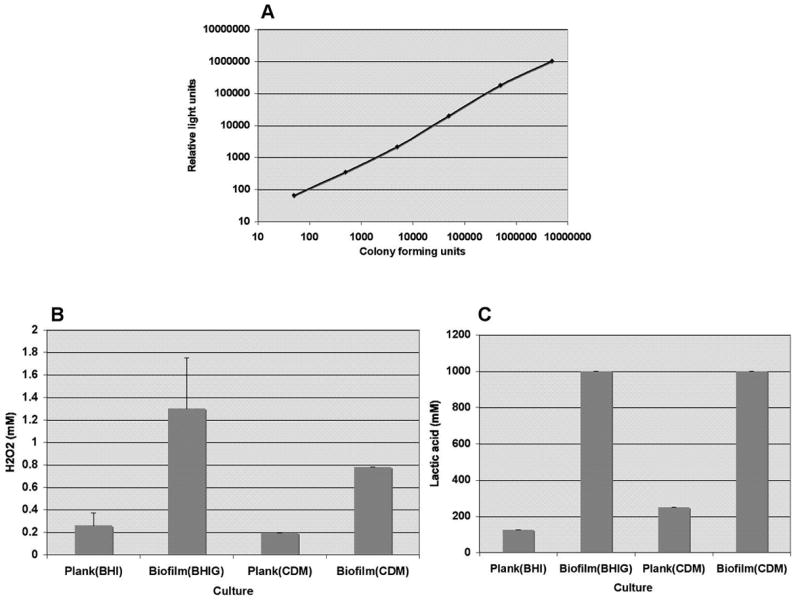
Panel A illustrates the direct correlation between the number of colony forming units (x-axis) and relative light units (y-axis) measured in the viability assay. In Panel B, the MBC90 of H2O2, or the concentration required to decrease viability of biofilm and planktonic cultures grown in BHI or CDM by at least 90% is shown. In Panel C, the concentration of lactic acid required to decrease viability of biofilm and planktonic cultures grown in BHI or CDM by at least 90% is shown. Bars in Panels B and C represent the averages of three separate experiments and error bars represent the standard deviations.
To better replicate the conditions that G. vaginalis would encounter in the vagina, we grew biofilms and planktonic cultures in CDM and tested the sensitivity of the cultures to H202 and lactic acid. As shown in Figure 4, biofilms grown in CDM were resistant to 4-fold higher concentrations of H2O2 and 4-fold higher concentrations of lactic acid.
Fig. 4. Confocal microscopy of G. vaginalis biofilm.
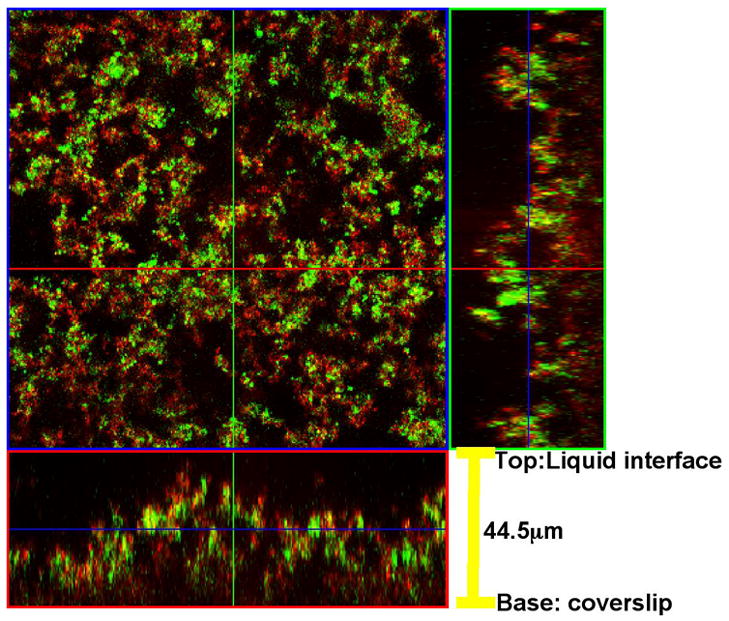
Biofilms were stained with SYTO® 9 and a fluorescent Alexa Fluor® 633-conjugate of WGA, and analyzed by confocal scanning laser microscopy. The large panel is a “bird’s-eye” view of the biofilm. The right panel is a side view from an x-axis section and the lower panel is a side-view from the y-axis section. The biofilm shown was formed by strain 465-5; biofilms formed by other strains yielded similar results.
Biochemical analysis of G. vaginalis biofilms
We hypothesized that biofilm dissolution would restore sensitivity to H202 and lactic acid. In order to design a method for biofilm dissolution, we preliminarily characterized the biochemical composition and structure of the biofilms. The crude biochemical composition of the extracellular matrix of G. vaginalis biofilms was characterized by extracting loosely associated material from biofilm and plaktonic bacteria. Biofilm extracts contained more than 4 times as much total carbohydrate per 106 bacterial cells compared to planktonic bacteria (Table I) but protein and nucleic acid concentrations were not significantly different (p>0.05 and p>0.5, respectively) suggesting that neither proteins nor nucleic acids are a principal component of the extracellular biofilm matrix.
TABLE I.
Biochemical analysis of extracts from planktonic and biofilm bacteria.
| mg protein/109 CFU | mg nucleic acid/109CFU* | mg carbohydrate/109 CFU | |
|---|---|---|---|
| Planktonic | 0.44 ± 0.06 | 0.13 ± 0.01 | 0.28 ± 0.09 |
| Biofilm | 0.21 ± 0.14 | 0.14 ± 0.02 | 1.23 ± 0.5† |
Nucleic acid concentrations in mg nucleic acid/10 CFU were calculated using the equation OD260Xdilution factorX0.05.
p<0.05 by Student’s t-test.
To further characterize the carbohydrate component of the biofilm matrix, we blotted extracts and probed them with various lectins. Glycine Max, which binds to N-acetylgalactosamine bound weakly to extracts from biofilms grown in CDM but not to planktonic extracts or extracts from biofilms grown in BHI (Fig 3B). Concanavalin A, which binds to α-mannose and α-glucose, did not bind to extracts from biofilms or planktonic bacteria (Fig. 3C). Wheat germ agglutinin, which binds to N-acetylglucosamine, reacted with all extracts, but biofilm bacteria grown in BHI contained approximately 25-fold more of the material that reacted with the lectin in comparison to planktonic bacteria grown in BHI (Fig. 3A) suggesting that N-acetylglucosamine is an important component of the biofilm matrix.
Fig. 3. Biofilm extracts react with Wheat germ agglutinin (WGA) but not with Concanavalin A (ConA) or Glycine max (Gly).
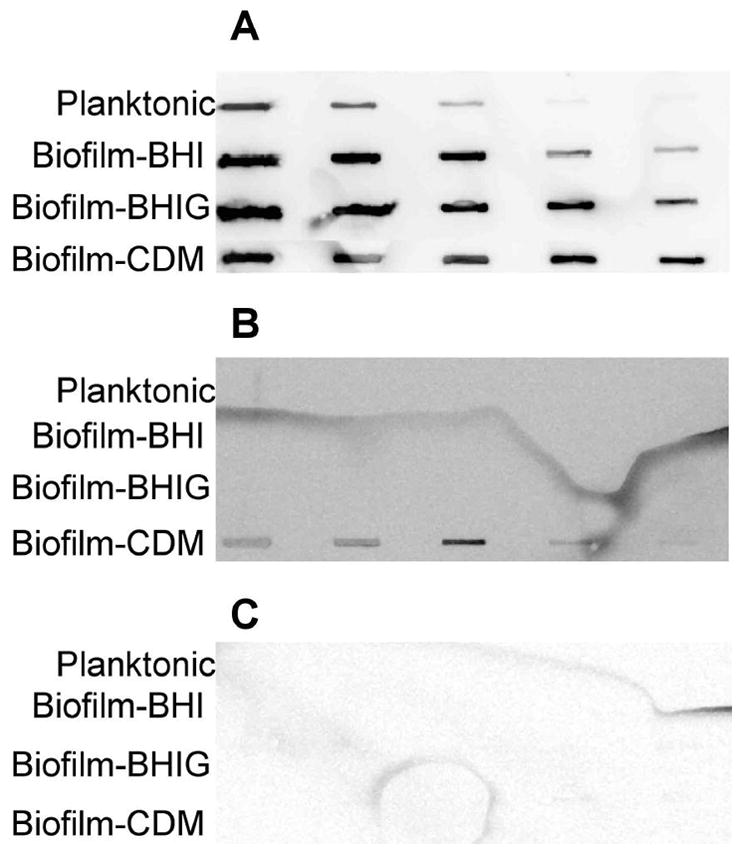
Serial 5-fold dilutions of extracts from biofilms grown in BHI, BHIG, or CDM and planktonic cultures grown in BHI or CDM were blotted onto nitrocellulose and probed with peroxidase-conjugated lectins. Panel A was probed with WGA-HRP, Panel B was probed with Gly-HRP, and Panel C was probed with ConA-HRP.
Structural analysis of G. vaginalis biofilms
Confocal microscopy was used to further analyze the N-acetylglucosamine-containing polysaccharide component of G. vaginalis biofilms cultured in BHIG. Biofilm bacteria were stained with the green nucleic acid stain SYTO® 9 and Alexa Fluor® 633-conjugate of WGA. Fig. 4 shows that the biofilm was ~44μm thick and exhibited a characteristic heterogeneous biofilm structure with peaks and “valleys” and microcolonies separated by channels. WGA adhered to material around the bacteria but did not co-localize with the nucleic acid stain suggesting that, as expected, the carbohydrate was extracellular and possibly only loosely associated with the cell surface.
Dissolution of G. vaginalis biofilms
We hypothesized that degradation of the exo-polysaccharide would lead to dissolution of the biofilms and would restore sensitivity to the different agents. To test this, biofilms were treated with sodium metaperiodate, which hydrolyzes polysaccharides, and chitinase, which degrades polymers of N-acetyl-glucosamine. As shown in Fig. 5, chitinase reduced the biofilm thickness only very slightly, even at the highest concentration tested, and sodium metaperiodate had no effect.
Fig. 5. Treatment of biofilms with chitinase and sodium metaperiodate.

G. vaginalis strain 465-5 was grown as a biofilm and treated for 20hr at 37°C with chitinase and sodium periodate. The reagents were serially diluted 5-fold horizontally across the plate. The biofilm wells were washed and stained with safranin to show biofilm dissolution as a decrease in red color. Biofilms formed by strains 5-1 and ATCC 49145 yielded similar results.
We then tested other catabolic enzymes and compounds on mature biofilms to determine their biofilm-dispersing activities (Fig. 6). Concentrations of Proteinase K and Trypsin as low as 32 μg/ml and 6.4 μg/ml (respectively) effectively disrupted the biofilms produced by all three strains. H2O2, lactic SDS, and DNaseI, which has been shown to disrupt Pseudomonas aeruginosa biofilms19, did not have an effect.
Fig. 6. Treatment of biofilms with proteases.
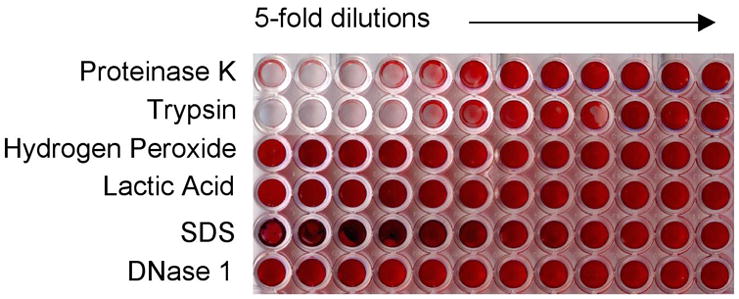
G. vaginalis strain 465-5 was grown as a biofilm and treated for 20hr at 37°C with proteinase K, trypsin, H2O2, lactic acid, SDS, or DNaseI. The reagents were serially diluted 5-fold horizontally across the plate. The biofilm wells were washed and stained with safranin to show biofilm dissolution as a decrease in red color. Biofilms formed by strains 5-1 and ATCC 49145 yielded similar results.
Effect of biofilm dissolution sensitivity
To determine whether proteolytic dissolution of biofilms would restore the MBC90 to levels associated with planktonic bacteria we pre-treated biofilms with proteinase K before adding H2O2 and lactic acid. Proteinase K alone did not affect bacterial viability; however, treatment of biofilms with the protease significantly reduced the sensitivities for H2O2 and lactic acid (Fig. 7).
Fig 7. Sensitivity of proteinase K-treated biofilms to H2O2 and lactic acid.
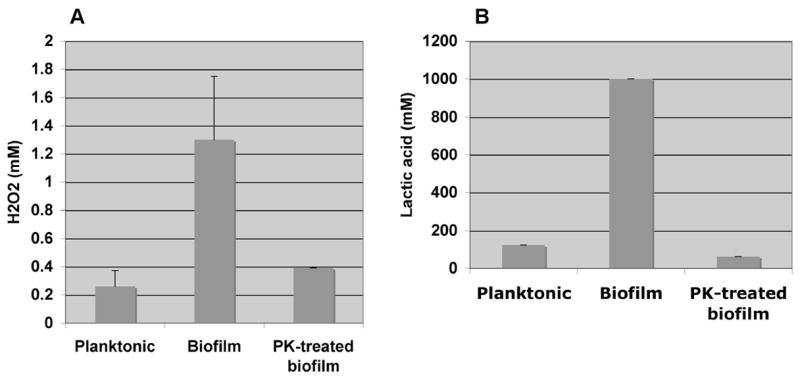
The concentrations of H2O2 (Panel A) and lactic acid (Panel B) required to decrease viability of planktonic cultures, biofilms and proteinase K-treated biofilms by at least 90% are shown. Proteinase K-treated biofilm cultures exhibited H2O2 and lactic acid sensitivities similar to that of planktonic cultures. Bars represent the averages of three separate experiments and error bars represent the standard deviations.
COMMENT
The etiology and pathogenesis of BV have remained enigmatic ever since initial reports of the condition in 195420. Confounding issues include the polymicrobial nature of BV and the role of host factors, both genetic and behavioral. The recent demonstration that BV is associated with the formation of a biofilm in which the predominant species is G. vaginalis12 is an important advance towards understanding its pathogenesis. We therefore sought to develop a simple model for studying G. vaginalis biofilms in vitro. We found that when cultured anaerobically in BHI+1% glucose, the bacteria formed a thick, tenacious biofilm on polystyrene.
Growth of G. vaginalis is normally suppressed by healthy lactobacilli. This inhibition is attributed to the production of lactic acid and H2O2. We hypothesized that the formation of a biofilm would increase the resistance of the bacteria to H2O2 and lactic acid leading to colonization even in the presence of lactobacilli. Bacterial sensitivity is normally assessed by analyzing the minimum inhibitory concentration (MIC) required to inhibit the growth of suspension of bacteria at low cell density. This type of assay can’t be used to measure biofilm sensitivities because the bacteria within biofilms are at a high cell density. We therefore measured bacterial viability of biofilms and high cell density planktonic cultures using a luciferase reagent that quantitatively measures ATP. Using this method, MBC90’s or the concentrations required to reduce viability by at least 90%, for planktonic bacteria were ~0.2mM H2O2 and ≥ 125–250mM lactic acid. The concentrations of lactic acid required to kill G. vaginalis were considerably higher than the amounts reported to be secreted by lactobacilli (~30–60mM)7, but since our assay measured bactericidal rather than bacteriostatic activity, it is possible that the concentration of lactic acid required to suppress growth is lower. Biofilm cultures were more resistant and tolerated 4–5 fold higher concentrations of H2O2 and 4–6 fold higher concentrations of lactic acid than planktonic cells. The finding that concentrations of H2O2 (0.8–1.3 mM) and lactic acid (1,000 mM) required to produce a 90% decrease in viability in biofilm bacteria were substantially greater than the MBC90’s for planktonic bacteria implies that the biofilm phenotype may contribute to vaginal colonization by G. vaginalis even in the presence of lactic acid- and H2O2-producing lactobacilli.
We hypothesized that enzymatic dissolution of biofilms would restore the sensitivity to H2O2 and lactic acid to planktonic levels. In order to find a compound to dissolve the biofilms, we preliminarily characterized the composition of the biofilm matrix. Biochemical analysis suggested that the extracellular matrix of the biofilms was predominantly composed of carbohydrate rather than protein and/or nucleic acid. Most previously characterized biofilms contain an exopolysaccharide intercellular adhesin21–24 so it is likely that the carbohydrate component we detected is a polysaccharide. Wheat germ agglutinin, a lectin from Triticum vulgaris bound to the matrix material suggesting that the matrix contains N-acetylglucosamine. We therefore tested chitinase, an enzyme that cleaves the β1-4-linkage between N-acetylglucosamine residues in chitin, for the ability to dissolve G. vaginalis biofilms. At the highest concentration tested, chitinase reduced the biofilm thickness only slightly. The extracellular polysaccharide adhesin involved in biofilm formation by a variety of unrelated bacterial species including Staphylococcus aureus and S. epidermidis, E. coli, and Actinobacillus actinomycetemcomitans is a β1-6-linked polymer of N-acetylglucosamine referred to as PGA or PNAG24–26. The β1-6-linkage of PNAG is resistant to chitinase, and it is possible that the polysaccharide matrix of G. vaginalis biofilms has a similar structure and is therefore not degraded by chitinase. We are currently conducting studies to determine if G. vaginalis elaborates a polysaccharide biochemically similar to PNAG. We also tested sodium metaperiodate, which cleaves polysaccharides by oxidation and chemical cleavage of carbon-carbon bonds for biofilm dissolving activity. Surprisingly, sodium metaperiodate did not affect the G. vaginalis biofilms. It is possible that a component the biofilms affected sodium metaperiodate activity or that other non-polysaccharide based adhesins are involved. Even though our biochemical analyses did not suggest a significant nucleic acid component to the biofilms, we tested DNaseI against G. vaginalis biofilms because it is known that P. aeruginosa biofilm matrix is composed to a large degree by nucleic acids, and that these biofilms, are dispersed by DNaseI19. However, no degradation of G. vaginalis biofilms by DNaseI was observed. Treatment of the biofilms with the proteases proteinase K and trypsin did, however, effectively remove the biofilm from the polystyrene wells. Biofilm extracts did not contain significantly more protein than planktonic bacteria suggesting that the extracellular matrix is not largely proteinaceous. It is possible however, that surface proteins are required to anchor the polysaccharide matrix to the bacteria or to somehow link the polysaccharides together. Cleavage of the protein anchors could, in this theoretical model, release the bacteria from the polysaccharide matrix and hence from the biofilm.
Dissolution of biofilms with proteinase K lowered sensitivities to both H2O2 and lactic acid. The finding that biofilm dispersal reduces sensitivity suggests that an enzyme that specifically targets the biofilm structure could be therapeutically useful. The use of probiotic therapy for BV, whereby healthy lactobacilli are re-introduced into the vagina, has been proposed, and this study suggests that G. vaginalis would be more sensitive to probiotic therapy if it were planktonic27. In addition to increasing the sensitivity of the bacteria to H2O2 and lactic acid, biofilm dissolution could increase the rate of expulsion of the bacteria from the vagina by the natural flow of mucous. Biofilm dispersal may also increase the susceptibility of the bacteria to the immune defenses. Proteinase K is a nonspecific protease that would likely damage the vaginal epithelium and would not be considered for clinical use. A more specific protease could, however, have therapeutic potential for BV.
Supplementary Material
Acknowledgments
We would like to thank Dr. Robin Ross for providing us with clinical isolates of G. vaginalis and Dr. Scott Henderson for assistance with the multi-photon scanning microscope.
Financial support: This work was supported by NIH grant R21AI61590-02 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sobel JD. What’s new in bacterial vaginosis and trichomoniasis? Infect Dis Clin North Am. 2005;19:387–406. doi: 10.1016/j.idc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Wilson J. Managing recurrent bacterial vaginosis. Sex Transm Infect. 2004;80:8–11. doi: 10.1136/sti.2002.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med. 1995;333:1737–42. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 4.Klebanoff MA, Hillier SL, Nugent RP, et al. Is bacterial vaginosis a stronger risk factor for preterm birth when it is diagnosed earlier in gestation? Am J Obstet Gynecol. 2005;192:470–7. doi: 10.1016/j.ajog.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Myer L, Denny L, Telerant R, Souza M, Wright TC, Jr, Kuhn L. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect Dis. 2005;192:1372–80. doi: 10.1086/462427. [DOI] [PubMed] [Google Scholar]
- 6.Taha TE, Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. Aids. 1998;12:1699–706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 7.McLean NW, McGroarty JA. Growth inhibition of metronidazole-susceptible and metronidazole-resistant strains of Gardnerella vaginalis by Lactobacilli in vitro. Appl Environ Microbiol. 1996;62:1089–92. doi: 10.1128/aem.62.3.1089-1092.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pybus V, Onderdonk AB. Microbial interactions in the vaginal ecosystem, with emphasis on the pathogenesis of bacterial vaginosis. Microbes Infect. 1999;1:285–92. doi: 10.1016/s1286-4579(99)80024-0. [DOI] [PubMed] [Google Scholar]
- 9.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 10.Gardner HL, Dukes CD. Haemophilus vaginalis vaginitis: a newly defined specific infection previously classified non-specific vaginitis. Am J Obstet Gynecol. 1955;69:962–76. [PubMed] [Google Scholar]
- 11.Criswell BS, Ladwig CL, Gardner HL, Dukes CD. Haemophilus vaginalis: vaginitis by inoculation from culture. Obstet Gynecol. 1969;33:195–9. [PubMed] [Google Scholar]
- 12.Swidsinski A, Mendling W, Loening-Baucke V, et al. Adherent Biofilms in Bacterial Vaginosis. Obstet Gynecol. 2005;106:1013–1023. doi: 10.1097/01.AOG.0000183594.45524.d2. [DOI] [PubMed] [Google Scholar]
- 13.Muli F, Struthers JK. Use of a continuous-culture biofilm system to study the antimicrobial susceptibilities of Gardnerella vaginalis and Lactobacillus acidophilus. Antimicrob Agents Chemother. 1998;42:1428–32. doi: 10.1128/aac.42.6.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen GD, Simpson WA, Younger JJ, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. Journal of Clinical Microbiology. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F. A colorimetric method for the determination of sugars. Nature. 1951;168:167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 16.Geshnizgani AM, Onderdonk AB. Defined medium simulating genital tract secretions for growth of vaginal microflora. J Clin Microbiol. 1992;30:1323–6. doi: 10.1128/jcm.30.5.1323-1326.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jefferson KK, Cerca N. Bacterial-bacterial cell interactions in biofilms: detection of polysaccharide intercellular adhesins by blotting and confocal microscopy. Methods Mol Biol. 2006;341:119–26. doi: 10.1385/1-59745-113-4:119. [DOI] [PubMed] [Google Scholar]
- 18.Hall-Stoodley L, Stoodley P. Developmental regulation of microbial biofilms. Curr Opin Biotechnol. 2002;13:228–33. doi: 10.1016/s0958-1669(02)00318-x. [DOI] [PubMed] [Google Scholar]
- 19.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- 20.Gardner HL, Dukes CD. New etiologic agent in nonspecific bacterial vaginitis. Science. 1954;120:853. doi: 10.1126/science.120.3125.853. [DOI] [PubMed] [Google Scholar]
- 21.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Gotz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Molecular Microbiology. 1996;20:1083–91. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan JB, Ragunath C, Velliyagounder K, Fine DH, Ramasubbu N. Enzymatic detachment of Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother. 2004;48:2633–6. doi: 10.1128/AAC.48.7.2633-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Overhage J, Schemionek M, Webb JS, Rehm BH. Expression of the psl operon in Pseudomonas aeruginosa PAO1 biofilms: PslA performs an essential function in biofilm formation. Appl Environ Microbiol. 2005;71:4407–13. doi: 10.1128/AEM.71.8.4407-4413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Preston JF, 3rd, Romeo T. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J Bacteriol. 2004;186:2724–34. doi: 10.1128/JB.186.9.2724-2734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenney D, Hubner J, Muller E, Wang Y, Goldmann DA, Pier GB. The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect Immun. 1998;66:4711–20. doi: 10.1128/iai.66.10.4711-4720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan JB, Velliyagounder K, Ragunath C, et al. Genes involved in the synthesis and degradation of matrix polysaccharide in Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae biofilms. J Bacteriol. 2004;186:8213–20. doi: 10.1128/JB.186.24.8213-8220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid G. Probiotic agents to protect the urogenital tract against infection. Am J Clin Nutr. 2001;73:437S–443S. doi: 10.1093/ajcn/73.2.437s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


