Abstract
Stem cells in various mammalian tissues retain the capacity to renew themselves and may be able to restore damaged tissue. Their existence has been proven by genetic tracer studies that demonstrate their differentiation into multiple tissue types and by their ability to self-renew through proliferation. Stem cells from the adult nervous system proliferate to form clonal floating colonies called spheres in vitro, and recent studies have demonstrated sphere formation by cells in the cochlea in addition to the vestibular system and the auditory ganglia, indicating that these tissues contain cells with stem cell properties. The presence of stem cells in the inner ear raises the hope of regeneration of mammalian inner ear cells but is difficult to correlate with the lack spontaneous regeneration seen in the inner ear after tissue damage. Loss of stem cells postnatally in the cochlea may correlate with the loss of regenerative capacity and may limit our ability to stimulate regeneration. Retention of sphere forming capacity in adult vestibular tissues suggests that the limited capacity for repair may be attributed to the continued presence of progenitor cells. Future strategies for regeneration must consider the distribution of endogenous stem cells in the inner ear and whether cells with the capacity for regeneration are retained.
Regeneration of inner ear
Potential therapeutic implications have spurred investigation of inner ear progenitor cells. Following reports that hair cells could regenerate in the sensory epithelium of birds and amphibians (Corwin et al., 1988; Ryals et al., 1988), several investigations had indicated that hair cells could regenerate in the mammalian vestibular system (Forge et al., 1993; Montcouquiol et al., 2001; Warchol et al., 1993). In the vestibular organs, cells that retain the capacity to divide and differentiate into more specialized cells appear to persist in the adult. However, several investigators have hypothesized that the complex epithelial organization of the organ of Corti only can occur after terminal differentiation and exit of cochlear cells from mitosis (Corwin et al., 1997; Kelley et al., 1995).
In fact, cell division in supporting cells of vestibular organs has been reported (Warchol et al., 1993), but cochlear supporting cells had an extremely limited ability to regenerate hair cells or to undergo cell division in early postnatal mice after lesioning (Kelley et al., 1995). The capacity for supporting cell division in mammalian vestibular organs can be enhanced by treatments with mitogenic growth factors, which are most effective in epithelia from neonates (Lambert, 1994). The differences in response to mitogens between mammals and birds is consistent with differences in the ability to regenerate hair cells throughout life (Montcouquiol et al., 2001). The basis for the reoccurrence of hair cells, albeit limited, in the damaged utricle seems to be cell proliferation, which is most readily observed after treatment with mitogenic growth factors. This regenerative ability has been proposed to be due to stem cells that reside in the sensory epithelium of the utricular macula (Li et al, 2003).
Recent studies have shown that adult vestibular stem cells are pluripotent: they differentiate into cell types corresponding to all three germ layers, endoderm, mesoderm, and ectoderm, and this includes neurons and hair cells in vitro (Li et al., 2003). This indicates that these endogenous cells are true stem cells. More recent data indicates that stem cells can be isolated from the neonatal cochlea as well (Oshima et al., 2006). The existence of stem cells in the mammalian cochlea opens the possibility that a damaged cochlea could be repaired by proliferation and differentiation of endogenous cells. However, despite the pluripotent differentiation potential of these cells, recovery doesn’t occur to any significant extent after damage to hair cells or neurons in the adult mammalian cochlea raising the possibility that cochlear stem cells disappear after birth or that they lose their stemness (i.e. the capacity to proliferate). The questions that remain are whether there are cells in vivo that might be available for replacement of hair cells and what mechanism could lead to proliferation and replacement of these cells. Recent reports on overexpression of a number of genes have provided some hope that adult cochlear cells may have an inherent capacity for regeneration when stimulated by the appropriate signals (Izumikawa et al., 2005), or that persistent stem cells or cells with stem cell-like properties might be directed toward a hair cell phenotype by gene expression in the appropriate spatial and temporal sequence. A new therapeutic approach would be possible if we could harness the pluripotency of potentially dormant progenitor cells to permit regeneration of hair cells in a damaged sensory epithelium or to generate neurons in situ that can reinnervate hair cells (Li et al., 2004).
Both neurons and hair cells are usually involved in the pathogenesis of hearing loss. A variety of environmental and genetic causes lead to hair cell loss. Spiral ganglion neurons undergo secondary degeneration in regions of hair cell loss in many types of sensorineural hearing loss (Liberman et al., 1978) and most hearing loss is permanent. Replacement of these cells will be a vital step in the treatment of inner ear lesions in which spared cells cannot supplant the function of lost cells. To replace lost cells two possible approaches can be envisaged: stem cell transplantation and stimulation of self-repair. For the latter approach endogenous stem cells would undergo proliferation and be converted into new hair cells. The demonstration that inner ear progenitor cells could differentiate into hair cells in vitro raises the possibility that they could regenerate in the inner ear. Here, we concentrate on mechanisms that could take advantage of endogenous cells for repair of the inner ear.
Adult tissue stem cells
The regeneration capacity of a tissue is determined in part by whether they contain endogenous stem cells. Some tissues that have a high turnover rate are thought to replace senescent cells by proliferation and differentiation of tissue stem cells. These tissues have stem cells that constantly replace lost cells in the adult, and their designation as adult tissue stem cells is based on their continued ability to self-renew and differentiate. Adult stem cells can only proliferate for a limited number of generations, and their response to differentiation signals declines after each generation. The failure of cells to regenerate could also be due to a tissue environment that either does not support or prevents regeneration (Horner et al., 2002). Tissues with high turnover as well as the capacity for regeneration, however, do not necessarily have stem cells that account for regeneration. In the liver, where oval cells have been shown to have progenitor cell properties, the regrowth of damaged tissue has been attributed largely to the ability of even fully differentiated cells to undergo mitosis with appropriate signaling (Fausto, 2004). The issue of whether an adult organ contains stem cells has been easier to settle in tissues with high turnover rates such as blood, skin and intestine (Alonso et al., 2003; Weissman, 2000) but more difficult to decide conclusively for liver, heart, pancreas, and the central and peripheral nervous systems (Bixby et al., 2002; Bonner-Weir et al., 2005; Dor et al., 2004; Fausto, 2004; Gage, 2002; Rubart et al., 2006; Urbanek et al., 2005). Both routes of replacement have been proposed in the pancreas: an absence of stem cells with only occasional cell division by existing pancreatic beta-cells (Dor et al., 2004); and replacement of lost cells from a usually quiescent progenitor cell compartment (Bonner-Weir et al., 2005).
Regrowth of fibers after tissue damage is known to occur in the peripheral nervous system. Olfactory neurons have been shown to regrow spontaneously (Pasterkamp et al., 1998), and motor neurons can regrow to their original targets (McMahan, 1990) at the motor endplate, but in these examples, the neuron survives the injury and damaged axons regrow to their targets. In contrast, replacement of lost neural cells has rarely been demonstrated. In the central nervous system, although there is little normal turnover, cells can be recruited to replace lost neurons (Gage, 2002). Neural stem cells are present in the subventricular zone of the lateral ventricles and in the dentate gyrus. These cells are capable of replacing lost neurons by cell division followed by migration and differentiation. The presence of these cells may contribute to the formation of new connections during normal activity, as well as permitting repair after neuronal injury and loss. The discovery of cells that could undergo self-renewal and cell division in the adult opened the way to understanding regeneration as well as growth of new circuitry in the adult central and peripheral nervous systems.
Thus, stem cells have been demonstrated in many adult tissues, but cell regeneration does not necessarily prove the existence of stem cells. The existence of populations of cells that proliferate and are capable of differentiation to multiple cell types can be demonstrated by cell recruitment, proliferation and differentiation in response to injury in vivo, usually by use of a genetic tracer to follow the lineage of a cell (Gage, 2002; Reynolds et al., 1992), but this can be difficult to demonstrate without the necessary tracers for a tissue specific stem cell. The existence of stem cells can now also be demonstrated by several in vitro criteria: in addition to the capacity for cell division and differentiation, these include expression of markers of stem cells, and the capacity to form neurospheres (Reynolds et al., 1992).
Progenitors for hair cells
Are hair cells capable of regeneration after damage from toxins or noise? Recovery of normal thresholds suggests that limited damage to hair cells after acoustic trauma may be repaired by the cell (Gale et al., 2002; Harding et al., 2002). However, no evidence for cell division leading to replacement of hair cells has been reported for the adult mammalian cochlea.
In addition to the vestibular system, the mammalian cochlea in neonates contains cells that have properties of stem cells based on sphere formation and self-renewal (Oshima et al., 2006) (Fig. 1). These cells differ from the normal post-mitotic cells present in the organ of Corti and vestibular system (Oshima et al., 2006). The cells form clonal spheres that can be propagated in culture and have from 1–3 stem cells per sphere. The capacity for sphere formation decreased with age: in the cochlea, sphere formation could be demonstrated in embryonic and newborn animals but few spheres were produced from the adult (Fig. 2). This is consistent with the finding that vestibular organs can regenerate some hair cells throughout life whereas the cochlea does not (Corwin et al., 1997; Kelley et al., 1995; Warchol et al., 1993).
Figure 1.
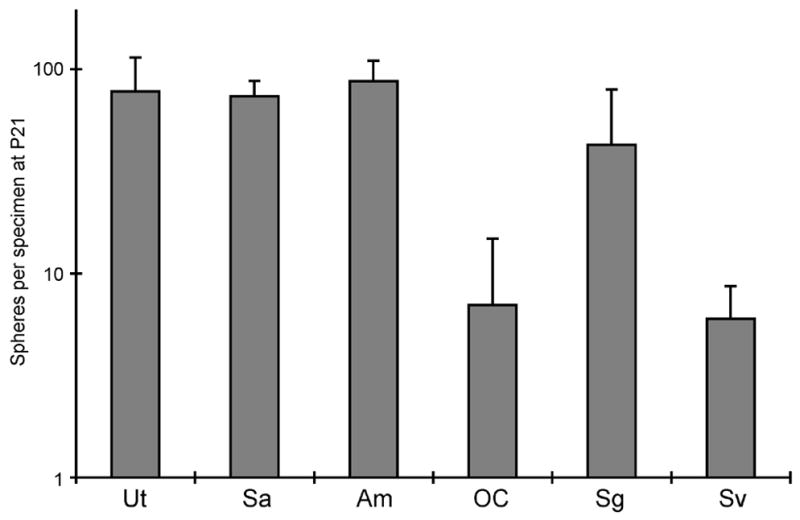
Inner ear stem cells from the vestibular and auditory systems of a P21 mouse. Spheres were generated from vestibular (Utricle, Ut; Sacculus, Sa; Ampula, Am) and cochlear (organ of Corti, oC; Spiral ganglion, Sg; Stria vascularis, Sv) organs by culture of dissociated cells for 7 days. The number of spheres was counted and expressed per single specimen (Ut, Sa, oC, Sc, Sv) or per 3 ampullae.
Figure 2.
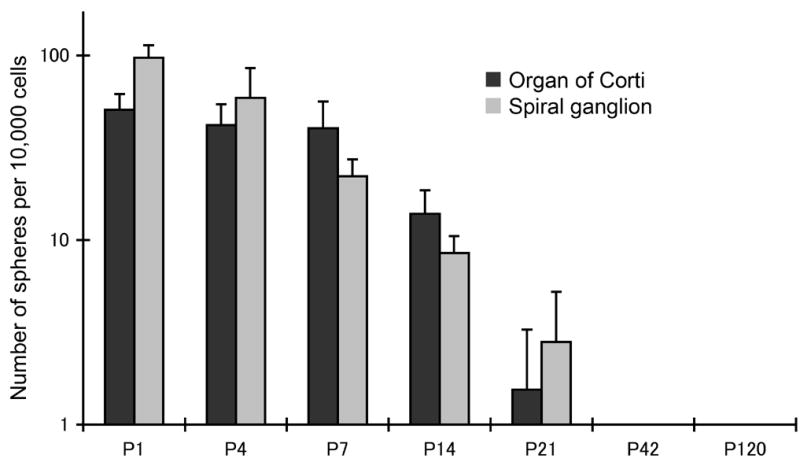
Number of spheres generated from cochlear tissues in newborn and postnatal mice. Formation of spheres from dissociated cells was quantified after 7 days in culture and was expressed as spheres per 104 cells. The generation of spheres from the organ of Corti decreased in the first few postnatal days.
Induction of differentiation of these cells resulted in the expression of markers for hair cells (Oshima et al., 2006), and these cells occurred in patches surrounded by cells with markers of supporting cells (Fig. 3).
Figure 3.
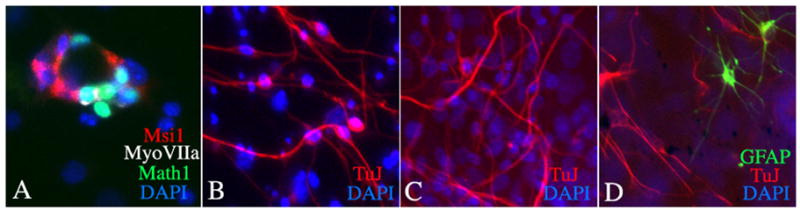
Culture of spheres leads to differentiation of inner ear cells. A. Generation of hair cell-like cells from organ of Corti spheres. Musashi 1 positive supporting cells appear to be adjacent to each cell expressing myosin VIIa and Math1 (hair cell marker positive cells) in this sphere differentiated for 2 weeks in culture. B. Differentiation of neurons from spheres obtained from spiral ganglion. The appearance of cells that were positive for β-III tubulin (stained with antibody TuJ) and had a neuronal morphology is shown after 1 week in culture. C. Neuronal differentiation from spheres derived from utricle. Neurons obtained from utricular spheres were β-III tubulin positive. D. Utricular spheres contain stem cells that differentiate to become β-III tubulin-positive neurons and GFAP-positive glia.
Where are the precursor cells that give rise to spheres in vitro? Several lines of evidence suggest that progenitor cells may be in the greater epithelial ridge in the newborn. GFP expressed under the control of the nestin promoter is found in the GER (Lopez et al., 2004) as well as in border cells, phalangeal cells and Deiters cells. Nestin was found in supporting cells in mice, although the expression level was lower at later time points and only a few Deiters cells were positive at P60. Could supporting cells be precursors? Supporting cells have the capacity to develop hair cell characteristics upon viral transduction with Atoh1 (Izumikawa et al., 2005; Zheng et al., 2000). The recent finding that supporting cells from the cochlea have the capacity to divide and differentiate into hair cells in vitro (White et al., 2006) further suggests that they could play a role in regeneration, but their ability to differentiate into other cells types in the cochlea was limited to newborn mice and did not occur in 2 week old mice. This is consistent with our data showing that stem cell numbers decrease in the first few postnatal weeks.
Progenitor cells in spiral ganglion
Degeneration of the spiral ganglion cell and its processes may occur as a secondary or a primary event (Zimmermann et al., 1995). Secondary cochlear neuronal degeneration is thought to follow a variety of insults to the cochlea. Primary cochlear neuronal degeneration has been described in a variety of pathologies that cause direct damage to neurons (Starr et al., 1996; Varga et al., 2003), and can occur in mice exposed to sound pressure levels that do not cause hair cell loss (Kujawa et al., 2006).
Spiral ganglion neurons have been reported to regrow fibers to varying extent after damage in different animal models. Several studies have suggested that the endings regrow after damage by glutamate toxicity in guinea pigs (Puel et al., 1997; Sekiya et al., 2003). Experimental sectioning of the auditory nerve in mice leads to extensive regrowth of fibers into the cochlea (Sugawara et al., 2005). However, in humans, the extent of regrowth is not sufficient to be clinically significant (Nadol, 1997).
Do endogenous cells undergo cell division in the spiral ganglion? The formation of spheres from spiral ganglion of mice in the early postnatal period suggests the possibility that the ganglion may have cells with stem cell properties (Oshima et al., 2006). These cells proliferate in spheres and have the capacity to differentiate into neurons in vitro (Fig. 3). In addition we find neurons after a similar differentiation protocol with spheres prepared from the organ of Corti or from the vestibular sensory epithelia (Fig. 3). These stem cells can give rise to hair cells and neurons and glia (Fig. 3) and, thus, may be similar in their capacity for differentiation to endogenous otocyst progenitors that appear to give rise to supporting cells, hair cells and neurons in the embryo. Others have demonstrated the presence of neural progenitor cells in the adult human auditory nerve, with the expression of nestin-positive neural progenitors that divide and express markers found in inner ear sensory neurons like TrkB and TrkC (Rask-Andersen et al., 2005).
Replacement of spiral ganglion neurons and reformation of neural connections to hair cells has been demonstrated using tissue from newborn animals as well as embryonic stem cells as sources of neurons for transplantation into in vitro (Martinez-Monedero et al., 2006) and in vivo models (Corrales et al., 2006).
Acknowledgments
Supported by grants F33 DC006789, RO1 DC007174, and DC006167 as well as P30 DC05209 from the National Institute on Deafness and other Communicative Disorders (NIDCD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17:1189–200. doi: 10.1101/gad.1086903. [DOI] [PubMed] [Google Scholar]
- Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002;35:643–56. doi: 10.1016/s0896-6273(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23:857–61. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- Corrales CE, Pan L, Li H, Liberman MC, Heller S, Edge AS. Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: Growth of processes into the organ of corti. J Neurobiol. 2006;66:1489–500. doi: 10.1002/neu.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–4. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Oberholtzer JC. Fish n' chicks: model recipes for hair-cell regeneration? Neuron. 1997;19:951–4. doi: 10.1016/s0896-6273(00)80386-4. [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–6. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–87. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259:1616–9. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–3. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JE, Meyers JR, Periasamy A, Corwin JT. Survival of bundleless hair cells and subsequent bundle replacement in the bullfrog's saccule. J Neurobiol. 2002;50:81–92. doi: 10.1002/neu.10002. [DOI] [PubMed] [Google Scholar]
- Harding GW, Bohne BA, Ahmad M. DPOAE level shifts and ABR threshold shifts compared to detailed analysis of histopathological damage from noise. Hear Res. 2002;174:158–71. doi: 10.1016/s0378-5955(02)00653-6. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Thallmair M, Gage FH. Defining the NG2-expressing cell of the adult CNS. J Neurocytol. 2002;31:469–80. doi: 10.1023/a:1025739630398. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–6. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Kelley MW, Talreja DR, Corwin JT. Replacement of hair cells after laser microbeam irradiation in cultured organs of corti from embryonic and neonatal mice. J Neurosci. 1995;15:3013–26. doi: 10.1523/JNEUROSCI.15-04-03013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26:2115–23. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert PR. Inner ear hair cell regeneration in a mammal: identification of a triggering factor. Laryngoscope. 1994;104:701–18. doi: 10.1288/00005537-199406000-00010. [DOI] [PubMed] [Google Scholar]
- Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9:1293–9. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- Li H, Corrales CE, Edge A, Heller S. Stem cells as therapy for hearing loss. Trends Mol Med. 2004;10:309–15. doi: 10.1016/j.molmed.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Kiang NY. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta Otolaryngol Suppl. 1978;358:1–63. [PubMed] [Google Scholar]
- Lopez IA, Zhao PM, Yamaguchi M, de Vellis J, Espinosa-Jeffrey A. Stem/progenitor cells in the postnatal inner ear of the GFP-nestin transgenic mouse. Int J Dev Neurosci. 2004;22:205–13. doi: 10.1016/j.ijdevneu.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Martinez-Monedero R, Corrales CE, Cuajungco MP, Heller S, Edge AS. Reinnervation of hair cells by auditory neurons after selective removal of spiral ganglion neurons. J Neurobiol. 2006;66:319–31. doi: 10.1002/neu.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan UJ. The agrin hypothesis. Cold Spring Harb Symp Quant Biol. 1990;55:407–18. doi: 10.1101/sqb.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Corwin JT. Brief treatments with forskolin enhance s-phase entry in balance epithelia from the ears of rats. J Neurosci. 2001;21:974–82. doi: 10.1523/JNEUROSCI.21-03-00974.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB., Jr Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg. 1997;117:220–8. doi: 10.1016/s0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- Oshima K, Grimm C, Corrales CE, Senn P, Martinez-Monedero R, Geleoc GSG, Edge A, Holt JR, Heller S. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. 2006 doi: 10.1007/s10162-006-0058-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, De Winter F, Holtmaat AJ, Verhaagen J. Evidence for a role of the chemorepellent semaphorin III and its receptor neuropilin-1 in the regeneration of primary olfactory axons. J Neurosci. 1998;18:9962–76. doi: 10.1523/JNEUROSCI.18-23-09962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel JL, d'Aldin C, Ruel J, Ladrech S, Pujol R. Synaptic repair mechanisms responsible for functional recovery in various cochlear pathologies. Acta Otolaryngol. 1997;117:214–8. doi: 10.3109/00016489709117773. [DOI] [PubMed] [Google Scholar]
- Rask-Andersen H, Bostrom M, Gerdin B, Kinnefors A, Nyberg G, Engstrand T, Miller JM, Lindholm D. Regeneration of human auditory nerve. In vitro/in video demonstration of neural progenitor cells in adult human and guinea pig spiral ganglion. Hear Res. 2005;203:180–91. doi: 10.1016/j.heares.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Rubart M, Field LJ. Cardiac regeneration: repopulating the heart. Annu Rev Physiol. 2006;68:29–49. doi: 10.1146/annurev.physiol.68.040104.124530. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–6. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Sekiya T, Shimamura N, Yagihashi A, Suzuki S. Effect of topically applied basic fibroblast growth factor on injured cochlear nerve. Neurosurgery. 2003;52:900–7. doi: 10.1227/01.neu.0000053509.98561.16. discussion 907. [DOI] [PubMed] [Google Scholar]
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996;119 ( Pt 3):741–53. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. J Assoc Res Otolaryngol. 2005;6:136–47. doi: 10.1007/s10162-004-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U S A. 2005;102:8692–7. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga R, Kelley PM, Keats BJ, Starr A, Leal SM, Cohn E, Kimberling WJ. Non-syndromic recessive auditory neuropathy is the result of mutations in the otoferlin (OTOF) gene. J Med Genet. 2003;40:45–50. doi: 10.1136/jmg.40.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993;259:1619–22. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–68. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–7. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–6. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zimmermann CE, Burgess BJ, Nadol JB., Jr Patterns of degeneration in the human cochlear nerve. Hear Res. 1995;90:192–201. doi: 10.1016/0378-5955(95)00165-1. [DOI] [PubMed] [Google Scholar]


