Abstract
Circadian clocks are intracellular molecular mechanisms designed to allow the cell, organ, and organism to prepare for an anticipated stimulus prior to its onset. In order for circadian clocks to maintain their selective advantage, they must be entrained to the environment. Light, sound, temperature, physical activity (including sleep/wake transitions), and food intake are among the strongest environmental factors influencing mammalian circadian clocks. Normal circadian rhythmicities in these environmental factors have become severely disrupted in our modern day society, concomitant with increased incidence of type 2 diabetes mellitus, obesity, and cardiovascular disease. Here, we review our current knowledge regarding the roles of peripheral circadian clocks, concentrating on those found within tissues directly involved in metabolic homeostasis and cardiovascular function. We propose that both inter- and intra- organ dyssynchronization, through alteration/impairment of peripheral circadian clocks, accelerates the development of cardiovascular disease risk factors associated with cardiometabolic syndrome.
Introduction
Organisms on earth have evolved multiple mechanisms that promote synchronization with the environment. One of these is the circadian clock, an intracellular molecular mechanism that allows the organism to prepare for an environmental event/stimulus prior to its onset.1 Circadian clocks have been found in almost all organisms, tissues, and cells investigated to date (with a few notable exceptions). Circadian rhythmicities in many of the dominant selective pressures that have influenced the evolution of humans (as well as other forms of terrestrial life), such as light, temperature, sound, physical activity, and food intake, have become disrupted in our modern day society, in which work, eating, exercise, and virtually any other activity can be performed independent of the time of day. Simultaneous with the loss of synchronization of humans with their environment has been a marked rise in so-called modern day epidemics, such as obesity, type 2 diabetes mellitus, and cardiovascular disease. Many of these diseases are associated with impaired circadian rhythmicities at multiple levels, ranging from the whole organism (e.g., behavior) to the individual cell (e.g., transcription). The purpose of the present article is to review our current knowledge of the roles of mammalian circadian clocks (focusing primarily on those within the cardiovascular system), and discuss the contribution of dyssynchronization of an organism with its environment, through circadian clock derangements, toward the development of cardiovascular disease.
Central versus Peripheral Circadian Clocks
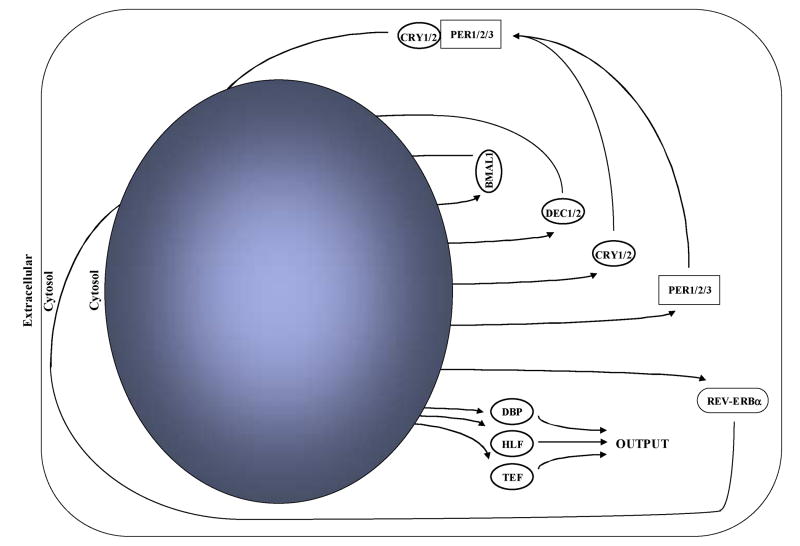
Circadian clocks can be defined as a set of proteins that generate self-sustained positive and negative transcriptional feedback loops with a free-running period of approximately 24 hours.1 The mammalian circadian clock is comprised of at least 10 core circadian clock proteins, as illustrated in Figure 1. At the heart of the circadian clock mechanism are the basic helix-loop-helix PER-ARNT-SIM transcription factors CLOCK (circadian locomoter output cycles kaput) and BMAL1 (brain- and muscle- ANRT-like protein 1). Upon heterodimerization, CLOCK and BMAL1 bind to E-boxes in the promoter of various target genes, including those encoding for negative (e.g., per1, per2, cry1, cry2, rev-erbα) and positive (e.g., bmal1) loop components. Similarly, the CLOCK/BMAL1 heterodimer induces other target genes in addition to the core clock components. These genes, known as clock-controlled or output genes (e.g., the PAR [rich in proline and acidic amino acid residues] transcription factors dbp, hlf, tef), may ultimately influence cellular and organ function over the course of the day. Furthermore, additional components of the circadian clock (e.g., REV-ERBα) influence the expression of clock output genes. For a more detailed overview of the molecular machinery of the mammalian circadian clock, please see other recent reviews.2-4
Figure 1. The intrinsic mammalian circadian clock.
Upon heterodimerization, CLOCK and BMAL1 induce the expression of various target genes, including components of negative (e.g., per1, per2, per3, cry1, cry2, dec1, dec2, rev-erbα) and positive (e.g., bmal1) feedback loops. Furthermore, the CLOCK:BMAL1 heterodimer can induce clock output genes, such as the family of PAR transcription factors (dbp, hlf, tef). The latter have the potential to influence multiple aspects of cellular function.
Mammalian circadian clocks can be divided into two major categories, namely central and peripheral circadian clocks.5-7 The central circadian clock is located within the suprachiasmatic nucleus (SCN), a collection of approximately 20,000 neurons found within the hypothalamus.8 In contrast, peripheral circadian clocks are those clocks found within non-SCN cells, including other regions of the central nervous system. To date, peripheral circadian clocks have been identified within all mammalians cells investigated, with the notable exception of the testis.9
The central clock, often referred to as the master clock, both directly and indirectly influences a number of facets of mammalian circadian biology. Surgical ablation of the SCN in rodents results in loss of circadian oscillations at multiple levels. This includes changes in behavior (e.g., locomotion, feeding, drinking), core body temperature, autocrine, paracrine and endocrine function, autonomic and sympathetic activity, intermediary metabolism, translation, and transcription.10 Several of these outcomes following SCN-ablation are the result of subsequent dyssynchrony of peripheral circadian clocks. The realization that peripheral circadian clocks directly modulate cellular and organ function over the course of the day has received increasing interest over the past several decades. For example, the circadian clock within the pancreatic β-cell directly regulates insulin gene and protein expression, likely in anticipation of increased requirement for insulin secretion upon feeding.11 Additionally, we have shown that the circadian clock within the cardiomyocyte directly regulates myocardial metabolic gene expression, likely in anticipation of sleep/wake and feeding/fasting cycles.12
SCN-ablation studies expose a hierarchy of mammalian circadian clocks, wherein loss of the central clock results in dyssynchrony of peripheral circadian clocks.6 The central circadian clock is entrained (reset) by light. Light-stimulation of the chromophore melanopsin within specialized ganglia of the retina, results in a direct transmission of this environmental signal to the SCN through the retino-hypothalamic tract.13 Substantial evidence suggests that the SCN subsequently entrains peripheral circadian clocks by influencing the levels of various neurohumoral factors. As such, central and peripheral circadian clocks can become dyssynchronized through manipulations that modulate neurohumoral factor oscillations. For example, restriction of feeding in rats to the light phase (a time at which these nocturnal mammals normally exhibit reduced food intake) results in a phase shifting of the peripheral, but not the central, circadian clocks.14 For this reason, it has been suggested that the timing of feeding (which is controlled, in part, by the SCN) is one of the strongest environmental factors influencing peripheral circadian clocks.
Various neurohumoral factors have been reported to entrain peripheral circadian clocks. These factors, which are termed zeitgebers (or time-keepers), include glucocorticoids, prostaglandins, epinephrine, norepinephrine, glucose, angiotensin II, and retinoic acid.15-21 Recent studies suggest that zeitgebers likely act in a cell type–specific manner. Evidence for this concept includes the observation that resynchronization of peripheral circadian clocks following manipulation of the light/dark cycle in mice occurs in a tissue-specific manner.22 Parabiosis experiments linking SCN-lesioned mice with normal littermates results in re-synchronization of some (e.g. liver), but not all (e.g. heart), peripheral circadian clocks.23 These data raise the possibility that inter-organ dyssynchronization of circadian clocks may occur during certain physiological and pathophysiological states. Furthermore, organs are comprised of multiple cell types (e.g. cardiomyocytes, vascular smooth muscle cells (VSMCs), endothelial cells, and fibroblasts, in the case of the heart), each of which have the potential to respond to neurohumoral factors in a cell type–specific manner, raising the possibility of intra-organ dyssynchrony.
Output from the circadian clock initially manifests at the level of transcription. As such, many studies have focused attention toward identification of those genes directly regulated by the circadian clock. Subsequent translation of mRNA to protein ultimately results in altered cellular and organ function. A primary role for the circadian clock is to allow the cell to anticipate extracellular stimuli prior to their onset. This raises the possibility that the circadian clock may prepare the cell for a stimulus that does not occur under a given set of conditions. The latter may be true more so for the laboratory rodent, that is housed under optimal conditions, with constant access to food, and lack of a predation threat. As is the case with zeitgebers, circadian clock output undoubtedly exhibits cell type–specificity. For example, the circadian clock influences insulin gene expression only within pancreatic β-cells.11
Two major environmental influences for mammals are sleep/wake and feeding/fasting cycles. Rapid responsiveness of the heart during periods of increased demand ultimately improves viability. For example, upon awakening, an adequate increase in cardiac output is required, supporting increased locomotor activity as the organism forages for food, and evades predation. Increased energy demand must be matched by increased intermediary metabolism, thereby maintaining intracellular adenosine triphosphate (ATP) levels for continued actin-myosin cross-bridge cycling and ion homeostasis. In contrast to sleep/wake cycles that generally follow a robust circadian pattern in mammals under normal conditions, feeding/fasting cycle transitions depend upon the success of foraging. For organisms in the wild, the sleep-phase fast may be prolonged for hours, days, weeks, or even months, depending upon food availability. Conversely, once an organism is successful in its forage for food, excess nutrients must be rapidly and appropriately utilized/stored. It would, therefore, be a selective advantage for the organism to simultaneously anticipate not only prolongation of the sleep-phase fast, but also increased nutrient availability upon successful foraging.
Circadian Clocks within the Cardiovascular System
Circadian clocks have been reported for all cardiovascular system components investigated to date. Numerous investigators have reported marked oscillations in the expression of circadian clock genes for hearts and blood vessels (e.g., aorta) isolated from rodents at different times of the day.3,17,19,20,23-33 Furthermore, these oscillations persist in isolated cardiomyocytes and VSMCs in culture, exposing the intrinsic nature of this cell-autonomous molecular mechanism.17,19,20 More recently, rhythmicity in circadian clock gene expression has been reported for vascular endothelial cells.34 How these circadian clocks are regulated by environmental factors, and which processes are directly influenced by these molecular mechanisms, are questions being actively investigated by a number of research groups.
Resetting Circadian Clocks within Cardiovascular Components
Given the importance of not only synchronizing an organism with its environment, but also inter- and intra- organ synchronization, it is of great importance to understand which zeitgebers affect the timing of specific peripheral circadian clocks. Few studies have been published to date, investigating zeitgebers specifically for circadian clocks within different cell types of the cardiovascular system. Of these, two zeitgebers have been reported for VSMCs, and only one for cardiomyocytes. McNamara et al. have shown that retinoic acid influences the timing of the circadian clock within VSMCs, potentially through a direct interaction of the retinoic acid receptor (RAR) with the CLOCK:BMAL1 heterodimer.20 Similarly, Nonaka et al. have shown that angiotensin II acts as a zeitgeber for the circadian clock within VSMCs, through an angiotensin II type 1 receptor-dependent mechanism.19 We have reported that norephinephrine acts as a zeitgeber for the circadian clock within the cardiomyocyte, suggesting that sympathetic stimulation may influence the timing of this peripheral circadian clock.17
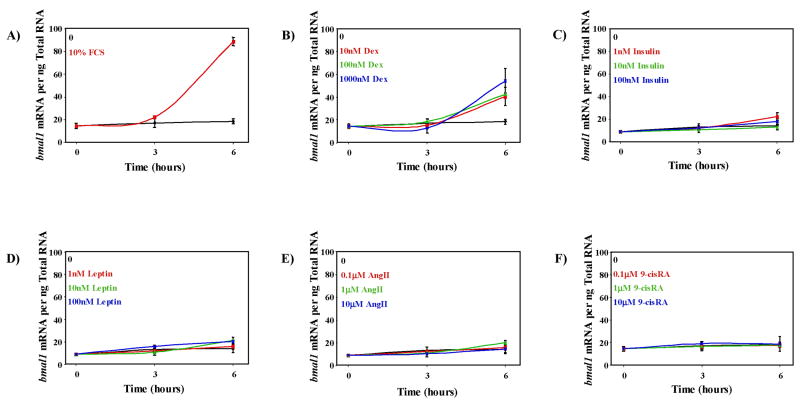
Given the relatively limited information available regarding the identities of zeitgebers for components of the cardiovascular system, we recently screened a number of neurohumoral factors as potential zeitgbers for the circadian clock within the cardiomyocyte. Among several factors examined, only the synthetic glucocorticoid dexamethasone induced the circadian clock genes bmal1 (Figure 2) and per2 (Young et al., unpublished observations), suggesting that cortisol is a strong zeitgeber for the circadian clock within the cardiomyocyte, as observed in other cell types.15 In contrast, neither leptin nor insulin influenced bmal1 gene expression in adult rat cardiomyocytes (ARCs) (Figure 2). Similarly, angiotensin II and retinoic acid did not influence expression of bmal1 in isolated cardiomyocytes (Figure 2), at concentrations in which they affect the circadian clock in VSMCs.19,20 Thus, cell type–specific zeitgebers likely exist within the cardiovascular system. Alterations of these (or other) zeitgebers during physiological and pathophysiological states, may, therefore, result in dyssychronization of circadian clocks within different components of the cardiovascular system.
Figure 2. Screening of potential zeitgbers for the circadian clock within adult rat cardiomyocytes (ARCs).
Following isolation, ARCs were plated to laminin-coated plates and cultured overnight in serum-free DMEM, as described previously.12 ARCs were next challenged with fetal calf serum (FCS; 10%), dexamethasone (Dex; 10, 100, or 1000 nM), insulin (1, 10, or 100 nM), leptin (1, 10, or 100 mM), angiotensin II (AngII; 0.1, 1, or 10 μM), or 9-cis retinoic acid (9-cisRA; 0.1, 1, or 10 μM). ARCs were terminated after either 3 or 6 hours of challenge, after which bmal1 expression was measured by real-time RT-PCR, as described previously.12 Control ARCs were cultured in DMEM supplemented with the test agent’s vehicle only. ARCs cultured in the presence of FCS served as positive controls. FCS and dexamethasone significantly (p<0.05) increased bmal1 expression at all concentrations investigated.
Identification of Circadian Clock-Regulated Processes
Given that the circadian clock is a transcriptionally based mechanism, output initially manifests at the level of altered transcription. As such, identification of those genes directly regulated by the circadian clock within a specific cell type will undoubtedly improve our understanding of those processes that are governed by this molecular mechanism. Several notable studies have investigated circadian rhythmicity of gene expression in two major components of the cardiovascular system (namely the heart and aorta) on a genome wide scale, through the use of microarrray analysis. Martino et al. reported that at least 13% of all genes expressed in the mouse heart exhibit significant circadian rhythmicity.26 Furthermore, these genes could be grouped into distinct categories, according to biological function and phase of oscillations. Synchronized oscillations were observed for genes encoding for proteins involved in cell signaling, protein synthesis, transcriptional regulation, and mitochondrial function.26 Similar results were reported by Storch et al., who investigated circadian oscillations of myocardial genes for mice maintained in dim light, suggesting that a large proportion of oscillating myocardial genes do so independent of light/dark cycles.35 More recently, Rudic et al. reported that 330 genes exhibit significant circadian oscillations in the mouse aorta, many of which were involved in the regulation of metabolism and vascular integrity.36
Circadian oscillations in gene expression could be due to either intracellular (i.e., the circadian clock) or extracellular (i.e., neurohumoral factors) influences.3 The microarray studies, described above, for intact hearts and aortas isolated from normal animals at different times of the day cannot differentiate between the potential contribution of these influences. Through the use of tissue and/or cell culture studies, the potential contribution of neurohumoral factors can be removed. Persistence of gene expression oscillations in vitro strongly suggests direct regulation by the intracellular circadian clock. We have demonstrated that the metabolic genes pyruvate dehydrogenase kinase 4 (pdk4) and uncoupling protein 3 (ucp3) exhibit marked circadian oscillations both in the intact rat heart, as well as ARCs, suggesting direct regulation of these genes by the circadian clock within the cardiomyocyte.17,37,38 A second potential strategy for dissecting intracellular versus extracellular influences is through alteration, impairment, and/or loss of circadian clock function in an in vivo setting. Critical early studies have utilized primarily light/dark cycle manipulations and/or genetically modified rodent models wherein circadian clocks are ubiquitously affected throughout the organism. Given that circadian clocks influence a plethora of biological processes, including behavioral and endocrine functions, studies involving the aforementioned models (e.g., CLOCK mutant mice) undoubtedly also influence oscillations in neurohumoral factors.39 As such, investigators have begun utilizing cell type–specific circadian clock–impaired models, as a means to identify organ-specific roles for these intracellular mechanisms. For example, through the use of an osteoblast-specific PER2 null mouse (on a PER1 null background), Fu et al. have recently shown that the circadian clock directly regulates bone density.40 Similarly, we have recently generated a cardiomyocyte-specific circadian clock mutant (CCM) mouse, wherein a dominant negative CLOCK mutant protein is specifically expressed within the cardiomyocytes of the heart.12 Hearts isolated from CCM mice exhibit severe loss of oscillations in circadian clock genes, and in the metabolic genes pdk4 and ucp3, confirming that the circadian clock within the cardiomyocyte directly regulates myocardial metabolic gene expression.12 Initial microarray studies involving CCM mouse hearts suggest that as many as 1,200 myocardial genes are regulated directly by the circadian clock within the cardiomyocyte, including genes influencing metabolism, cellular signaling, ion homeostasis, and transcriptional regulation.41 Comparable models in VSMCs and endothelial cells will undoubtedly improve our understanding of the roles of the circadian clock within these critical cardiovascular components.
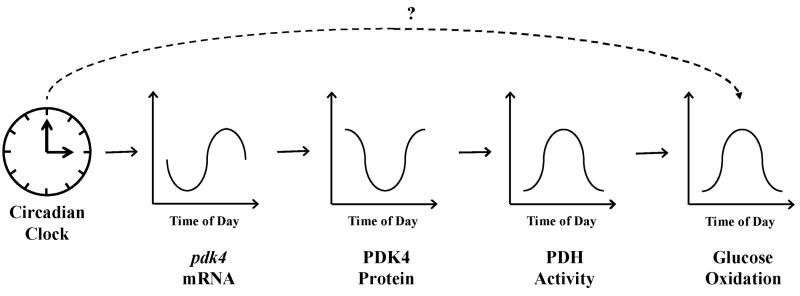
One commonality between cardiovascular function, sleep/wake cycles, feeding/fasting cycles, and the circadian clock is metabolism. For example, the circadian clock within the cardiomyocyte directly regulates myocardial metabolic gene expression. Consistent with an approximate 3-6 hour lag between pdk4 gene and PDK4 protein expression, pyruvate dehydrogenase, which is phosphorylated and inhibited by PDK4, exhibits increased activity in the rat heart at the light (less active) to dark (more active) phase transition.3 The latter correlates with increased reliance of the rat heart for carbohydrate as a fuel during the dark phase.37 This series of events, which is depicted in Figure 3, likely allows the heart to anticipate increased energy demand upon awakening. Indeed, the heart primarily matches increased energetic demand during periods of acute increased workload by increasing carbohydrate oxidation.42
Figure 3. Hypothetical mechanism by which the circadian clock within the cardiomyocyte influences myocardial metabolism over the course of the day.
Repression of pdk4 during the light phase likely promotes pyruvate dehydrogenase (PDH) activity and carbohydrate metabolism at the light-to-dark phase transition. ‘?’ represents additional unknown factors potentially linking the intramyocellular circadian clock to myocardial metabolism.
Recent studies strongly support the idea that the circadian clock within the cardiomyocyte allows the heart to anticipate feeding/fasting cycles. Through the use of both in vivo (e.g., light/dark cycle manipulations and the CCM mouse) and in vitro (e.g., isolated ARCs) models, a direct relationship between the circadian clock within the cardiomyocyte and responsiveness of the heart to prolongation of the sleep-phase fast was established.12 The rodent heart exhibits greater responsiveness to fatty acids (in terms of fatty acid oxidation gene induction) during the dark phase, a property that is mediated by the circadian clock within the cardiomyocyte.12,38 This enables the heart to adequately utilize fatty acids when the sleep-phase fast is prolonged. In addition, we find that diacylglycerol acyltransferase 2 (DGAT2; a critical enzyme promoting triglyceride synthesis) and adiponutrin (ADPN; a triglyceride lipase) are directly regulated by the circadian clock within the cardiomyocyte.12,43,44 Impairment of the circadian clock within the cardiomyocyte (i.e., CCM mice) results in chronic elevation of myocardial adpn expression, chronic repression of dgat2 expression, and prevention of fasting-induced myocardial triglyceride synthesis (despite increased circulating fatty acid levels).12 Taken together, these data expose a role for the circadian clock within the cardiomyocyte in allowing rapid adaptation of the heart to prolongation of fasting.
Relatively little is known regarding the identity of those genes (and, therefore, those processes) regulated by circadian clocks within non-cardiomyocytes of the cardiovascular system. Maemura et al. provide strong evidence in support of the hypothesis that the thrombolytic factor plasminogen-activated inhibitor-1 (PAI-1) is regulated directly by the circadian clock within vascular endothelial cells.45 Cycle-like factor 1 (CLIF1; also known as BMAL2) is a BMAL1 homologue that is highly expressed in endothelial cells. Heterodimerization of CLIF1 with CLOCK results in induction of pai-1 expression, exposing pai-1 as a direct clock output gene. Given the strong circadian rhythmicity in the onset of myocardial infarctions, which correlates with circulating PAI-1 levels, Maemura et al. have proposed a direct relationship between the circadian clock within vascular endothelial cells and circadian rhythmicity in myocardial infarctions.46
Other Peripheral Circadian Clocks Potentially Influencing Cardiovascular Function
Cardiovascular disease clearly occurs as a consequence of both intra- and inter-organ dysregulation. Diabetes mellitus, obesity, hypertension, sleep apnea, and shift work are all risk factors for cardiovascular disease, which may cause, or result from, inter-organ dyssynchrony. For example, type 2 diabetes mellitus, the primary form of diabetes in humans and the greatest risk factor for the development of cardiovascular disease, results in large part to dyssnchrony between the hypothalamus, skeletal muscle, liver, pancreas, adipose tissue, and immune system. All these organs possess intracellular circadian clocks, with the potential of influencing multiple facets of cellular and organ function. Loss of synchrony between any one of these organs, either with the environment and/or other organs, could potentially lead to increased risk of cardiovascular disease. We will briefly consider the potential roles of intracellular circadian clocks within hepatocytes, skeletal myocytes, and adipocytes. In addition, given their strong association with cardiovascular physiology and pathophysiology, circadian rhythms in neurohumoral factors will be discussed.
Hepatocytes
Oscillations in circadian clock components have been characterized for rodent livers, both at the gene and protein expression levels.14,47 Like other peripheral circadian clocks, few direct circadian clock-controlled genes (in addition to the PAR transcription factors DBP, HLF, and TEF) have been identified for the liver. Those that have been identified include angiotensinogen, steroid 15α-hydroxylase, and coumarin 7-hydroxylase.48,49 As with other organs, microarray studies have exposed extensive and divergent circadian rhythmicity in gene expression for the liver.35,50 More recently, Reddy et al. have taken a proteomic-based approach, revealing that up to 20% of soluble proteins in the mouse liver are subject to circadian control.51 Many of the hepatic proteins shown to oscillate over the course of the day clustered into metabolic pathways, including those of carbohydrate metabolism. Many of these metabolic proteins exhibit increased expression during the dark phase, consistent with a role for rapid postprandial nutrient homeostasis. Microarray studies have also shown that many of the circadian oscillations in hepatic gene expression are abolished in CLOCK mutant mice.52 Whether this is due to impairment of the circadian clock within the hepatocyte or altered neurohumoral factor circadian rhythms (or both) has yet to be established.
Skeletal myocytes
As with the liver and heart, skeletal muscle exhibits marked circadian rhythmicities in metabolism. For example, skeletal muscle insulin sensitivity is higher at the beginning of the dark phase for the rat, a property that persists ex vivo.53 To date, these observations have been attributed primarily to diurnal variations in neurohumoral factors, such as cortisol and growth hormone. More recently, we have begun to investigate circadian oscillations in both circadian clock components and metabolic genes in rodent skeletal muscle.38,54 We find that similar to the heart, rodent skeletal muscle exhibits increased responsiveness to fatty acids during the active (dark) phase for the rat.38 Given that the circadian clock within the cardiomyocyte directly influences fatty acid responsiveness of the heart, it is likely that the circadian clock within skeletal myocytes mediates this property of skeletal muscle. Furthermore, it is possible that the circadian clock within skeletal myocytes mediates circadian rhythmicities in contractile performance and insulin sensitivity. The latter hypotheses are currently under investigation through the use of a skeletal myocyte-specific circadian clock mutant (SMCM) mouse recently generated in our laboratories.
Adipocytes
Excess adiposity is a major determinant of many diseases including type 2 diabetes mellitus, coronary heart disease, stroke, several types of cancer, and respiratory disorders. Through research conducted in the past two decades, we now know that adipose tissue functions as an active endocrine organ, secreting a number of adipose-specific molecules implicated in glucose homeostasis, adiposity, hypertension, and sleep behavior. We and others have now confirmed the presence of a fully functional circadian clock mechanism within adipose.55-57 In addition to clock component genes, a number of adipocyte-specific factors, many of which are known to modulate cardiovascular function, have also been demonstrated to exhibit circadian expression, including leptin (lep), acylation stimulating protein (asp), adipsin (df), resistin (rstn), adiponectin (apm1), and visfatin (pbef1).55,56 Rev-erbα, a central circadian clock component, has been shown to promote adipogenesis by facilitating gene expression of PPARγ target genes, including ap2 and c/ebpα, suggesting that the propensity to accumulate adipose varies throughout the course of a day.58 We have recently shown that the metabolic genes fatty acid transport protein 1 (fatp1), long-chain fatty acyl-CoA synthetase 1 (acsl1), and adipocyte differentiation-related protein (adrp) exhibit diurnal variations in expression with fluctuations greater than two-fold (nadir-to-zenith) within adipose.56 Future studies will undoubtedly identify adipokines and metabolic genes that are regulated directly by the circadian clock within the adipocyte. Such studies are currently being performed through the use of an adipocyte-specific circadian clock mutant (ACM) mouse recently generated in our laboratories.
Neurohumoral factors
Neurohumoral factors not only play a central role in peripheral circadian clock synchrony/dyssynchrony but are critical influences/mediators of cardiovascular physiology and pathophysiology.3 Sympathetic and autonomic nervous stimulation, cortisol, interleukin 6 (IL6), atrial natriuretic factor, vasopressin, and components of the rennin-angiotensin system all undoubtedly influence normal cardiovascular function over the course of the day.59-67 These factors, many of which are regulated by peripheral circadian clocks, have also been implicated in the onset of adverse cardiovascular events. As mentioned above, circulating levels of PAI-1, a known circadian clock–regulated thrombolytic factor, are closely correlated with the occurrence of myocardial infarctions in humans.46 Circadian rhythmicity of many of these neurohumoral factors are markedly altered in both animal models and human cardiovascular disease states, suggesting that impairment of neurohumoral circadian rhythms will not only influence synchrony of peripheral circadian clocks but also contributes to the pathogenesis of cardiovascular disease (see below).
Impairment of Circadian Clocks as an Accelerant for Cardiovascular Disease Progression
An important question that should be addressed relates to the likelihood that impairment of circadian clocks within cardiovascular components (or other peripheral tissues) contributes toward the pathogenesis of cardiovascular disease. This question can be subdivided as follows: 1) when might circadian clocks become impaired; and 2) would dyssynchrony of peripheral circadian clocks between different cell types and/or the environment adversely affect cardiovascular function? Using our current knowledge, each of these questions will be addressed in turn.
When Might Circadian Clocks Become Impaired?
The circadian clock within the heart is altered in various animal models of human disease, including hypertension, diabetes, myocardial infarction, as well as simulated shift work. For example, hypertrophy induced by pressure overload results in a marked attenuation in circadian clock output in the rat heart.31 Consistent with these observations, feeding the Dahl salt sensitive rat a high salt diet (thereby causing hypertension) attenuates oscillations in circadian clock genes in the heart.27 In contrast, hearts isolated from the spontaneously hypertensive rat (SHR; an additional genetic model of hypertension) exhibit augmented oscillations in circadian clock genes.28 Streptozotocin-induced diabetes (an animal model of type I diabetes mellitus) results in a phase shift in the circadian clock within the heart, as well as numerous other peripheral clocks.30,32 In an animal model of type 2 diabetes mellitus (the db/db mouse), oscillations in circadian clock genes were attenuated and/or phase shifted in the liver (although the heart was not investigated).68 More recently, we have found that a 30-minute occlusion of the left main coronary artery in a rat in vivo, followed by reperfusion (i.e., a model of acute myocardial infarction) results in a rapid and severe impairment of the circadian clock within the ischemic region of the left ventricle (Young et al., unpublished observations). A 12-hour phase shift in the light/dark cycle leads to re-entrainment of the circadian clock within the rat heart, which requires at least five days for completion.12 In contrast, this light/dark cycle manipulation results in re-entrainment of heart rate and blood pressure circadian rhythms within 1-2 days, in both rodents and humans.69-71 As such, the circadian clock within the heart is dyssynchronous from its environment for between 3 to 4 days following reversal of the light/dark cycle. This has significant implications for shift workers, who routinely alter their light/dark cycle, and exhibit increased risk for cardiovascular disease development.72,73
Peripheral circadian clocks are likely to be affected by additional cardiovascular disease risk factors, include obesity, aging, and certain sleep disorders (e.g., sleep apnea). Consistent with this idea, Ando et al. report attenuated oscillations in the expression of circadian clock genes in livers and adipose tissue of obese KK-A(y) mice.55 Kunieda et al. recently reported that senescence of human aortic VSMCs was also associated with attenuated circadian clock gene oscillations.74 Whether obesity or aging influence the circadian clock within the cardiomyocyte is currently unknown. Disrupted sleep patterns resulting from shift work, jet travel, or other factors (e.g., sleep apnea) represent conditions in which the normal synchrony between the light/dark phase transitions, sleeping, and eating is significantly disturbed. Multiple studies have reported altered levels of plasma lipids, altered glucose metabolism, lower levels of antioxidants, and alterations in adipokines following disrupted sleep.75-77 At the molecular level, such metabolic alterations may ultimately result directly from disruptions of the circadian clock mechanisms.
Altered sleep/wake patterns have the potential to disrupt both central and peripheral circadian clocks by at least three means: constant or arrhythmic exposure to light, altered feeding patterns, and altered patterns of physical activity. Reduction or extinction of the nighttime period through altered sleep has the potential to directly affect the resetting of the central clock, ultimately resulting in downstream disruptions of the peripheral clocks. In addition to alterations in exposure to light, recent studies suggest that one of the strongest entraining influences for peripheral tissues is the timing of feeding.14,78 Natural patterns of feeding are disrupted by alterations in sleep/wake patterns, in particular by extended periods of wakefulness. Recently, timing of exercise has been shown to produce phase shifts in normal circadian rhythms (as measured by nocturnal onset of plasma melatonin), with subjects who exercised at moderate or intense levels in the late evening/night experiencing a phase delay in nocturnal melatonin secretion the following day, while early evening exercise resulted in a phase advance of melatonin secretion.79-81 Exercise in the early morning or afternoon had no effect on normal circadian rhythms. These results suggest that vigorous work or activity in the late evening or at night has the potential to produce dyssynchrony in central and peripheral circadian clocks.
Does Circadian Clock Dyssynchrony Contribute Towards the Pathogenesis of Cardiovascular Disease?
As discussed above, all cardiovascular disease risk factors investigated to date (e.g., hypertension, diabetes, obesity, shift work, aging) result in alterations in peripheral (as well as central in certain instances) circadian clocks, including those found within cardiovascular components. These observations suggest that during disease states, circadian clocks within peripheral tissues exhibit dyssynchrony not only with the environment but also between one another. The question, therefore, arises as to whether such dyssynchrony accelerates the progression of cardiovascular disease development.
It is becoming increasingly clear that circadian clocks and metabolism directly influence one another. Circadian clocks regulate metabolism in the heart, skeletal muscle, liver, and likely adipose tissue, all of which are major organs involved in wholebody metabolic homeostasis. Of these, the circadian clock within the cardiomyocyte has been shown to be essential for responsiveness of the heart to fatty acids.12 An inability of the cell to adequately increase fatty acid utilization in the face of increased fatty acid availability results in accumulation of detrimental intracellular long-chain fatty acid (LCFA) derivatives. The latter cause contractile dysfunction of the heart through influences on ion channels, protein kinase C activity, reactive oxygen species production, ceramide generation, and induction of apoptosis.82 Accumulation of these LCFA derivatives within skeletal myocytes, hepatocytes, pancreastic β-cells, and VSMCs, is associated with insulin resistance, glucose intolerance, dyslipidemia, insulin insufficiency, and increased vascular resistance.83-85 Assuming circadian clocks within non-cardiac tissues also regulate responsiveness to fatty acids, it is easy to envisage how disruption of peripheral circadian clocks would contribute to various hallmarks of cardiometabolic syndrome, including cardiovascular disease.
CLOCK mutant mice exhibit dyssynchrony with their environment. Circadian oscillations in locomotion, feeding, and various neurohumoral factors are severely impaired in CLOCK mutant mice. Turek et al. recently suggested that these mice possess many characteristics of cardiometabolic syndrome, including dyslipidemia, and increased predisposition to the development of obesity.39 To date, cardiovascular function has not been characterized formally in these mice. Similar to CLOCK mutant mice, Ribeiro et al. have shown that simulated shift work in humans (which undoubtedly dyssychronizes peripheral circadian clocks), results in postprandial dyslipidemia.86 The direct effects that this form of dyssychronization has on cardiovascular morbidity and mortality was highlighted by Penev et al., who reported decreased survival of cardiomyopathetic hamsters when subjected to a continuous shifting of the light/dark cycle (thereby mimicking shift work).87 Such observations strongly support the concept that dyssynchronization of peripheral circadian clocks accelerates many of the common diseases plaguing modern day society, potentially through loss of metabolic homeostasis. This concept is illustrated in Figure 4. Future strategies for the treatment of not only cardiovascular disease but also obesity and diabetes, may, therefore, focus on resynchronization of peripheral circadian clocks with the environment, through lifestyle changes and/or pharmacological interventions. In the latter case, for example, Scheer et al. have shown that daily nighttime melatonin administration in patients with essential hypertension restores circadian oscillations in blood pressure.88
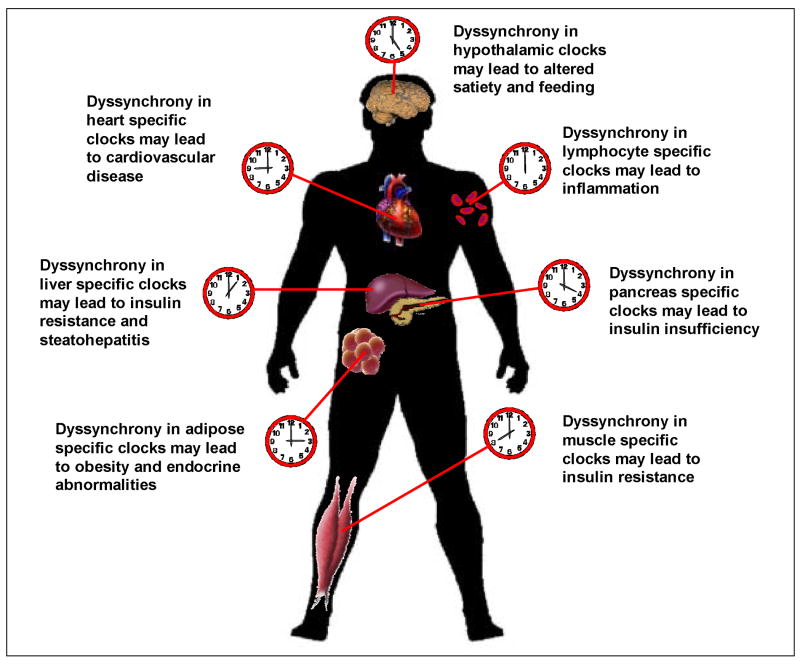
Figure 4. Dyssynchronization of peripheral circadian clocks potentially contribute to the pathogenesis of multiple facets of cardiometabolic syndrome.
Disruption of normal synchronization between skeletal muscle, adipose tissue, liver, hypothalamus, and pancreas likely accelerates insulin resistance, insulin insufficiency, and dyslipidemia, the hallmarks of cardiometabolic syndrome. Disruption of circadian clocks within the heart and vasculature, in the face of a dyssynchronized metabolic milieu, would accelerate cardiovascular disease.
Conclusion
Circadian rhythms are an integral component of virtually all aspects of life, including death. Rhythms in cardiovascular physiology and pathophysiology are perfect examples of this concept, which have been attributed primarily to diurnal variations in neurohumoral factors.3,62,89-92 What has become increasingly clear is that almost every cell in humans possesses an intracellular circadian clock, capable of altering both cellular and organ function over the course of the day. Circadian clocks are entrained by a plethora of extracellular influences, in a cell type–specific manner. As such, during certain pathophysiological conditions, including sleep-related disorders, both intra- and inter- organ dyssynchronization can occur. This in turn will lead to metabolic dysregulation, and consequent cardiometabolic syndrome–related diseases, such as obesity, diabetes, and cardiovascular disease. Future strategies for the treatment of these modern day epidemics will likely target resynchonization of peripheral circadian clocks, through both lifestyle changes and pharmacological interventions.
Acknowledgments
We wish to thank David J. Durgan for help with the isolated adult rat cardiomyocyte studies presented in Figure 2. This work was supported by the National Heart, Lung, and Blood Institute (HL-074259-01) and the USDA (6250-51000-046-02).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edery I. Circadian rhythms in a nutshell. Physiol Genomics. 2000;3:59–74. doi: 10.1152/physiolgenomics.2000.3.2.59. [DOI] [PubMed] [Google Scholar]
- 2.Hardin PE. Transcription regulation within the circadian clock: the E-box and beyond. J Biol Rhythms. 2004;19:348–360. doi: 10.1177/0748730404268052. [DOI] [PubMed] [Google Scholar]
- 3.Young ME. The circadian clock within the heart: potential influence on myocardial gene expression, metabolism, and function. Am J Physiol Heart Circ Physiol. 2006;290:H1–H16. doi: 10.1152/ajpheart.00582.2005. [DOI] [PubMed] [Google Scholar]
- 4.Dunlap JC. Molecular Basis of Circadian Clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 5.Cermakian N, Sassone-Corsi P. Multilevel Regulation of the Circadian Clock. Nat Rev Mol Cell Biol. 2000;1(1):59–67. doi: 10.1038/35036078. [DOI] [PubMed] [Google Scholar]
- 6.Hirota T, Fukada Y. Resetting mechanism of central and peripheral circadian clocks in mammals. Zoological Sci. 2004;21:359–368. doi: 10.2108/zsj.21.359. [DOI] [PubMed] [Google Scholar]
- 7.Ripperger JA, Schibler U. Circadian regulation of gene expression in animals. Curr Opin Cell Biol. 2001;13:357–362. doi: 10.1016/s0955-0674(00)00220-9. [DOI] [PubMed] [Google Scholar]
- 8.Hastings MH, Herzog ED. Clock genes, oscillators, and cellular networks in the suprachiasmatic nuclei. J Biol Rhythms. 2004;19:400–413. doi: 10.1177/0748730404268786. [DOI] [PubMed] [Google Scholar]
- 9.Morse D, Cermakian N, Brancorsini S, Parvinen M, Sassone-Corsi P. No circadian rhythms in testis: Period1 expression is clock independent and developmentally regulated in the mouse. Mol Endocrinol. 2003;17:141–151. doi: 10.1210/me.2002-0184. [DOI] [PubMed] [Google Scholar]
- 10.Gachon F, Nagoshi E, Brown SA, Ripperger JA, Schibler U. The mammalian circadian timing system: from gene expression to physiology. Chromosoma. 2004;113:103–112. doi: 10.1007/s00412-004-0296-2. [DOI] [PubMed] [Google Scholar]
- 11.Allaman-Pillet N, Roduit R, Oberson A, Abdelli S, Ruiz J, Beckmann JS, Schorderet DF, Bonny C. Circadian regulation of islet genes involved in insulin production and secretion. Mol Cell Endocrinol. 2004;226:59–66. doi: 10.1016/j.mce.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Durgan DJ, Trexler NA, Egbejimi O, McElfresh TA, Suk HY, Petterson LE, Shaw CA, Hardin PE, Bray MS, Chandler MP, Chow CW, Young ME. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J Biol Chem. 2006;281:24254–24269. doi: 10.1074/jbc.M601704200. [DOI] [PubMed] [Google Scholar]
- 13.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 14.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchiya Y, Minami I, Kadotani H, Nishida E. Resetting of peripheral circadian clock by prostaglandin E2. EMBO Reports. 2005;6:256–261. doi: 10.1038/sj.embor.7400356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durgan DJ, Hotze MA, Tomlin TM, Egbejimi O, Graveleau C, Abel ED, Shaw CA, Bray MS, Hardin PE, Young ME. The intrinsic circadian clock within the cardiomyocyte. Am J Physiol Heart Circ Physiol. 2005;289:H1530–H1541. doi: 10.1152/ajpheart.00406.2005. [DOI] [PubMed] [Google Scholar]
- 18.Hirota T, Okano T, Kokame K, Shirotani-Ikejima H, Miyata T, Fukada Y. Glucose down-regulates Per1 and Per2 mRNA levels and induces circadian gene expression in cultured Rat-1 fibroblasts. J Biol Chem. 2002;277:44244–44251. doi: 10.1074/jbc.M206233200. [DOI] [PubMed] [Google Scholar]
- 19.Nonaka H, Emoto N, Ikeda K, Fukuya H, Rohman MS, Raharjo SB, Yagita K, Okamura H, Yokoyama M. Angiotensin II induces circadian gene expression of clock genes in cultured vascular smooth muscle cells. Circulation. 2001;104:1746–1748. doi: 10.1161/hc4001.098048. [DOI] [PubMed] [Google Scholar]
- 20.McNamara P, Seo SP, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001;105:877–889. doi: 10.1016/s0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- 21.Terazono H, Mutoh T, Yamaguchi S, Kobayashi M, Akiyama M, Udo R, Ohdo S, Okamura H, Shibata S. Adrenergic regulation of clock gene expression in mouse liver. Proc Natl Aad Sci USA. 2003;100:6795–6800. doi: 10.1073/pnas.0936797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 23.Guo H, Brewer JM, Champhekar A, Harris RBS, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc Natl Aad Sci USA. 2005;102:3111–3116. doi: 10.1073/pnas.0409734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtis AM, Seo SB, Westgate EJ, Rudic RD, Smyth EM, Chakravarti D, FitzGerald GA, McNamara P. Histone acetyltransferase-dependent chromatin remodeling and the vascular clock. J Biol Chem. 2004;279:7091–1097. doi: 10.1074/jbc.M311973200. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi H, Oishi K, Hanai S, Ishida N. Effect of feeding on peripheral circadian rhythms and behaviour in mammals. Genes to Cells. 2004;9:857–864. doi: 10.1111/j.1365-2443.2004.00769.x. [DOI] [PubMed] [Google Scholar]
- 26.Martino T, Arab S, Straume M, Belsham DD, Tata N, Cai F, Liu P, Trivieri M, Ralph M, Sole MJ. Day/night rhythms in gene expression of the normal murine heart. J Mol Med. 2004;82:256–264. doi: 10.1007/s00109-003-0520-1. [DOI] [PubMed] [Google Scholar]
- 27.Mohri T, Emoto N, Nonaka H, Fukuya H, Yagita K, Okamura H, Yokoyama M. Alterations of circadian expressions of clock genes in dahl salt-sensitive rats fed a high-salt diet. Hypertension. 2003;42:189–194. doi: 10.1161/01.HYP.0000082766.63952.49. [DOI] [PubMed] [Google Scholar]
- 28.Naito Y, Tsujino T, Fujioka Y, Ohyanagi M, Iwasaki T. Augmented diurnal variations of the cardiac renin-angiotensin system in hypertensive rats. Hypertension. 2002;40:827–833. doi: 10.1161/01.hyp.0000039960.66987.89. [DOI] [PubMed] [Google Scholar]
- 29.Naito Y, Tsujino T, Kawasaki D, Okumura T, Morimoto S, Masai M, Sakoda T, Fujioka Y, Ohyanagi M, Iwasaki T. Circadian gene expression of clock genes and plasminogen activator inhibitor-1 in heart and aorta of spontaneously hypertensive and Wistar-Kyoto rats. J Hypertens. 2003;21:1107–1115. doi: 10.1097/00004872-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Oishi K, Kasamatsu M, Ishida N. Gene- and tissue-specific alterations of circadian clock gene expression in streptozotocin-induced diabetic mice under restricted feeding. Biochem Biophys Res Commun. 2004;317:330–334. doi: 10.1016/j.bbrc.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 31.Young ME, Razeghi P, Taegtmeyer H. Clock genes in the heart: characterization and attenuation with hypertrophy. Circ Res. 2001;88:1142–1150. doi: 10.1161/hh1101.091190. [DOI] [PubMed] [Google Scholar]
- 32.Young ME, Wilson CR, Razeghi P, Guthrie PH, Taegtmeyer H. Alterations of the Circadian Clock in the Heart by Streptozotocin-induced Diabetes. J Mol Cell Cardiol. 2002;34:223–231. doi: 10.1006/jmcc.2001.1504. [DOI] [PubMed] [Google Scholar]
- 33.Davidson AJ, London B, Block GD, Menaker M. Cardiovascular tissues contain independent circadian clocks. Clin Exp Hypertens. 2005;27:307–311. [PubMed] [Google Scholar]
- 34.Takeda N, Horie S, Imai Y, Harada T, Kawanami D, Saito T, Nojiri T, Oishi K, Ishida N, Maemura K. Peripheral clock regulates carcadian thrombomodulin gene expression in vascular endothelial cells. Circulation. 2005;112:II–139. [Google Scholar]
- 35.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 36.Rudic RD, McNamara P, Reilly D, Grosser T, Curtis AM, Price TS, Panda S, Hogenesch JB, FitzGerald GA. Bioinformatic analysis of circadian gene oscillation in mouse aorta. Circulation. 2005;112:2716–2724. doi: 10.1161/CIRCULATIONAHA.105.568626. [DOI] [PubMed] [Google Scholar]
- 37.Young ME, Razeghi P, Cedars AM, Guthrie PH, Taegtmeyer H. Intrinsic diurnal variations in cardiac metabolism and contractile function. Circ Res. 2001;89:1199–1208. doi: 10.1161/hh2401.100741. [DOI] [PubMed] [Google Scholar]
- 38.Stavinoha MA, RaySpellicy JW, Hart-Sailors ML, Mersmann HJ, Bray MS, Young ME. Diurnal variations in the responsiveness of cardiac and skeletal muscle to fatty acids. Am J Physiol. 2004;287:E878–E887. doi: 10.1152/ajpendo.00189.2004. [DOI] [PubMed] [Google Scholar]
- 39.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon EL, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803–815. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 41.Fuller MK, Ha N, Shaw CA, Chow CW, Bray MS, Young ME. Identification of genes directly regulated by the intrinsic circadian clock within the cardiomyocyte. Circulation. 2006;114:II–231. [Google Scholar]
- 42.Goodwin GW, Taylor CS, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem. 1998;273:29530–29539. doi: 10.1074/jbc.273.45.29530. [DOI] [PubMed] [Google Scholar]
- 43.Lake AC, Sun Y, Li JL, Kim JE, Johnson JW, Li D, Revett T, Shih HH, Liu W, Paulsen JE, Gimeno RE. Expression, regulation, and triglyceride hydrolase activity of Adiponutrin family members. J Lipid Res. 2005;46:2477–2487. doi: 10.1194/jlr.M500290-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, Farese RVJ. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem. 2004;279:11767–11776. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- 45.Maemura K, de la Monte SM, Chin MT, Layne MD, Hsieh CM, Yet SF, Perrella MA, Lee ME. CLIF, a novel cycle-like factor, regulates the circadian oscillation of plasminogen activator inhibitor-1 gene expression. J Biol Chem. 2000;275:36847–36851. doi: 10.1074/jbc.C000629200. [DOI] [PubMed] [Google Scholar]
- 46.Maemura K, Layne MD, Watanabe M, Perrell MA, Nagai R, Lee ME. Molecular mechanisms of morning onset of myocardial infarction. Ann N Y Acad Sci. 2001;947:398–402. doi: 10.1111/j.1749-6632.2001.tb03972.x. [DOI] [PubMed] [Google Scholar]
- 47.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 48.Narayanan CS, Cui Y, Kumar A. DBP binds to the proximal promoter and regulates liver-specific expression of the human angiotensinogen gene. Biochem Biophys Res Commun. 1998;251:388–393. doi: 10.1006/bbrc.1998.9430. [DOI] [PubMed] [Google Scholar]
- 49.Lavery DJ, Lopez-Molina L, Margueron R, Fleury-Olela F, Conquet F, Schibler U, Bonfils C. Circadian expression of the steroid 15 alpha-hydroxylase (Cyp2a4) and coumarin 7-hydroxylase (Cyp2a5) genes in mouse liver is regulated by the PAR leucine zipper transcription factor DBP. Mol Cell Biol. 1999;19:6488–6499. doi: 10.1128/mcb.19.10.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12:540–550. doi: 10.1016/s0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- 51.Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, O’Neill JS, Wong GK, Chesham J, Odell M, Lilley KS, Kyriacou CP, Hastings MH. Circadian orchestration of the hepatic proteome. Curr Biol. 2006;16:1107–1115. doi: 10.1016/j.cub.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 52.Oishi K, Miyazaki K, Kadota K, Kikuno R, Nagase T, Atsumi GI, Ohkura N, Azama T, Mesaki M, Yukimasa S, Kobayashi H, Iitaka C, Umehara T, Horikoshi M, Kudo T, Shimizu Y, Yano M, Monden M, Machida K, Matsuda J, Shuichi H, Todo T, Ishida N. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J Biol Chem. 2003;278:41519–41527. doi: 10.1074/jbc.M304564200. [DOI] [PubMed] [Google Scholar]
- 53.Leighton B, Kowalchuk JM, Challiss RA, Newsholme EA. Circadian rhythm in sensitivity of glucose metabolism to insulin in rat soleus muscle. Am J Physiol. 1988;255:E41–E45. doi: 10.1152/ajpendo.1988.255.1.E41. [DOI] [PubMed] [Google Scholar]
- 54.Stavinoha MA, RaySpellicy JW, Essop MF, Graveleau C, Abel ED, Hart-Sailors ML, Mersmann HJ, Bray MS, Young ME. Evidence for mitochondrial thioesterase 1 as a peroxisome proliferator-activated receptor-alpha-regulated gene in cardiac and skeletal muscle. Am J Physiol. 2004;287:E888–E895. doi: 10.1152/ajpendo.00190.2004. [DOI] [PubMed] [Google Scholar]
- 55.Ando H, Yanagihara H, Hayashi Y, Obi Y, Tsuruoka S, Takamura T, Kaneko S, Fujimura A. Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology. 2005;146:5631–5636. doi: 10.1210/en.2005-0771. [DOI] [PubMed] [Google Scholar]
- 56.Bray MS, Young ME. Circadian rhythms in the development of obesity: potential role for the circadian clock within the adipocyte. Obesity Reviews. 2006;7 doi: 10.1111/j.1467-789X.2006.00277.x. In Press. [DOI] [PubMed] [Google Scholar]
- 57.Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55:962–970. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]
- 58.Fontaine C, Dubois G, Duguay Y, Helledie T, Vu-Dac N, Gervois P, Soncin F, Mandrup S, Fruchart JC, Fruchart-Najib J, Staels B. The orphan nuclear receptor Rev-Erbalpha is a peroxisome proliferator-activated receptor (PPAR) gamma target gene and promotes PPARgamma-induced adipocyte differentiation. J Biol Chem. 2003;278:37672–37680. doi: 10.1074/jbc.M304664200. [DOI] [PubMed] [Google Scholar]
- 59.Gordon RD, Wolfe LK, Island DP, Liddle GW. A diurnal rhythm in plasma renin activity in man. J Clin Invest. 1966;45:1587–1592. doi: 10.1172/JCI105464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katz FH, Romfh P, Smith JA. Diurnal variation of plasma aldosterone, cortisol and renin activity in supine man. J Clin Endocrinol Metab. 1975;40:125–134. doi: 10.1210/jcem-40-1-125. [DOI] [PubMed] [Google Scholar]
- 61.Katz FH, Smith JA, Lock JP, Loeffel DE. Plasma vasopressin variation and renin activity in normal active humans. Horm Res. 1979;10:289–302. doi: 10.1159/000179011. [DOI] [PubMed] [Google Scholar]
- 62.Linsell CR, Lightman SL, Mullen PE, Brown MJ, Causon RC. Circadian rhythms of epinephrine and norepinephrine in man. J Clin Endocrinol Metab. 1985;60:1210–1215. doi: 10.1210/jcem-60-6-1210. [DOI] [PubMed] [Google Scholar]
- 63.Portaluppi F, Montanari L, Bagni B, degli Uberti E, Trasforini G, Margutti A. Circadian rhythms of atrial natriuretic peptide, blood pressure and heart rate in normal subjects. Cardiology. 1989;76:428–432. doi: 10.1159/000174529. [DOI] [PubMed] [Google Scholar]
- 64.Richards AM, Nicholls MG, Espiner EA, Ikram H, Cullens M, Hinton D. Diurnal patterns of blood pressure, heart rate and vasoactive hormones in normal man. Clin Exp Hypertens. 1986;8:153–166. doi: 10.3109/10641968609074769. [DOI] [PubMed] [Google Scholar]
- 65.Stern N, Beahm E, McGinty D, Eggena P, Littner M, Nyby M, Catania R, Sowers JR. Dissociation of 24-hour catecholamine levels from blood pressure in older men. Hypertension. 1985;7:1023–1029. doi: 10.1161/01.hyp.7.6.1023. [DOI] [PubMed] [Google Scholar]
- 66.Tuck ML, Stern N, Sowers JR. Enhanced 24-hour norepinephrine and renin secretion in young patients with essential hypertension: relation with the circadian pattern of arterial blood pressure. Am J Cardiol. 1985;55:112–115. doi: 10.1016/0002-9149(85)90310-8. [DOI] [PubMed] [Google Scholar]
- 67.Fantidis P, Perez De Prada T, Fernandez-Ortiz A, Carcia-Touchard A, Alfonso F, Sabate M, Hernandez R, Escaned J, Bauelos C, Macaya C. Morning cortisol production in coronary heart disease patients. Eur J Clin Invest. 2002;32:304–308. doi: 10.1046/j.1365-2362.2002.00988.x. [DOI] [PubMed] [Google Scholar]
- 68.Kudo T, Akiyama M, Kuriyama K, Sudo M, Moriya T, Shibata S. Night-time restricted feeding normalises clock genes and Pai-1 gene expression in the db/db mouse liver. Diabetologia. 2004;47:1425–1436. doi: 10.1007/s00125-004-1461-0. [DOI] [PubMed] [Google Scholar]
- 69.Chau NP, Mallion JM, de Gaudemaris R, Ruche E, Siche JP, Pelen O, Mathern G. Twenty-four-hour ambulatory blood pressure in shift workers. Circulation. 1989;80:341–347. doi: 10.1161/01.cir.80.2.341. [DOI] [PubMed] [Google Scholar]
- 70.Sundberg S, Kohvakka A, Gordin A. Rapid reversal of circadian blood pressure rhythm in shift workers. J Hypertens. 1988;6:393–396. [PubMed] [Google Scholar]
- 71.van den Buuse M. Circadian rhythms of blood pressure and heart rate in conscious rats: effects of light cycle shift and timed feeding. Physiol Behav. 1999;68:9–15. doi: 10.1016/s0031-9384(99)00148-1. [DOI] [PubMed] [Google Scholar]
- 72.Knutsson A, Akerstedt T, Jonsson BG, Orth-Gomer K. Increased risk of ischaemic heart disease in shift workers. Lancet. 1986;12:89–92. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- 73.Harma MI, Ilmarinen JE. Towards the 24-hour society--new approaches for aging shift workers? Scand J Work Envion Health. 1999;25:610–615. doi: 10.5271/sjweh.488. [DOI] [PubMed] [Google Scholar]
- 74.Kunieda T, Minamino T, Katsuno T, Tateno K, Nishi J, Miyauchi H, Orimo M, Okada S, Komuro I. Cellular senescence impairs circadian expression of clock genes in vitro and in vivo. Cir Res. 2006;98:532–539. doi: 10.1161/01.RES.0000204504.25798.a8. [DOI] [PubMed] [Google Scholar]
- 75.Karlsson BH, Knutsson AK, Lindahl BO, Alfredsson LS. Metabolic disturbances in male workers with rotating three-shift work. Results of the WOLF study. Int Arch Occup Environ Health. 2003;76:424–430. doi: 10.1007/s00420-003-0440-y. [DOI] [PubMed] [Google Scholar]
- 76.Sharifian A, Farahani S, Pasalar P, Gharavi M, Aminian O. Shift work as an oxidative stressor. J Cir Rhythms. 2005;3:15–17. doi: 10.1186/1740-3391-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. 2005;90:2537–2544. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 79.Buxton OM, Lee CW, L’Hermite-Baleriaux M, Turek FW, Van Cauter E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R714–R724. doi: 10.1152/ajpregu.00355.2002. [DOI] [PubMed] [Google Scholar]
- 80.Buxton OM, L’Hermite-Baleriaux M, Hirschfeld U, Cauter E. Acute and delayed effects of exercise on human melatonin secretion. J Biol Rhythms. 1997;12:568–574. doi: 10.1177/074873049701200611. [DOI] [PubMed] [Google Scholar]
- 81.Buxton OM, Frank SA, L’Hermite-Baleriaux M, Leproult R, Turek FW, Van Cauter E. Roles of intensity and duration of nocturnal exercise in causing phase delays of human circadian rhythms. Am J Physiol. 1997;273:E536–E542. doi: 10.1152/ajpendo.1997.273.3.E536. [DOI] [PubMed] [Google Scholar]
- 82.Young ME, McNulty PH, Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: Part II: Potential mechanisms. Circulation. 2002;105:1861–1870. doi: 10.1161/01.cir.0000012467.61045.87. [DOI] [PubMed] [Google Scholar]
- 83.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 84.Shimabukuro M, Zhou Y, Levi M, Unger R. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Egan BM, Greene EL, Goodfriend TL. Insulin resistance and cardiovascular disease. Am J Hypertens. 2001;14:116S–125S. doi: 10.1016/s0895-7061(01)02078-7. [DOI] [PubMed] [Google Scholar]
- 86.Ribeiro DCO, Hampton SM, Morgan L, Deacon S, Arendt J. Altered postprandial hormone and metabolic responses in a simulated shift work environment. J Endocrinology. 1998;158:305–310. doi: 10.1677/joe.0.1580305. [DOI] [PubMed] [Google Scholar]
- 87.Penev PD, Kolker DE, Zee PC, Turek FW. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am J Physiol. 1998;275:H2334–H2337. doi: 10.1152/ajpheart.1998.275.6.H2334. [DOI] [PubMed] [Google Scholar]
- 88.Scheer FAJL, Van Montfrans GA, van Someren EJW, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004;43:192–197. doi: 10.1161/01.HYP.0000113293.15186.3b. [DOI] [PubMed] [Google Scholar]
- 89.Degaute JP, van de Borne P, Linkowski P, Van Cauter E. Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men. Hypertension. 1991;18:199–210. doi: 10.1161/01.hyp.18.2.199. [DOI] [PubMed] [Google Scholar]
- 90.Hill L. On rest, sleep and work and the concomitant changes in the circulation of the blood. Lancet. 1898;1:282–285. [Google Scholar]
- 91.Khatri IM, Freis ED. Hemodynamic changes during sleep. J Appl Physiol. 1967;22:867–873. doi: 10.1152/jappl.1967.22.5.867. [DOI] [PubMed] [Google Scholar]
- 92.Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79:733–743. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]