Abstract
Rationale: Our study has shown that plasma levels of ghrelin, a stomach-derived peptide, are significantly reduced in sepsis, and that ghrelin administration improves organ blood flow via a nuclear factor (NF)-κB–dependent pathway. However, it remains unknown whether ghrelin has any protective effects on severe sepsis–induced acute lung injury (ALI) and, if so, whether inhibition of NF-κB plays any role in it.
Objectives: To test the hypothesis that ghrelin reduces severe sepsis–induced ALI and mortality through inhibition of NF-κB.
Methods: Sepsis was induced in rats by cecal ligation and puncture (CLP). Five hours after CLP, a bolus intravenous injection of 2 nmol of ghrelin was followed by continuous infusion of 12 nmol of ghrelin via a minipump for 15 hours. Samples were harvested 20 hours post-CLP (i.e., severe sepsis). Pulmonary levels of ghrelin and proinflammatory cytokines were measured by ELISA. NF-κB p65 and IκBα expression and NF-κB activity were measured by Western blot analysis and ELISA, respectively. Pulmonary blood flow was measured with radioactive microspheres. In additional animals, the necrotic cecum was excised 20 hours post-CLP and 10-day survival was recorded.
Measurements and Main Results: Pulmonary levels of ghrelin decreased significantly 20 hours post-CLP. Ghrelin administration restored pulmonary levels of ghrelin, reduced lung injury, increased pulmonary blood flow, down-regulated proinflammatory cytokines, inhibited NF-κB activation, and improved survival in sepsis. Administration of a specific ghrelin receptor antagonist worsened the survival rate after CLP and cecal excision.
Conclusions: Ghrelin can be developed as a novel treatment for severe sepsis–induced ALI. The protective effect of ghrelin is mediated through inhibition of NF-κB.
Keywords: peptide, cytokine, nuclear factor-κB
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Levels of ghrelin, an endogenous ligand for the growth hormone secretagogue receptor, are reduced in sepsis. However, there is only limited information on the protective effects of ghrelin in sepsis.
What This Study Adds to the Field
Ghrelin has a beneficial effect on sepsis-induced lung injury via a mechanism that involves inhibition of the nuclear factor-κB pathway in the lungs.
Despite significant advances in the understanding of the pathogenesis of sepsis and its management, the mortality rate of severe sepsis remains unacceptably high (1). The ultimate cause of death in patients with severe sepsis is multiple organ failure. The lung is frequently the first failing organ during the sequential development of multiple organ dysfunction in sepsis (2, 3). Acute lung injury (ALI) that clinically manifests as acute respiratory distress syndrome is the primary cause of death under these conditions (3). The pathophysiological sequelae of sepsis are caused by an overreaction of the immune system to microorganisms and their products (4). Excessive cytokine-mediated inflammation plays a fundamental role in the pathogenesis of sepsis-induced ALI. On the other hand, immunosuppression is also evident at the onset of sepsis (5). Therefore, direct antiinflammatory strategies have produced modest clinical effects in critically ill patients (6).
The signals that lead to increased gene expression and biosynthesis of proinflammatory mediators by inflammatory cells are thus of considerable interest. In this regard, the activation of nuclear factor (NF)-κB has been implicated as an important step in the development of ALI (7). Increased activation of NF-κB is found in alveolar macrophages, peripheral blood mononuclear cells, and neutrophils from patients with sepsis (8–10). A greater or more persistent nuclear accumulation of NF-κB is associated with higher mortality and more persistent organ dysfunction, including pulmonary injury (9). Therefore, NF-κB activation is likely to be a logical therapeutic target for ALI (7).
Ghrelin, a novel endogenous ligand for the growth hormone secretagogue receptor (GHSR)-1a, is a 28–amino acid acylated peptide produced predominantly by the stomach (11). In addition to its growth hormone–releasing properties (12), ghrelin has now been proved to possess other endocrine and nonendocrine activities reflecting central and peripheral GHSR-1a distribution (13, 14). Li and coworkers reported that ghrelin mitigates proinflammatory cytokine production and mononuclear cell binding through inhibition of NF-κB in human endothelial cells (15). Our studies have shown that circulating levels of ghrelin decreased significantly in a rat model of polymicrobial sepsis induced by cecal ligation and puncture (CLP) (16), and that ghrelin administration increases cardiac output, reduces total peripheral resistance, and improves organ blood flow under such conditions (17). The improvement of tissue perfusion by ghrelin in severe sepsis appears to be mediated by down-regulation of endothelin-1 in endothelial cells, involving an NF-κB–dependent pathway (17). However, it remains unknown whether ghrelin has any protective effects on severe sepsis–induced ALI and, if so, whether inhibition of NF-κB plays any role in it. The present study was conducted to test the hypothesis that ghrelin attenuates severe sepsis–induced ALI and mortality through inhibition of NF-κB. Some of the results of this study were previously reported in the form of an abstract (18).
METHODS
Animal Model of Sepsis
Male Sprague-Dawley rats (275–325 g) were housed in a temperature-controlled room on a 12-h light:dark cycle and fed a standard Purina rat chow diet (LabDiet 5001; PMI Nutrition, St. Louis, MO). Before the induction of sepsis, rats were fasted overnight but allowed water ad libitum. Rats were anesthetized by isoflurane inhalation and the ventral neck, abdomen, and groin were shaved and washed with 10% povidone-iodine. CLP was performed as we previously described (19), and the animals were randomly assigned to various groups. Sham-operated animals (i.e., control animals) underwent the same procedure with the exception that the cecum was neither ligated nor punctured. The animals were resuscitated by subcutaneous administration of normal saline (3 ml/100 g body weight) immediately after surgery. Animals were then anesthetized at various intervals after CLP or sham operation for collection of tissue samples. All experiments were performed in accordance with the National Institutes of Health (Bethesda, MD) guidelines for the use of experimental animals. This project was approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research (Manhasset, NY).
Administration of Ghrelin
Rat ghrelin (Phoenix Pharmaceuticals, Belmont, CA) was dissolved in normal saline to a final concentration of 100 μM. Endotoxin levels in the preparations of ghrelin were measured by the Limulus amebocyte lysate method as previously described (20). Our results showed that the levels of endotoxin in the preparations were not detectable. Two hundred-microliter Alzet minipumps (infusion rate, 8 μl/h; DURECT, Cupertino, CA) were primed with ghrelin solution or vehicle (normal saline) for 3 hours before implantation. After a slow intravenous bolus injection of 2 nmol of ghrelin (or 200 μl of vehicle) 5 hours after CLP, the minipump was then connected to a jugular venous catheter and implanted subcutaneously. Twenty hours after CLP (i.e., 15 h after implantation of the minipump), the rats were killed and blood and tissue samples were collected. The total dose of ghrelin each rat received was 45 nmol/kg body weight.
Water Content Determination and Histologic Examination
Lung edema was estimated by comparing wet-to-dry weight ratios. Lung tissues were dried in a 70°C oven for 48 hours. Lung water content was calculated as percent H2O = (1 – dry wt/wet wt) × 100%. The morphologic alterations in the lungs were examined 20 hours after the onset of sepsis by light microscopy. Tissue samples were obtained from sham-operated or septic animals and submerged in 10% Formalin in neutral buffered solution (Sigma, St. Louis, MO) for immediate fixation and later embedded in paraffin. The tissue blocks were then sectioned at a thickness of 5 μm, floated on warm water, and transferred to glass slides, where they were stained with hematoxylin and eosin (H&E), dehydrated, and coverslipped. Morphologic examinations were performed by light microscopy and documented by photographs. A scoring system to grade the degree of lung injury was employed, based on the following histologic features: edema, hyperemia and congestion, neutrophil margination and tissue infiltration, intraalveolar hemorrhage and debris, and cellular hyperplasia. Each feature was graded as absent, mild, moderate, or severe, with a score of 0–3. A total score was calculated for each animal (21).
Determination of Pulmonary Blood Flow
Twenty hours after CLP or sham operation, the animals were anesthetized again by isoflurane inhalation. Both the right femoral artery and vein were cannulated with polyethylene-50 tubing. An additional polyethylene-50 catheter was inserted into the left ventricle via the right carotid artery. Cerium-141–labeled microspheres (PerkinElmer Life and Analytical Sciences, Waltham, MA) were suspended in 15% dextran, containing 0.05% Tween 80 surfactant to prevent aggregation. The microspheres were dispensed with a vortex shaker for 3 minutes and a 0.2- to 0.25-ml suspension of microspheres with an activity of approximately 4 μCi/rat was infused into the left ventricle over a period of 20 seconds at a constant rate. An estimated 150,000 microspheres were injected into each rat. The reference blood sample was withdrawn from the femoral arterial catheter beginning 20 seconds before microsphere infusion and continued for 80 seconds at a rate of 0.7 ml/minute. Normal saline (0.8 ml) was infused through the left ventricular catheter immediately after microsphere infusion over a period of 40 seconds. Mean arterial pressure and heart rate were monitored before the injection of radioactive microspheres by using a blood pressure analyzer (Digi-Med; Micro-Med, Louisville, KY). At the end of the experiment, the rat was killed with an overdose of isoflurane. The lungs were harvested, washed with normal saline, and gently blotted on filter paper. The lungs were then weighed and placed in a counting tube, and the radioactivity was counted in a γ counter (Cobra II series auto-γ counter; Packard BioScience/PerkinElmer Life and Analytical Sciences). The reference blood sample was transferred into a counting tube and counted. The remaining microspheres, which were left in the syringe after injection, were also counted. Pulmonary blood perfusion was calculated as previously described (22).
Bacterial Load
To determine the bacterial load in the peritoneum, the peritoneal cavity was lavaged with 5 ml of sterile saline. Serial log dilutions were made. To determine the bacterial load in the blood, 100 μl of blood was collected and serially diluted with sterile saline. To determine the pulmonary bacterial load, the lungs were harvested and equal amounts of wet tissue were homogenized and briefly centrifuged to remove gross particulate matter. Serial log dilutions of tissue homogenates were applied. Five hundred microliters of each dilution was then plated on chocolate agar plates (Fisher Scientific, Pittsburgh, PA) and incubated at 37°C for 24 hours under aerobic conditions. Colony-forming units were counted. Results were expressed as colony-forming units per milliliter or milligram of wet tissue.
Survival Study
In additional groups of animals, ghrelin or vehicle was administered as described above, 5 hours after CLP. Twenty hours after CLP, the necrotic cecum was excised and the abdominal cavity was washed twice with 40 ml of warm, sterilized normal saline solution. The abdominal incision was then closed in layers. The procedure of cecal excision in CLP animals was performed to mimic the clinical situation, in which the septic focus is removed whenever possible. The animals were then allowed food and water ad libitum and were monitored for 10 days to record body weight changes and survival.
Determination of Pulmonary Ghrelin, Tumor Necrosis Factor-α, and IL-6
Twenty hours after CLP or sham operation, the lungs were rapidly harvested. The tissues were excised, rinsed of blood, homogenized in homogenization buffer (phosphate-buffered saline solution, containing 0.05% Triton X-100 and a protease inhibitor cocktail; pH 7.2; 4°C), using a Polytron (Kinematica, Lucerne, Switzerland), and sonicated for 10 seconds. Homogenates were centrifuged at 12,000 × g for 10 minutes, and ghrelin, tumor necrosis factor (TNF)-α, and IL-6 levels were quantified with an ELISA kit specifically for human and rat active ghrelin (100% cross-reactivity with rat active ghrelin; Linco Research/Millipore, Billerica, MA), rat TNF-α or IL-6 (BD Biosciences Pharmingen, San Diego, CA). The assay was performed according to the instructions provided by the manufacturer. Pulmonary levels of ghrelin, TNF-α, and IL-6 were normalized to the protein concentration in the sample.
Western Blotting Analysis of NF-κB Nuclear Translocation
The lungs were snap-frozen in liquid nitrogen and pulverized 20 hours after CLP or sham operation. Nuclear and cytoplasmic extracts were prepared for Western blot analysis of NF-κB p65 (nuclear) and inhibitor of NF-κB (IκBα, cytoplasmic) expression (total p44/p42 mitogen-activated protein kinase [MAPK] and β-actin were used as controls for nuclear and cytoplasmic proteins, respectively [23]). Equal amounts of lung homogenates (25 μg/lane) were fractionated on 4–12% NuPAGE Bis-Tris gels (Invitrogen, Carlsbad, CA) and transferred to 0.2-μm nitrocellulose membrane. Nitrocellulose blots were blocked by incubation in TBST (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.1% Tween 20) containing 5% milk for 1 hour. Blots were incubated with rabbit NF-κB p65 and IκBα polyclonal antibodies (diluted 1:500; Santa Cruz Biotechnology; Santa Cruz, CA) overnight at 4°C. The blots were then washed in TBST five times for 10 minutes. Blots were incubated with horseradish peroxidase–linked anti–rabbit IgG (Cell Signaling Technology, Danvers, MA) for 1 hour at room temperature, and then washed five times in TBST for 10 minutes. A chemiluminescent peroxidase substrate (ECL; GE Healthcare Bio-Sciences, Piscataway, NJ) was applied according to the manufacturer's instructions, and the membranes were exposed briefly to X-ray film. The band densities were determined with an imaging system (Bio-Rad, Hercules, CA). The blots were then stripped and incubated with an anti–total p44/p42 MAPK antibody (for nuclear protein, diluted 1:200; Santa Cruz Biotechnology) or anti–β-actin antibody (for cytoplasmic protein, diluted 1:200; Santa Cruz Biotechnology) to ensure equal loading. The ratios of the bands are shown.
Evaluation of NF-κB Activity
The DNA-binding activity of NF-κB in lung tissues was quantified by ELISA, using the TransAM NF-κB p65 transcription factor assay kit (Active Motif North America, Carlsbad, CA) (24). Briefly, fresh lung tissues were harvested 20 hours after CLP. Nuclear extracts were prepared with a nuclear extract kit (Active Motif North America) according to the manufacturer's instructions. Nuclear extracts were incubated in 96-well plates coated with immobilized oligonucleotide containing a consensus (5′-GGGACTTTCC-3′) binding site for the p65 subunit of NF-κB. NF-κB binding to the target oligonucleotide was detected by incubation with primary antibody specific for the activated form of p65 (Active Motif North America), visualized by anti-IgG–horseradish peroxidase conjugate and developing solution, and quantified at 450 nm with a reference wavelength of 655 nm. A standard curve was generated with recombinant NF-κB p65 proteins (Active Motif North America). NF-κB activities in the samples were expressed as micrograms per milligram of protein.
Administration of Ghrelin Receptor Antagonist [d-Arg1,d-Phe5,d-Trp7,9,Leu11]Substance P
To further define the role of ghrelin deficiency in lung pathophysiology, a specific and potent ghrelin receptor antagonist, [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P (Bachem, Torrance, CA) (25), was administered to normal animals. Briefly, the animals were anesthetized by isoflurane inhalation. The femoral vein was carefully separated from the artery and cannulated with polyethylene-50 tubing. [d-Arg1,d-Phe5,d-Trp7, 9,Leu11]Substance P (700 nmol/kg body weight in 1 ml of normal saline) or normal saline (vehicle, 1 ml) was infused into normal animals over a period of 1 hour through a pump (Harvard Bioscience, Holliston, MA). The pulmonary samples were collected at the end of infusion. Pulmonary levels of TNF-α and IL-6 were measured by ELISA as described above. Moreover, to further define the role of ghrelin deficiency in severe sepsis–induced ALI, [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P was administered to CLP animals. Briefly, [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P was dissolved in normal saline to a final concentration of 1.5 mM. The endotoxin levels in the preparations of [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P were measured by the Limulus amebocyte lysate method, as previously described (20). Our results showed that the levels of endotoxin in the preparations were not detectable. Two hundred-microliter Alzet minipumps (infusion rate; 8 μl/h; DURECT) were primed with [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P solution for 3 hours before implantation. Five hours after CLP, the animals were anesthetized again by isoflurane inhalation. After a slow intravenous bolus injection of 30 nmol of [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P, the minipump was then connected to a jugular venous catheter and implanted subcutaneously. The necrotic cecum was excised 20 hours after CLP. Survival was recorded for 10 days thereafter as described above. The total dose of [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P each rat received was 700 nmol/kg body weight.
Statistical Analysis
All data are expressed as means ± SE and compared by one-way analysis of variance and the Student-Newman-Keuls method for multiple group analysis or by Student t test for two-group analysis. Kruskal-Wallis one-way analysis of variance on ranks and the Student-Newman-Keuls method were used for statistical evaluation of the histopathologic scores. The survival rate was estimated by the Kaplan-Meier method and compared by log-rank test. Differences in values were considered significant at P < 0.05.
RESULTS
Alterations in Pulmonary Levels of Ghrelin after CLP
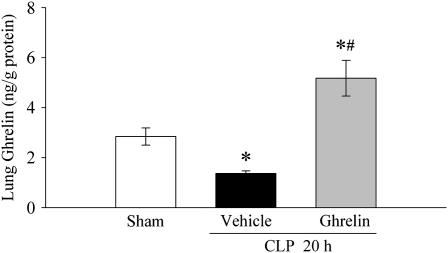
As shown in Figure 1, pulmonary levels of ghrelin 20 hours after sham operation were 2.8 ± 0.3 ng/g protein. Twenty hours after CLP, however, pulmonary levels of ghrelin decreased by 52% (P < 0.05). Intravenous administration of ghrelin increased pulmonary levels of ghrelin to 5.2 ± 0.7 ng/g protein.
Figure 1.
Alterations in pulmonary levels of ghrelin in sham-operated animals and septic animals treated with normal saline (vehicle) or ghrelin 20 hours after cecal ligation and puncture (CLP). Data are presented as means ± SE (n = 6) and compared by one-way analysis of variance (ANOVA) and the Student-Newman-Keuls method: *P < 0.05 versus sham group; #P < 0.05 versus vehicle group.
Effects of Ghrelin Administration on Acute Lung Injury
As shown in Figure 2, rats subjected to sepsis had a significant increase in lung water content as compared with sham-operated animals (P < 0.05). When septic animals were treated with ghrelin, lung water content was reduced and there was no statistically significant difference in lung water content between sham-operated and CLP ghrelin-treated animals. In terms of histopathological changes, lung injury (characterized by disruption of lung architecture, extravasation of red blood cells, and inflammatory cells in the alveolar space) was present in CLP animals treated with vehicle (Figure 3B). Ghrelin-treated rats revealed a marked reduction of infiltrated inflammatory cells and a significant improvement in lung architecture (Figure 3C). As indicated in Figure 3D, the lung injury score was significantly increased 20 hours after CLP and vehicle treatment as compared with that in sham-operated animals (P < 0.05). Administration of ghrelin after CLP markedly reduced the lung injury score by 39% (P < 0.05). Pulmonary blood perfusion decreased by 33%, from 148.1 ± 14.4 to 99.7 ± 11.6 ml/minute per 100 g of tissue 20 hours after CLP (P < 0.05; Figure 4). Ghrelin treatment significantly increased pulmonary blood perfusion by 42% to 141.9 ± 14.1 ml/minute per 100 g of tissue (P < 0.05; Figure 4).
Figure 2.
Alterations in lung water content in sham-operated animals and septic animals treated with normal saline (vehicle) or ghrelin 20 hours after cecal ligation and puncture (CLP). Data are presented as means ± SE (n = 6) and compared by one-way analysis of variance and the Student-Newman-Keuls method: *P < 0.05 versus sham group; #P < 0.05 versus vehicle group.
Figure 3.
Morphologic alterations of the lungs as determined by photomicrography. (A) Photomicrograph of a pulmonary section from a sham-operated rat. (B) Photomicrograph of a lung section from a septic rat 20 hours after cecal ligation and puncture (CLP) treated with vehicle. (C) Photomicrograph of a lung section from a septic rat 20 hours after CLP treated with ghrelin. (D) Histopathologic scoring of lung injury in sham-operated animals and septic animals treated with normal saline (vehicle) or ghrelin 20 hours after CLP. Insets: Images of boxed areas at higher magnification. Data are presented as means ± SE (n = 5) and compared by Kruskal-Wallis one-way analysis of variance on ranks and the Student-Newman-Keuls method: *P < 0.05 versus sham group; #P < 0.05 versus vehicle group. Original magnification (A–D): ×100; insets: ×400.
Figure 4.
Alterations in pulmonary blood flow in sham-operated animals and septic animals treated with normal saline (vehicle) or ghrelin 20 hours after cecal ligation and puncture (CLP). Data are presented as means ± SE (n = 6 or 7) and compared by one-way analysis of variance and the Student-Newman-Keuls method: *P < 0.05 versus sham group; #P < 0.05 versus vehicle group.
Effects of Ghrelin Administration on Bacterial Load
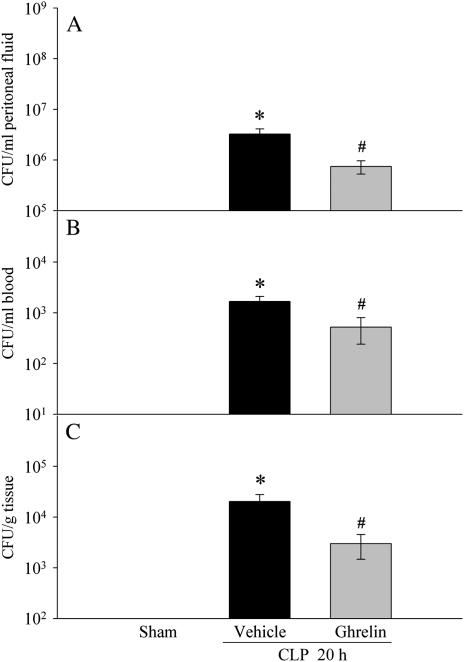
As indicated in Figures 5A–5C, colony-forming units were determined for peritoneal fluid (Figure 5A), blood (Figure 5B), and lungs (Figure 5C) in all rats 20 hours after CLP. However, the numbers of colony-forming units was significantly lower in the septic rats receiving ghrelin treatment than in those receiving vehicle treatment.
Figure 5.
Alterations in bacterial load in the peritoneal fluid (A), blood (B), and lungs (C) in sham-operated animals and septic animals treated with normal saline (vehicle) or ghrelin 20 hours after cecal ligation and puncture (CLP). Data are presented as means ± SE (n = 5–8) and compared by one-way analysis of variance and the Student-Newman-Keuls method: *P < 0.05 versus sham group; #P < 0.05 versus vehicle group.
Effects of Ghrelin Administration on Survival Rate
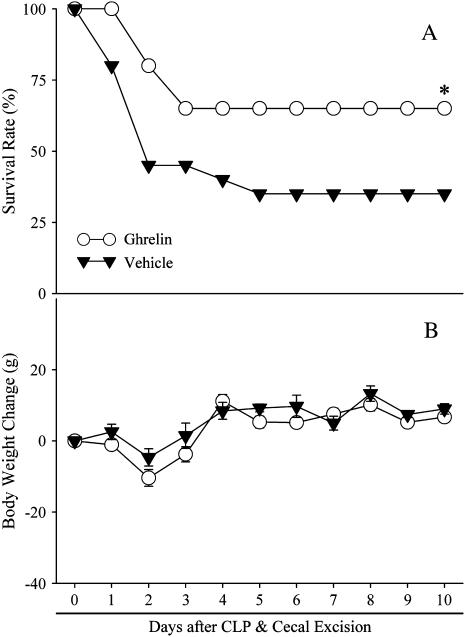
The survival rate after CLP and cecal excision with vehicle administration was 45% on Day 2 and decreased to 35% on Days 6–10 (Figure 6A). Treatment with ghrelin, however, improved the survival rate to 80% on Day 2 and to 65% on Days 4–10, which was significantly higher than for the CLP group treated with vehicle (P < 0.05; Figure 6A). Although ghrelin is an appetite stimulant, we did not find any significant differences in body weight changes between septic animals treated with vehicle and ghrelin on Days 1–10 after CLP and cecal excision (Figure 6B).
Figure 6.
Alterations in the survival rate (A) and body weight (B) 10 days after cecal ligation and puncture (CLP) and cecal excision with normal saline treatment (solid triangles) and CLP with ghrelin treatment (open circles). There were 20 animals in each group. The survival rate was estimated by the Kaplan-Meier method and compared by log-rank test. *P < 0.05 versus CLP plus vehicle.
Effects of Ghrelin Administration on Pulmonary Levels of Proinflammatory Cytokines
As indicated in Figure 7A, TNF-α levels in the lungs were increased by 77% 20 hours after CLP (P < 0.05). Treatment with ghrelin reduced pulmonary TNF-α levels by 33% in CLP animals (P < 0.05), and there was no significant difference in pulmonary TNF-α levels between sham-operated and CLP ghrelin-treated animals (Figure 7A). Similarly, pulmonary levels of IL-6 increased by 8.5-fold 20 hours after CLP. Ghrelin treatment decreased them by 41% (P < 0.05; Figure 7B).
Figure 7.
Alterations in pulmonary levels of tumor necrosis factor (TNF)-α (A) and IL-6 (B) in sham-operated animals and septic animals treated with normal saline (vehicle) or ghrelin 20 hours after cecal ligation and puncture (CLP). Data are presented as means ± SE (n = 6) and compared by one-way analysis of variance and the Student-Newman-Keuls method: *P < 0.05 versus sham group; #P < 0.05 versus vehicle group.
Ghrelin Inhibits Sepsis-induced NF-κB Translocation in Lungs
To study whether ghrelin has any effect on NF-κB translocation in the lungs, NF-κB p65 expression in the nucleus and IκBα expression in the cytoplasm were determined 20 hours after CLP by Western blot analysis. As demonstrated in Figures 8A and 8B, NF-κB p65 levels in the nucleus increased by 69% 20 hours after CLP (P < 0.05), which was associated with a 59% decrease in the cytoplasmic levels of IκBα (P < 0.05). Treatment with ghrelin, however, resulted in a 21% decrease in the nuclear levels of NF-κB (Figure 8A) and a 76% increase in the cytoplasmic levels of IκBα (Figure 8B). This result indicates that ghrelin treatment inhibits the degradation of IκBα and prevents the translocation of NF-κB into the nucleus. Because Western blot analysis can only measure the levels of protein expression, we repeated the measurement with an ELISA-based kit. This ELISA-based method can detect not only the expression, but also the activity, of NF-κB (i.e., binding with a discrete nucleotide sequence). Our ELISA result indicated that NF-κB activity in the lungs increased by 1.7-fold 20 hours after CLP (Figure 8C). Ghrelin treatment decreased NF-κB activity by 59% (Figure 8C).
Figure 8.
Alterations in nuclear levels of nuclear factor (NF)-κB p65 (A), cytoplasm levels of IκBα (B), and NF-κB activities (C) in the lungs in sham-operated animals and septic animals treated with normal saline (vehicle) or ghrelin 20 hours after cecal ligation and puncture (CLP). Total p44/42 MAPK and β-actin were served as loading control for nuclear and cytoplasmic proteins, respectively. Representative blots are also presented. Data are expressed as means ± SE (n = 4–6 per group) and compared by one-way analysis of variance and the Student-Newman-Keuls method: *P < 0.05 versus sham-operated animals; #P < 0.05 versus CLP animals treated with vehicle.
Effects of Ghrelin Receptor Antagonist on Pulmonary Levels of Proinflammatory Cytokines in Normal Animals and on Survival of Septic Animals
As indicated in Figure 9A, TNF-α levels in the lungs were increased by 59% after intravenous infusion of ghrelin receptor antagonist [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P (P < 0.05) for 1 hour in normal animals. Similarly, pulmonary levels of IL-6 were also elevated by 89% after [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P administration in normal animals (P < 0.05; Figure 9B). As indicated in Figure 10A, administration of [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P to CLP animals decreased the 10-day survival rate from 35 to 15% (P < 0.05). There was no difference in body weight changes between septic animals treated with vehicle and ghrelin receptor antagonist on Days 1–10 after CLP and cecal excision (Figure 10B).
Figure 9.
Alterations in pulmonary levels of tumor necrosis factor (TNF)-α (A) and IL-6 (B) in normal animals 1 hour after normal saline (vehicle) or [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P (D-SP) treatment. Data are expressed as means ± SE (n = 6 per group) and compared by Student t test: *P < 0.05 versus vehicle group.
Figure 10.
Alterations in the survival rate (A) and body weight (B) 10 days after cecal ligation and puncture (CLP) and cecal excision with normal saline treatment (solid triangles, derived from Figure 6) and CLP with [d-Arg1,d-Phe5,d-Trp7,9,Leu11]substance P treatment (open triangles). There were 20 animals in each group. The survival rate was estimated by the Kaplan-Meier method and compared by log-rank test. *P < 0.05 versus CLP plus vehicle.
DISCUSSION
The lung is frequently the first failing organ during the sequential development of multiple organ dysfunction in sepsis. Severe sepsis, when accompanied by acute respiratory distress syndrome, continues to be the leading causes of death in intensive care units, with a mortality that has remained over 40% (26). As such, the development of novel strategies for the treatment of ALI is critical for the improvement of patient outcome in severe sepsis.
Ghrelin, an acylated peptide produced predominantly in the stomach, was discovered to be a natural ligand of the GHSR-1a in 1999 (11). Given the broad distribution of ghrelin and functional GHSR-1a in various tissues (27), it is now widely accepted that this peptide exerts multiple paracrine, autocrine, and endocrine biological activities. Studies have indicated that ghrelin appears to play a determinant role in fetal lung development (28). Ghrelin receptor–binding sites have been demonstrated in the adult lung parenchyma and pulmonary artery wall (29, 30). In addition, ghrelin administration has been shown to attenuate monocrotaline-induced pulmonary hypertension, pulmonary vascular remodeling, and right ventricular hypertrophy in rats (31). Therefore, it seems reasonable to propose that ghrelin plays a prevailing role in physiological and pathophysiological functions of the lungs.
Our present study shows that lung injuries, characterized by increased lung water content, disruption of lung architecture, extravasation of red blood cells, accumulation of inflammatory cells, and decreased pulmonary blood flow, are present 20 hours after CLP in vehicle-treated animals. These injuries are associated with decreased levels of ghrelin in the pulmonary tissues. In this regard, administration of ghrelin may be a useful adjunct in the treatment of severe sepsis–induced lung injury. However, ghrelin is a small peptide with a relatively short half-life. Our study has indicated that the half-life of ghrelin is 11 minutes in the normal rat and 17 minutes during the late stage of sepsis (32). As such, a continuous infusion of ghrelin is required to maintain an effective in vivo concentration. Therefore, we used an osmotic minipump to infuse ghrelin intravenously after a slow bolus injection. Administration of ghrelin in this way has been shown to maintain relatively high levels of ghrelin in the circulation (17). In this study, we also found that pulmonary levels of ghrelin 20 hours after CLP in ghrelin-treated animals were 86% higher than those in sham-operated animals despite a 52% decrease after CLP. The increase in pulmonary levels of ghrelin is associated with mitigated lung injury, decreased bacterial load, increased pulmonary blood flow, and improved survival after CLP. Moreover, the fact that administration of ghrelin receptor antagonist decreased the 10-day survival rate after CLP and cecal excision confirms the notion that the beneficial effect of ghrelin in sepsis is mediated through its receptors. Thus, ghrelin may be an effective treatment for severe sepsis–induced ALI.
It is now widely accepted that ALI in the setting of sepsis is the result of the actions of an integrated network of soluble inflammatory mediators and a variety of inflammatory cells (33). The formation of proinflammatory mediators such as TNF-α and IL-6 plays an important role in the pathophysiology of ALI. TNF-α is present in the bronchoalveolar lavage fluid of patients at risk for acute respiratory distress syndrome (ARDS) and with established ARDS (34). The highest concentrations of TNF-α are found in the bronchoalveolar lavage fluid from patients with sustained ARDS. Raised levels of IL-6 have been described in a number of acute conditions such as burns, major surgery, and sepsis (35). Circulating levels of IL-6 have been shown to be excellent predictors of the severity of ARDS of various etiologies, including sepsis (36) and acute pancreatitis (37). In patients with ARDS, nonsurvivors have significantly higher ratios of bronchoalveolar lavage fluid to plasma cytokine concentrations than survivors (38). This suggests the critical role of lung inflammation in mortality. In this regard, the fact that ghrelin administration down-regulated pulmonary levels of TNF-α and IL-6 suggests another possible mechanism by which ghrelin exerts a beneficial effect in severe sepsis–induced ALI. In addition, the antiinflammatory property of ghrelin was confirmed by the result showing that acute ghrelin receptor inhibition with a specific ghrelin receptor antagonist increased pulmonary levels of TNF-α and IL-6. This result suggests the important role of ghrelin in the regulation of cytokine responses even under normal conditions.
Injection of TNF-α into experimental animals causes a syndrome that is indistinguishable from septic shock (39). Administration of IL-6 to normal rats decreases myocardial contractility and organ blood flow in a dose-dependent manner (40). Therefore, increased levels of proinflammatory cytokines cause deterioration in cardiovascular function. On the other hand, increases in cardiac output and organ blood flow do not prevent organ injury and inflammation in sepsis (41). In this regard, decreased inflammation appears to improve pathophysiology.
Expression of proinflammatory genes is regulated by transcriptional mechanisms. NF-κB is one critical transcription factor required for maximal expression of many cytokines involved in the pathogenesis of acute lung injury. Our previous study has shown that ghrelin inhibits TNF-α–induced NF-κB nuclear translocation in cultured endothelial cells (17). The result from the present study provide novel in vivo evidence showing that ghrelin inhibits the degradation of IκBα and prevents the translocation of NF-κB into the nucleus in the lungs. More importantly, the activity of NF-κB (i.e., binding with a discrete nucleotide sequence) was also decreased by ghrelin treatment. Activation of NF-κB appears to play a central role in the development of pulmonary inflammation and acute lung injury (7). In vitro studies have shown that NF-κB regulates gene expression of cytokines, chemokines, and adhesion molecules (42). All of these factors play an important role in lung inflammatory injury (43). In addition, an in vivo animal study has shown an association between NF-κB activation and the expression of cytokines, chemokines, and vascular adhesion molecules (44–46). These findings are supported by a study of patients with ARDS, which showed enhanced NF-κB activation in alveolar macrophages recovered by bronchoalveolar lavage (8). In vivo studies demonstrated that suppression of lung NF-κB activation results in decreased proinflammatory mediator expression and reduced inflammatory injury (7). Therefore, the beneficial effect of ghrelin in sepsis-induced ALI appears to be mediated through inhibition of NF-κB activity. Although the molecular mechanisms and cellular targets leading to inhibition of NF-κB activation by ghrelin remain to be determined, our study has shed some light on this issue. Our results have shown that ghrelin does not directly inhibit TNF-α and IL-6 release from endotoxin-stimulated Kupffer cells or peritoneal macrophages. The intact vagus nerve is essential for the inhibitory effect of ghrelin on the release of proinflammatory cytokines (47). Vagus nerve signaling is a critical component of the afferent loop that modulates immune responses to systemic inflammation (48). Acetylcholine released by stimulation of the vagus nerve binds to the α7 subunit of the nicotinic acetylcholine receptor expressed on macrophages, suppressing proinflammatory cytokine production by an NF-κB–dependent pathway (49, 50). Ghrelin receptors are expressed at high density in the brainstem (51). Our preliminary study has shown that intravenous administration of ghrelin after CLP activates the nucleus of the solitary tract in the brainstem, which controls vagal activity. Moreover, intracerebroventricular administration of ghrelin (1 nmol in 10 μl) reduced serum levels of TNF-α by 65% in endotoxemia (intravenous injection of lipopolysaccharide, 15 mg/kg body weight). Thus, it appears that the beneficial effect of ghrelin in sepsis is mediated through stimulation of the vagus nerve. In this study, we also found that ghrelin administration decreased bacterial load after CLP. This could be due to the general improvement in the animals' condition, thereby leading to increased bacterial phagocytosis by macrophages and neutrophils. However, the precise mechanism responsible for the effect of ghrelin on bacterial load warrants further investigation.
In summary, pulmonary levels of ghrelin decreased significantly 20 hours after CLP. Intravenous administration of ghrelin after the onset of sepsis increased pulmonary levels of ghrelin, ameliorated lung histology, reduced lung water content, elevated lung blood flow, improved survival, and decreased pulmonary levels of proinflammatory cytokines. Thus, ghrelin can be developed as a novel treatment for severe sepsis–induced ALI. This protective effect appears to be mediated through inhibition of NF-κB.
Supported by National Institutes of Health grants R01 GM053008 and R01 GM057468 (P.W.). R.W. was supported by Postdoctoral Fellowship 0325802T from the American Heart Association (the Heritage Affiliate).
Originally Published in Press as DOI: 10.1164/rccm.200604-511OC on July 12, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Vincent JL, Abraham E. The last 100 years of sepsis. Am J Respir Crit Care Med 2006;173:256–263. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–1310. [DOI] [PubMed] [Google Scholar]
- 3.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138–150. [DOI] [PubMed] [Google Scholar]
- 5.Heidecke CD, Hensler T, Weighardt H, Zantl N, Wagner H, Siewert JR, Holzmann B. Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. Am J Surg 1999;178:288–292. [DOI] [PubMed] [Google Scholar]
- 6.Eichacker PQ, Parent C, Kalil A, Esposito C, Cui X, Banks SM, Gerstenberger EP, Fitz Y, Danner RL, Natanson C. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med 2002;166:1197–1205. [DOI] [PubMed] [Google Scholar]
- 7.Fan J, Ye RD, Malik AB. Transcriptional mechanisms of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2001;281:L1037–L1050. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz MD, Moore EE, Moore FA, Shenkar R, Moine P, Haenel JB, Abraham E. Nuclear factor-κB is activated in alveolar macrophages from patients with acute respiratory distress syndrome. Crit Care Med 1996;24:1285–1292. [DOI] [PubMed] [Google Scholar]
- 9.Arnalich F, Garcia-Palomero E, Lopez J, Jimenez M, Madero R, Renart J, Vazquez JJ, Montiel C. Predictive value of nuclear factor κB activity and plasma cytokine levels in patients with sepsis. Infect Immun 2000;68:1942–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang KY, Arcaroli JJ, Abraham E. Early alterations in neutrophil activation are associated with outcome in acute lung injury. Am J Respir Crit Care Med 2003;167:1567–1574. [DOI] [PubMed] [Google Scholar]
- 11.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone–releasing acylated peptide from stomach. Nature 1999;402:656–660. [DOI] [PubMed] [Google Scholar]
- 12.Arvat E, Di Vito L, Broglio F, Papotti M, Muccioli G, Dieguez C, Casanueva FF, Deghenghi R, Camanni F, Ghigo E. Preliminary evidence that ghrelin, the natural GH secretagogue (GHS)–receptor ligand, strongly stimulates GH secretion in humans. J Endocrinol Invest 2000;23:493–495. [DOI] [PubMed] [Google Scholar]
- 13.Cowley MA, Grove KL. Ghrelin: satisfying a hunger for the mechanism. Endocrinology 2004;145:2604–2606. [DOI] [PubMed] [Google Scholar]
- 14.Wu JT, Kral JG. Ghrelin: integrative neuroendocrine peptide in health and disease. Ann Surg 2004;239:464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, McCormick ML, Sigmund CD, Tang C, Weintraub NL. Ghrelin inhibits proinflammatory responses and nuclear factor-κB activation in human endothelial cells. Circulation 2004;109:2221–2226. [DOI] [PubMed] [Google Scholar]
- 16.Wu R, Zhou M, Cui X, Simms HH, Wang P. Upregulation of cardiovascular ghrelin receptor occurs in the hyperdynamic phase of sepsis. Am J Physiol Heart Circ Physiol 2004;287:H1296–H1302. [DOI] [PubMed] [Google Scholar]
- 17.Wu R, Dong W, Zhou M, Cui X, Hank SH, Wang P. Ghrelin improves tissue perfusion in severe sepsis via downregulation of endothelin-1. Cardiovasc Res 2005;68:318–326. [DOI] [PubMed] [Google Scholar]
- 18.Wu R, Dong W, Ji Y, Cui Y, Yang D, Zhou M, Marini CP, Ravikumar TS, Wang P. Ghrelin attenuates gut and lung injury after intestinal ischemia and reperfusion [abstract]. Crit Care Med 2006;34:A16. [Google Scholar]
- 19.Yang S, Lim YP, Zhou M, Salvemini P, Schwinn H, Josic D, Koo DJ, Chaudry IH, Wang P. Administration of human inter-α-inhibitors maintains hemodynamic stability and improves survival during sepsis. Crit Care Med 2002;30:617–622. [DOI] [PubMed] [Google Scholar]
- 20.Yang S, Zhou M, Chaudry IH, Wang P. The role of lipopolysaccharide in stimulating adrenomedullin production during polymicrobial sepsis. Biochim Biophys Acta 2001;1537:167–174. [DOI] [PubMed] [Google Scholar]
- 21.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med 1982;3:35–56. [PubMed] [Google Scholar]
- 22.Cui X, Wu R, Zhou M, Simms HH, Wang P. Differential expression of cytochrome P450 isoforms in the lungs of septic animals. Crit Care Med 2004;32:1186–1191. [DOI] [PubMed] [Google Scholar]
- 23.Saeed RW, Varma S, Peng T, Tracey KJ, Sherry B, Metz CN. Ethanol blocks leukocyte recruitment and endothelial cell activation in vivo and in vitro. J Immunol 2004;173:6376–6383. [DOI] [PubMed] [Google Scholar]
- 24.Renard P, Ernest I, Houbion A, Art M, Le CH, Raes M, Remacle J. Development of a sensitive multi-well colorimetric assay for active NFκB. Nucleic Acids Res 2001;29:E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asakawa A, Inui A, Kaga T, Katsuura G, Fujimiya M, Fujino MA, Kasuga M. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 2003;52:947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693. [DOI] [PubMed] [Google Scholar]
- 27.Hattori N, Saito T, Yagyu T, Jiang BH, Kitagawa K, Inagaki CGH. GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab 2001;86:4284–4291. [DOI] [PubMed] [Google Scholar]
- 28.Volante M, Fulcheri E, Allia E, Cerrato M, Pucci A, Papotti M. Ghrelin expression in fetal, infant, and adult human lung. J Histochem Cytochem 2002;50:1013–1021. [DOI] [PubMed] [Google Scholar]
- 29.Katugampola SD, Pallikaros Z, Davenport AP. [125I-His9]-ghrelin, a novel radioligand for localizing GHS orphan receptors in human and rat tissue: up-regulation of receptors with athersclerosis. Br J Pharmacol 2001;134:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papotti M, Ghe C, Cassoni P, Catapano F, Deghenghi R, Ghigo E, Muccioli G. Growth hormone secretagogue binding sites in peripheral human tissues. J Clin Endocrinol Metab 2000;85:3803–3807. [DOI] [PubMed] [Google Scholar]
- 31.Henriques-Coelho T, Correia-Pinto J, Roncon-Albuquerque R Jr, Baptista MJ, Lourenco AP, Oliveira SM, Brandao-Nogueira A, Teles A, Fortunato JM, Leite-Moreira AF. Endogenous production of ghrelin and beneficial effects of its exogenous administration in monocrotaline-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 2004;287:H2885–H2890. [DOI] [PubMed] [Google Scholar]
- 32.Wu R, Zhou M, Cui X, Simms HH, Wang P. Ghrelin clearance is reduced at the late stage of polymicrobial sepsis. Int J Mol Med 2003;12:777–781. [PubMed] [Google Scholar]
- 33.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol 2004;202:145–156. [DOI] [PubMed] [Google Scholar]
- 34.Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F, Park DR, Pugin J, Skerrett SJ, Hudson LD, Martin TR. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;164:1896–1903. [DOI] [PubMed] [Google Scholar]
- 35.Nijsten MWN, Hack CE, Helle M, Ten Duis HJ, Klasen HJ, Aarden LA. Interleukin-6 and its relation to the humoral immune response and clinical parameters in burned patients. Surgery 1991;109:761–767. [PubMed] [Google Scholar]
- 36.Leser HG, Gross V, Scheibenbogen C, Heinisch A, Salm R, Lausen M, Ruckauer K, Andreesen R, Farthmann EH, Scholmerich J. Elevation of serum interleukin-6 concentration precedes acute-phase response and reflects severity in acute pancreatitis. Gastroenterology 1991;101:782–785. [DOI] [PubMed] [Google Scholar]
- 37.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock 2002;17:463–467. [DOI] [PubMed] [Google Scholar]
- 38.Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS: persistent elevation over time predicts poor outcome. Chest 1995;108:1303–1314. [DOI] [PubMed] [Google Scholar]
- 39.Natanson C, Eichenholz PW, Danner RL, Eichacker PQ, Hoffman WD, Kuo GC, Banks SM, MacVittie TJ, Parrillo JE. Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J Exp Med 1989;169:823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janssen SP, Gayan-Ramirez G, Van den Bergh A, Herijgers P, Maes K, Verbeken E, Decramer M. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation 2005;111:996–1005. [DOI] [PubMed] [Google Scholar]
- 41.Wang P, Ba ZF, Ayala A, Chaudry IH. Hepatocellular dysfunction persists during early sepsis despite increased volume of crystalloid resuscitation. J Trauma 1992;32:389–397. [DOI] [PubMed] [Google Scholar]
- 42.Blackwell TS, Christman JW. The role of nuclear factor-κB in cytokine gene regulation. Am J Respir Cell Mol Biol 1997;17:3–9. [DOI] [PubMed] [Google Scholar]
- 43.Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four κB-like motifs and of constitutive and inducible forms of NF-κB. Mol Cell Biol 1990;10:1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riedemann NC, Guo RF, Bernacki KD, Reuben JS, Laudes IJ, Neff TA, Gao H, Speyer C, Sarma VJ, Zetoune FS, et al. Regulation by C5a of neutrophil activation during sepsis. Immunity 2003;19:193–202. [DOI] [PubMed] [Google Scholar]
- 45.Tzeng HP, Ho FM, Chao KF, Kuo ML, Lin-Shiau SY, Liu SH. β-Lapachone reduces endotoxin-induced macrophage activation and lung edema and mortality. Am J Respir Crit Care Med 2003;168:85–91. [DOI] [PubMed] [Google Scholar]
- 46.Manning AM, Bell FP, Rosenbloom CL, Chosay JG, Simmons CA, Northrup JL, Shebuski RJ, Dunn CJ, Anderson DC. NF-κB is activated during acute inflammation in vivo in association with elevated endothelial cell adhesion molecule gene expression and leukocyte recruitment. J Inflamm 1995;45:283–296. [PubMed] [Google Scholar]
- 47.Wu R, Dong W, Cui X, Zhou M, Simms HH, Ravikumar TS, Wang P. Ghrelin down-regulates proinflammatory cytokines in sepsis through activation of the vagus nerve. Ann Surg 2007;245:480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tracey KJ. The inflammatory reflex. Nature 2002;420:853–859. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 2003;421:384–388. [DOI] [PubMed] [Google Scholar]
- 50.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 2007;117:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin Y, Matsumura K, Fukuhara M, Kagiyama S, Fujii K, Iida M. Ghrelin acts at the nucleus of the solitary tract to decrease arterial pressure in rats. Hypertension 2004;43:977–982. [DOI] [PubMed] [Google Scholar]