Abstract
Rationale: Decreased lung function has been linked to increased inflammation and oxidative stress. Statins have demonstrated antiinflammatory and antioxidant properties.
Objectives: We investigated the effect of statin use on decline in lung function in the elderly, and whether smoking modified this effect.
Methods: Our study population included 2,136 measurements on 803 elderly men from the Normative Aging Study whose lung function (FVC and FEV1) was measured two to four times between 1995 and 2005. Subjects indicated statin use and smoking history at each visit. We used mixed linear models to estimate the effects of each covariate, adjusting for subject and possible confounders.
Measurements and Main Results: For those not using statins, the estimated decline in FEV1 was 23.9 ml/year (95% confidence interval [CI], −27.8 to −20.1 ml/yr), whereas those taking statins had an estimated 10.9-ml/year decline in FEV1 (95% CI, −16.9 to −5.0 ml/yr). We also examined the effect of statins with smoking by dividing the cohort into four groups: never-smokers, longtime quitters (quit ≥ 10 yr ago), recent quitters (quit < 10 yr ago), and current smokers. We found a significant three-way interaction between time since first visit, statin use, and smoking status (P < 0.001). Within each smoking category, the effect of statins was always estimated to be beneficial, but the size of the improvement in the decline rate varied among smoking groups. We found similar results for FVC decline.
Conclusions: Our results indicate that statin use attenuates decline in lung function in the elderly, with the size of the beneficial effect modified by smoking status.
Keywords: statins, lung function, FVC, FEV1, smoking
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Inflammation and oxidative stress are associated with decreased lung function. Statins have demonstrated antiinflammatory and antioxidant properties.
What This Study Adds to the Field
Statin use is associated with slower rates of lung function decline in the elderly. This suggests a possible treatment for those with impaired lung function that should be investigated further.
Lung function has been shown to predict both cardiovascular mortality and total mortality (1, 2). In addition, a higher rate of decline in lung function has been associated with increased risks of both mortality (3, 4) and hospitalizations related to chronic obstructive pulmonary disease (COPD) (3). COPD, a condition characterized by airway limitation, inflammation, and long-term lung function decline, is a leading cause of mortality in the United States and the world (5).
Research has demonstrated that statins have many pleiotropic effects, including antiinflammatory and antioxidant effects. Statins have been shown to reduce serum levels of C-reactive protein (CRP), a marker of systemic inflammation (6). In addition, statins have been shown to reduce lung inflammation in transplant recipients (7). Studies have also demonstrated that statins reduce oxidative stress (8). Inflammation is considered central to the pathogenesis of COPD and oxidative stress is also believed to be important in its development (5). No study has previously investigated the effects of statin use on decline in lung function in smokers and nonsmokers.
We hypothesized that statins would have a protective effect on decline in lung function. This study examines whether the use of statins affects the rate of lung function decline in the elderly. In addition, we investigated whether smoking history modified this effect of statins.
METHODS
Study Population
Subjects in this study were part of the Veterans Administration (VA) Normative Aging Study, a longitudinal study established in 1963, details of which have been published previously (9). Briefly, 2,280 men from the greater Boston area, ages 21 to 80 years, enrolled in the study after an initial health screening determined that they were free of known chronic medical conditions. Participants visited the study center every 3 years to undergo physical examinations and fill out questionnaires. Our analyses included 803 subjects whose lung function was measured at least twice between January 1995 and June 2005.
Study center visits took place in the morning, after an overnight fast and abstinence from smoking. Physical examinations included measurement of height and lung function (FVC and FEV1). Subjects indicated pulmonary disorders (asthma, chronic bronchitis, emphysema) by a questionnaire based on the American Thoracic Society Division of Lung Diseases 1978 questionnaire (10). Information on smoking habits and medication use was also collected by questionnaire, with responses confirmed by a trained interviewer. Descriptive statistics for these data divided by statin use are listed in Table 1. Note that because of the observational nature of our study, some subjects began using statins during the study period and are thus in both groups, depending on the visit date. Because our groups are not independent, we provide Table 1 for a more general comparison of the group characteristics and did not calculate statistically significant differences between the groups.
TABLE 1.
DESCRIPTIVE STATISTICS
| Variable | Statin Users* (n = 571) | Not Using Statins* (n = 1,565) |
|---|---|---|
| Age, yr | 71.7 (6.5) | 70.5 (7.4) |
| Height, m | 1.74 (0.066) | 1.74 (0.070) |
| Ethnic background, n (%) | ||
| White | 558 (98.4) | 1518 (98.0) |
| Black | 9 (1.6) | 31 (2.0) |
| Smoking status, n (%) | ||
| Never-smoker | 162 (28.6) | 483 (31.1) |
| Longtime quitter (quit ≥ 10 yr before visit) | 353 (62.4) | 858 (55.2) |
| Recent quitter (quit < 10 yr before visit) | 38 (6.7) | 130 (8.4) |
| Current smoker | 13 (2.3) | 83 (5.3) |
| Lifetime smoking, pack-years† | 30.9 (23.0) | 28.8 (23.8) |
| Chronic pulmonary diseases, n (%) | ||
| Doctor-confirmed asthma | 29 (5.0) | 107 (6.8) |
| Unconfirmed asthma | 2 (0.35) | 8 (0.51) |
| Doctor-confirmed chronic bronchitis | 35 (6.2) | 101 (6.49) |
| Unconfirmed chronic bronchitis | 2 (0.35) | 12 (0.77) |
| Doctor-confirmed emphysema | 12 (2.1) | 45 (2.9) |
| Unconfirmed emphysema | 2 (0.35) | 5 (0.32) |
| Coronary heart disease, n (%) | 327 (57.3) | 279 (17.8) |
| FVC, L | 3.27 (0.66) | 3.44 (0.68) |
| FEV1, L | 2.45 (0.59) | 2.57 (0.59) |
Values are listed as mean (SD) or number (%), where indicated. This table includes data collected at each visit for all measurements on all subjects.
Some subjects are in both groups (began taking statins during study period).
Pack-years for former or current smokers.
Lung Function Data
Pulmonary function tests were performed as previously reported (11). Briefly, a water-filled recording spirometer was used to obtain measures of FVC and FEV1, with values adjusted by body temperature and pressure. These spirometric tests were performed in accordance with American Thoracic Society guidelines. The average levels for FVC and FEV1 by smoking category are listed in Table 2.
TABLE 2.
LUNG FUNCTION BY SMOKING STATUS CATEGORY
| FEV1 (L)
|
FVC (L)
|
||||
|---|---|---|---|---|---|
| Smoking Status | n | Mean | SD | Mean | SD |
| Never-smoker | 645 | 2.61 | 0.54 | 3.41 | 0.67 |
| Longtime quitter (quit ≥ 10 yr before visit) | 1,211 | 2.55 | 0.59 | 3.41 | 0.69 |
| Recent quitter (quit < 10 yr before visit) | 168 | 2.36 | 0.66 | 3.29 | 0.69 |
| Current smoker | 96 | 2.25 | 0.59 | 3.24 | 0.65 |
Statistical Methods
We chose the following variables a priori and included them in all of our models regardless of statistical significance: baseline age, height at visit, race, cigarette smoking at visit (current smoker, long-ago quitter, recent quitter, and pack-years), chronic lung conditions at visit (asthma, emphysema, and chronic bronchitis), season, weekday, and year of visit.
Measurements of FVC and FEV1 were taken for each subject on up to four visits, with 377 patients measured twice, 322 measured three times, and 104 measured four times, between January 1995 and June 2005. We used a mixed linear model for our analysis to account for both the repeated measurements on each subject as well as the observational nature of our study. An association between the dependent variable and a covariate was considered to be significant if the covariate had a P value of less than 0.05 in the model.
We estimated the annual decline in lung function by statin use via an interaction term in the model. We present the estimated effect of statin use on decline in lung function as the change in milliliters per year of FVC or FEV1. These changes were calculated by Δyrs × β, with 95% confidence intervals (CI) Δyrs × (β ± 1.96 × SE), where Δyrs is the change in years (1 yr for our model), β is the estimated regression coefficient for time, and SE is the standard error of β.
RESULTS
Descriptive statistics for the cohort are shown in Table 1. Subjects using statins had more diagnosed coronary disease than nonusers, but otherwise the groups differed little. Although lung function was somewhat lower for statin users than for nonusers, the use of statins increased over time. Hence, these measurements were generally made at an older age than the measurements without statins. Models that control for age or time are then required to examine the association between lung function and statin use.
All the models presented here include both white and black subjects, using a dummy variable to account for race. Because fewer than 2% of subjects were black, we also ran these models including only white subjects and produced equivalent results (data not shown). Thus, we included both black and white subjects in the models to increase power. We considered whether bronchodilator and/or corticosteroid use might be influencing these results. The use of these medications was low (only 3% of observations), and when we ran our model excluding those patients taking either medication we produced equivalent results (data not shown). Thus, we kept these subjects in the models presented here.
We first examined the effect of statins on lung function decline for the entire cohort. For subjects not using statins, the estimated annual decline in FEV1 was 23.9 ml/year (95% CI, −27.8 to −20.1 ml/yr), whereas the decline was estimated at 10.9 ml/year (95% CI, −16.9 to −5.0 ml/yr) for nonusers (P value for interaction < 0.001). Similarly, the estimated decline in FVC was 36.2 ml/year (95% CI, −41.5 to −30.8 ml/yr) for subjects not taking statins, whereas those taking statins were estimated to have a 14.0-ml/year decline in FVC (95% CI, −22.2 to −5.9 ml/yr) (P value for interaction < 0.001).
For comparison, we also ran unadjusted models with time as the only covariate. We ran the models separately for statin users and nonusers to obtain unadjusted estimates of change in lung function for each group, with results listed in Table 3. These unadjusted estimates were very similar to the estimates from our model adjusting for covariates.
TABLE 3.
UNADJUSTED CHANGES IN LUNG FUNCTION BY STATIN USE
| Effect by Statin Use | Change in FEV1 (ml/yr) | 95% Confidence Interval | Change in FVC (ml/yr) | 95% Confidence Interval |
|---|---|---|---|---|
| Not using statins | −24.0 | −27.1 to −20.8 | −34.5 | −38.8 to −30.2 |
| Using statins | −12.4 | −18.9 to −5.9 | −14.9 | −24.2 to −5.6 |
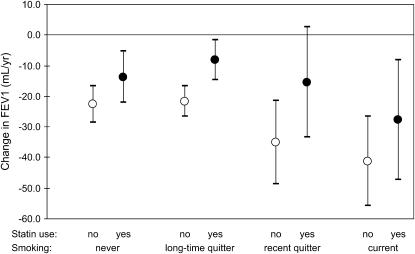
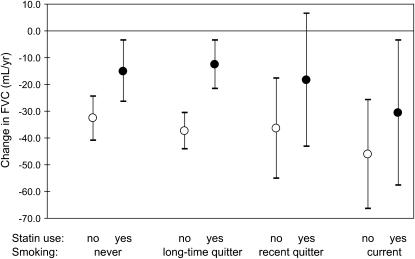
In our first model, we controlled for the effects of smoking (see Statistical Methods), because smoking is known to cause more rapid lung function decline. In our second model, we examined whether smoking status modified the estimated effect of statins. We divided the cohort into four groups: (1) never-smokers, (2) longtime quitters (quit ≥ 10 yr ago), (3) recent quitters (quit < 10 yr ago), and (4) current smokers. The three-way interaction among time since first visit, statin use, and smoking status was significant for FEV1 (P value for interaction < 0.001) and for FVC (P value for interaction < 0.001). We obtained separate estimates for statin users and nonusers in each smoking category (Table 4; Figures 1 and 2). Within each smoking group, those not taking statins were estimated to experience faster declines in FEV1 and FVC than those taking statins, but the size of the effect varied somewhat with smoking status.
TABLE 4.
DECLINE IN FVC AND FEV1 BY STATIN USE AND SMOKING STATUS
| Effect by Statin Use and Smoking Status
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Smoking Status | Statin Use | n | Change in FEV1 (ml/yr) | 95% CI | P Value | Change in FVC (ml/yr) | 95% CI | P Value |
| Never | No | 483 | −22.6 | −28.6 to −16.5 | 0.069 | −32.7 | −41.0 to −24.4 | 0.008 |
| Never | Yes | 162 | −13.7 | −22.0 to −5.4 | −15.0 | −26.5 to −3.6 | ||
| Longtime quitter | No | 858 | −21.6 | −26.6 to −16.6 | <0.001 | −37.4 | −44.3 to −30.5 | <0.001 |
| Longtime quitter | Yes | 353 | −8.2 | −14.8 to −1.6 | −12.7 | −21.8 to −3.6 | ||
| Recent quitter | No | 130 | −35.1 | −48.7 to −21.5 | 0.059 | −36.5 | −55.2 to −17.7 | 0.211 |
| Recent quitter | Yes | 38 | −15.4 | −33.5 to 2.7 | −18.5 | −43.2 to 6.3 | ||
| Current | No | 83 | −41.2 | −55.9 to −26.4 | 0.238 | −46.1 | −66.5 to −25.6 | 0.324 |
| Current | Yes | 13 | −27.8 | −47.4 to −8.3 | −30.6 | −57.7 to −3.6 | ||
Definition of abbreviation: CI = confidence interval.
P values represent significance of pairwise interactions.
Figure 1.
Decline in FEV1 by statin use and smoking status.
Figure 2.
Decline in FVC by statin use and smoking status.
Because of the uneven groupings within our study population and the resulting overlaps of confidence intervals, the estimates presented here are only suggestive of any actual differences among subgroups. In general, all subgroups appear to experience a beneficial effect of statins, and the differences in the size of that effect do not appear to be extreme. In terms of the net difference in rates of decline between statin nonusers and users within each subgroup, the recent quitters had the greatest net difference for FEV1 (19.7 ml/yr) and longtime quitters had the greatest net difference for FVC (24.7 ml/yr). In terms of the fold-difference in rates of decline, the longtime quitter group had the highest fold difference for both FEV1 (∼2.5) and FVC (∼3). Our results suggest (weakly) that longtime quitters and recent quitters may be able to benefit more from statin use than other groups.
DISCUSSION
Our analyses support our hypothesis that statin use has a protective effect on decline in lung function. We found that a noticeably attenuated lung function decline is predicted for those who are taking statins compared with those who are not taking statins. In addition, we looked at smoking status and found a significant three-way interaction among year, statin use, and smoking status in predicting lung function. Our results examining effect modification by smoking status suggested that longtime quitters and recent quitters may experience a larger beneficial effect from statins as compared with never-smokers and current smokers, but additional studies including more current smokers would be needed to confirm that finding.
To our knowledge, this is the first study to report a beneficial effect of statins on the rate of lung function decline. Although several promising medications are under investigation, no treatment for patients with COPD other than for smoking cessation has been consistently shown to improve the long-term rate of lung function decline (5). Our results point to a treatment that could potentially benefit those with COPD in addition to the benefits of stopped smoking. We observed that the rates of declines of FVC and FEV1 are cut at least in half in longtime quitters and recent quitters who are taking statins compared with those not taking statins. Although clinical trials will be needed to determine the actual size of the effect, our estimates suggest that, if these effects are confirmed, statin use could have a noticeable clinical benefit. Also, the link between lung function and mortality and the reduced levels of lung function in the elderly indicate the importance of a possibility of reducing the rate of decline.
Very little research has been published that examines the effect of statins on lung function. A recent abstract published for the Chest 2006 conference examined the effects of statin use on lung function in 485 elderly subjects who were current or former smokers (12). This abstract reported reduced declines in FVC and FEV1 for statin users compared with nonusers. Although we do not know the details of this study as only an abstract has been published, these preliminary results are consistent with the effects we identified in this study for current and former smokers. The scope of our study is broader by including never-smokers and comparing these effects to categories of smoking history, and our study population is larger in size.
Another relevant study examined the effect of statin use on lung transplant recipients (7). This study found that among double-lung and heart–lung recipients, statin users (n = 9) had significantly better post-transplantation spirometry than nonusers (n = 64). When considering the single-lung recipients, the study found improved lung function in statin users, but the effect was smaller and the researchers observed high variability between baseline and subsequent pulmonary function tests. This study also examined inflammatory cells in the bronchoalveolar lavage fluid and found significantly lower percentages of neutrophils and lymphocytes in statin users compared with nonusers. Although this study of post-transplantation effects of statin is not representative of a larger population in a normal aging process, the beneficial effects of statins observed are consistent with our findings, and the inflammatory cell counts suggest that the improved lung function seen in statin users could result from reduced inflammation in the lung.
One possible mechanism for the protective effect of statins relates to lung inflammation. A recent review suggests that, given the wide-ranging antiinflammatory properties of statins, there is a potential for the clinical use of statins in treating respiratory disease that merits further investigation (13). Animal studies have found that statins reduce neutrophil levels in lung tissue when lipopolysaccharides are used to induce an inflammatory response in the lung (14, 15). Another study on rats showed that statins protected against smoking-induced lung damage and exhibited some antiinflammatory effects on the lung (16). In humans, the study by Johnson and colleagues mentioned above found reduced levels of neutrophils and lymphocytes in the bronchoalveolar lavage of statin users compared with nonusers (7). Statins have also been shown to suppress or reduce the concentrations of other cells related to inflammatory responses, including Th1 cells, interferon-γ cells (17), and natural killer cells (18), and IL-8 production in human lung tissue (19, 20).
Decreasing systemic inflammation may also be a possible mechanism for the effect of statins on lung function. Statins are known to reduce serum levels of CRP (6). Cross-sectional studies have found an inverse relationship between CRP and lung function, in which higher levels of CRP are associated with lower lung function, even in healthy subjects (21). Two recent studies have examined the relationship of CRP to lung function decline using both cross-sectional and longitudinal analyses. Both studies found the usual inverse relationship between serum CRP and FVC and/or FEV1 in cross-sectional analyses (22, 23). In longitudinal analyses, although neither study found a significant association between baseline serum CRP and decline in FVC and/or FEV1 between the two study visits, one of them found that increases in CRP levels between visits were associated with greater declines in FEV1 (23).
Inflammation and lung function decline are also linked in studies of COPD. COPD is characterized by chronic airway inflammation and causes long-term lung function decline (5), and several studies have found higher levels of CRP in those with COPD compared with control subjects (24). Recently, CRP was found to be predictive of COPD morbidity and mortality (25). In addition, statin use has been associated with reduced mortality in subjects with COPD, including those without diagnosed ischemic heart disease (although some subjects probably had undiagnosed heart conditions) (26). Therefore, the antiinflammatory effects of statins could be responsible for the attenuated declines in lung function we observed.
Statins may decrease inflammation by decreasing oxidative stress in the lung. Studies have found that nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, expressed in the lung epithelium, produces reactive oxygen species in human bronchial epithelial cells (27, 28). Statins have been shown to inhibit activation of NADPH oxidase in monocytes (29) and possibly also in neutrophils (30). Thus, the protective effect of statins on lung function could be related to reducing reactive oxygen species through inhibition of NADPH oxidase activation. Of course, more research is needed to identify whether this is the probable mechanism.
One limitation of this study is the demographics of the study population. Study subjects were all elderly men, most of them white. Because lung function decline is believed to vary by sex, age, and race, we cannot readily extrapolate our results to populations of different demographics. Also, our study population includes fewer current smokers and recent quitters than longtime quitters and never-smokers, so the estimates for the effects of those may not be representative of larger populations. Still, the significance of the interaction indicates differences in statin effect among the smoking categories, and this model gives a suggestion of what the difference in effect might be. Additional research on a study population that included more smokers and recent quitters would be needed to determine more specific effects for statin use within these various groups.
Another consideration is that this study is not a randomized clinical trail. We must consider how the nonrandom selection of those taking statins may have influenced our results. Because statins are usually prescribed to lower cholesterol, we do not expect that statin users would be predisposed to a smaller lung function decline than nonstatin users because of this condition. To test whether statin use was acting as a surrogate for coronary heart disease, we changed our interaction model to estimate the annual decline in lung function by coronary heart disease instead of statin use, and found no significant association between them (results not shown).
We also considered that those taking statin medications may see a doctor more regularly and therefore take more medications than other patients, which could mean that we are seeing an effect of a combination of medicines. The most common medications that may improve lung function would be bronchodilators and corticosteroids, but, as explained in Results, the use of these medications was low, and our model produced equivalent estimates for the modification effect of statins when those measurements were excluded. Thus, we do not believe that the effect seen in statins is influenced by these medications.
If those taking statins also see a doctor more regularly, it is possible that these subjects may engage in other health-conscious behavior that could confound our results. This “healthy user” effect may be a source of confounding in results of the benefits of statin use (31). Although we cannot definitively rule this out, we attempted to check on this by using our measure of omega 3 fatty acid intake. We first added omega 3 intake into the model and found that controlling for it did not change the estimated effect of statins. We also divided subjects at the highest quartile of omega 3 intake and found no significant interaction with time for this categorization (results not shown). This, of course, does not mean that subjects are not participating in healthy lifestyle activities that we have not measured, which is why these findings need to be confirmed by randomized clinical trials before conclusions can be generalized to larger populations.
Finally, we note that, in Table 1, the descriptive statistics show that the mean FVC and FEV1 levels are slightly lower in the statin group than in the control group. Part of this difference can be explained by the higher mean age for the statin group. Some of the difference could be related to the higher levels of heart disease and hypertension in the control group, which could lead to taking medications such as β-blockers that lower lung function. Adding β-blocker use to our model did not change the estimates for the effects of statins, and we found no association between use of β-blockers and lung function decline. Thus, we do not expect that any conditions causing the slightly lower initial lung function in statin users have an impact on our findings for the effect of statins. We also considered the issue of a survivor bias. Those with very low lung function who remain in the study may be declining more slowly, which could be a factor in our estimates because the lung function levels appear to be slightly lower in statin users than in nonusers and are lower in those with more smoking history. To check whether this may be affecting our estimates, we excluded subjects in the lowest 5% of lung function and re-ran our analyses. The new estimates for the effect of statin use on FEV1 and FVC decline were the same as those above (results not shown), so we do not think those with low lung function are biasing the effect of statins. Of course, the issue of survivor bias is always a concern, but we think the effect on our estimates is minimal.
Although a randomized clinical trial is needed to confirm our findings, the results of our prospective analyses indicate that statin use reduces the severity of lung function decline in the elderly, and these beneficial effects seem to be present regardless of smoking history. This research adds to the growing body of knowledge indicating the positive effects of statin use beyond its cholesterol-lowering properties.
Acknowledgments
The authors thank Elaine R. Dibbs, Shelly Amberg, and Jordan Awerbach for their invaluable contributions to the VA Normative Aging Study.
Supported by U.S. Environmental Protection Agency grants R827353 and R832416 and National Institute of Environmental Health Sciences grants ES015172-01 and ES0002. The VA Normative Aging Study is supported by the Cooperative Studies Program/Epidemiology Research and Information Center of the U.S. Department of Veterans Affairs and is a component of the Massachusetts Veterans Epidemiology Research and Information Center, Boston, Massachusetts.
Originally Published in Press as DOI: 10.1164/rccm.200705-656OC on August 2, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Schunemann HJ, Dorn J, Grant BJ, Winkelstein W Jr, Trevisan M. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest 2000;118:656–664. [DOI] [PubMed] [Google Scholar]
- 2.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest 2005;127:1952–1959. [DOI] [PubMed] [Google Scholar]
- 3.Mannino DM, Davis KJ. Lung function decline and outcomes in an elderly population. Thorax 2006;61:472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan G, Knuiman MW, Divitini ML, James A, Musk AW, Bartholomew HC. Decline in lung function and mortality: the Busselton Health Study. J Epidemiol Community Health 1999;53:230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med 2001;163:1256–1276. [DOI] [PubMed] [Google Scholar]
- 6.Prasad K. C-reactive protein (CRP)-lowering agents. Cardiovasc Drug Rev 2006;24:33–50. [DOI] [PubMed] [Google Scholar]
- 7.Johnson BA, Iacono AT, Zeevi A, McCurry KR, Duncan SR. Statin use is associated with improved function and survival of lung allografts. Am J Respir Crit Care Med 2003;167:1271–1278. [DOI] [PubMed] [Google Scholar]
- 8.Kurian KC, Rai P, Sankaran S, Jacob B, Chiong J, Miller AB. The effect of statins in heart failure: beyond its cholesterol-lowering effect. J Card Fail 2006;12:473–478. [DOI] [PubMed] [Google Scholar]
- 9.Bell B, Rose C, Damon A. The Normative Aging Study: an interdisciplinary and longitudinal study of health and aging. Aging Hum Dev 1972;3:4–17. [Google Scholar]
- 10.Ferris BG. Epidemiology standardization project (American Thoracic Society). Am Rev Respir Dis 1978;118:1–120. [PubMed] [Google Scholar]
- 11.Sparrow D, O'Connor G, Colton T, Barry CL, Weiss ST. The relationship of nonspecific bronchial resp to the occurrence of respiratory symptoms and decreased levels of pulmonary function: the Normative Aging Study. Am Rev Respir Dis 1987;135:1255–1260. [DOI] [PubMed] [Google Scholar]
- 12.Younis WG, Chbeir EA, Daher NN, Dernaika TA, Kinasewitz GT, Keddissi JI. Statins protect smokers from lung disease. Chest Meeting Abstracts 2006;130:180S. [Google Scholar]
- 13.Hothersall E, McSharry C, Thomson NC. Potential therapeutic role for statins in respiratory disease. Thorax 2006;61:729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao HW, Mao LG, Zhu JP. Protective effects of pravastatin in murine lipopolysaccharide-induced acute lung injury. Clin Exp Pharmacol Physiol 2006;33:793–797. [DOI] [PubMed] [Google Scholar]
- 15.Fessler MB, Young SK, Jeyaseelan S, Lieber JG, Arndt PG, Nick JA, Worthen GS. A role for hydroxy-methylglutaryl coenzyme a reductase in pulmonary inflammation and host defense. Am J Respir Crit Care Med 2005;171:606–615. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Lee DS, Kim EK, Choe KH, Oh YM, Shim TS, Kim SE, Lee YS, Lee SD. Simvastatin inhibits cigarette smoking–induced emphysema and pulmonary hypertension in rat lungs. Am J Respir Crit Care Med 2005;172:987–993. [DOI] [PubMed] [Google Scholar]
- 17.McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I, Capell HA, Sattar N. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet 2004;363:2015–2021. [DOI] [PubMed] [Google Scholar]
- 18.Katznelson S, Wang XM, Chia D, Ozawa M, Zhong HP, Hirata M, Terasaki PI, Kobashigawa JA. The inhibitory effects of pravastatin on natural killer cell activity in vivo and on cytotoxic T lymphocyte activity in vitro. J Heart Lung Transplant 1998;17:335–340. [PubMed] [Google Scholar]
- 19.Hayden J, Swartfiguer J, Szelinger S. e al. Lysophosphatidylcholine stimulation of alveolar epithelial cell interleukin-8 production and neutrophil chemotaxis is inhibited by statin treatment [abstract]. Proc Am Thorac Soc 2005;2:A72. [Google Scholar]
- 20.Gaugler MH, Vereycken-Holler V, Squiban C, Vandamme M, Vozenin-Brotons MC, Benderitter M. Pravastatin limits endothelial activation after irradiation and decreases the resulting inflammatory and thrombotic responses. Radiat Res 2005;163:479–487. [DOI] [PubMed] [Google Scholar]
- 21.Aronson D, Roterman I, Yigla M, Kerner A, Avizohar O, Sella R, Bartha P, Levy Y, Markiewicz W. Inverse association between pulmonary function and C-reactive protein in apparently healthy subjects. Am J Respir Crit Care Med 2006;174:626–632. [DOI] [PubMed] [Google Scholar]
- 22.Fogarty AW, Britton JR, Jones S, Lewis SA, McKeever T. Systemic inflammation and decline in lung function in a general population: a prospective study. Thorax 2007;62:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaaban R, Kony S, Driss F, Leynaert B, Soussan D, Pin I, Neukirch F, Zureik M. Change in C-reactive protein levels and FEV1 decline: a longitudinal population-based study. Respir Med 2006;100:2112–2120. [DOI] [PubMed] [Google Scholar]
- 24.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004;59:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:250–255. [DOI] [PubMed] [Google Scholar]
- 26.Soyseth V, Brekke PH, Smith P, Omland T. Statin use is associated with reduced mortality in COPD. Eur Respir J 2007;29:279–283. [DOI] [PubMed] [Google Scholar]
- 27.Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J 2003;17:1502–1504. [DOI] [PubMed] [Google Scholar]
- 28.Shao MX, Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA 2005;102:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delbosc S, Morena M, Djouad F, Ledoucen C, Descomps B, Cristol JP. Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, are able to reduce superoxide anion production by NADPH oxidase in THP-1-derived monocytes. J Cardiovasc Pharmacol 2002;40:611–617. [DOI] [PubMed] [Google Scholar]
- 30.Bandoh T, Sato EF, Mitani H, Nakashima A, Hoshi K, Inoue M. Antioxidative potential of fluvastatin via the inhibition of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity. Biol Pharm Bull 2003;26:818–822. [DOI] [PubMed] [Google Scholar]
- 31.Majumdar SR, McAlister FA, Eurich DT, Padwal RS, Marrie TJ. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ 2006;333:999. [DOI] [PMC free article] [PubMed] [Google Scholar]