Abstract
Rationale: Cleaning work and professional use of certain cleaning products have been associated with asthma, but respiratory effects of nonprofessional home cleaning have rarely been studied.
Objectives: To investigate the risk of new-onset asthma in relation to the use of common household cleaners.
Methods: Within the follow-up of the European Community Respiratory Health Survey in 10 countries, we identified 3,503 persons doing the cleaning in their homes and who were free of asthma at baseline. Frequency of use of 15 types of cleaning products was obtained in a face-to-face interview at follow-up. We studied the incidence of asthma defined as physician diagnosis and as symptoms or medication usage at follow-up. Associations between asthma and the use of cleaning products were evaluated using multivariable Cox proportional hazards or log-binomial regression analysis.
Measurements and Main Results: The use of cleaning sprays at least weekly (42% of participants) was associated with the incidence of asthma symptoms or medication (relative risk [RR], 1.49; 95% confidence interval [CI], 1.12−1.99) and wheeze (RR, 1.39; 95% CI, 1.06−1.80). The incidence of physician-diagnosed asthma was higher among those using sprays at least 4 days per week (RR, 2.11; 95% CI, 1.15−3.89). These associations were consistent for subgroups and not modified by atopy. Dose–response relationships (P < 0.05) were apparent for the frequency of use and the number of different sprays. Risks were predominantly found for the commonly used glass-cleaning, furniture, and air-refreshing sprays. Cleaning products not applied in spray form were not associated with asthma.
Conclusions: Frequent use of common household cleaning sprays may be an important risk factor for adult asthma.
Keywords: airway irritants, epidemiology, incidence, ECRHS
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Several studies have provided evidence for adverse respiratory health effects related to professional cleaning exposures. However, potential risks of nonprofessional uses in private homes have not been evaluated.
What This Study Adds to the Field
Common, nonprofessional use of household cleaning products in spray form is associated with new-onset asthma in adults.
There is growing evidence that cleaning workers are at increased risk for asthma (1–3), in some areas being particularly apparent for those employed in domestic cleaning (4). Epidemiologic studies have identified specific professional cleaning products associated with asthma, including bleach (5) and sprays (6). Many products used in professional cleaning are also widely applied in private households. Analysis of data from the first phase of European Community Respiratory Health Survey (ECRHS I) showed that homemakers had a small but statistically significant excess risk of asthma, and it was hypothesized that this may be related to the use of cleaning products at home (1). Several studies have reported chronic respiratory disorders after accidental inhalation related to (the mixing of) household cleaners (7–10), but we are not aware of studies that have evaluated respiratory effects of common use of domestic cleaning products. The aim of this study was to investigate the risk of new-onset asthma in relation to the use of common household cleaners within the follow-up of the ECRHS. Some of the results of these studies have been previously reported in the form of an abstract (11).
METHODS
The methodology of the ECRHS II has been described elsewhere (12). Briefly, 29 study centers did a follow-up investigation on asthma and allergy and their known or suspected risk factors in a random population sample of men and women, who were 20 to 44 years of age at the baseline survey (i.e., ECRHS I). Twenty-two centers from 10 European countries agreed to take part in the assessment of selected occupational and domestic exposures at follow-up using modular questionnaires. All local institutional committees on ethical practice approved the study protocol, and participants provided written, informed consent.
At the face-to-face interview of ECRHS II, 4,267 (59% of all) participants indicated doing or having done the cleaning and/or washing in their homes during the follow-up period. To study new-onset asthma, we excluded 764 individuals with asthma at baseline according to a definition with a high sensitivity (13) (those who had reported a history of asthma and/or having had nocturnal attacks of shortness of breath in the last 12 mo, and/or wheeze when not having a cold in the last 12 mo in ECRHS I).
Asthma at follow-up was evaluated in several ways. Current asthma was defined as having had an attack of asthma in the last 12 months and/or having had nocturnal attacks of shortness of breath in the last 12 months and/or using current medication for asthma (12). Current wheeze was defined as wheezing or whistling in the chest at any time in the last 12 months when not having a cold. Participants who reported that they had ever had asthma were in addition asked (1) whether asthma was confirmed by a doctor and (2) how old they were when they had their first attack of asthma. Physician-diagnosed asthma was defined as reported asthma confirmed by a doctor with reported first asthma attack between ECRHS I and II.
In a face-to-face interview, participants were asked about the use of products for domestic cleaning and washing during the follow-up period (www.ecrhs.org). A short questionnaire was developed based on a previous study in Spanish cleaners (14), adapted for cleaning activities in the participant's own home, and pilot-tested in one center (15). The frequency of use of 15 different products was recorded as never, less than 1 day per week, 1 to 3 days per week, or 4 to 7 days per week.
Procedures and equipment for clinical testing were identical at both surveys. FEV1 was recorded by means of spirometry using a standardized method (12). Methacholine challenge was performed with a dosimeter (Mefar, Brescia, Italy). Bronchial hyperresponsiveness (BHR) was defined as a 20% fall in FEV1 associated with a methacholine dose of 1 mg or less. Atopy was defined as a specific serum IgE level of greater than 0.35 kU/L to house dust mite, cat, timothy grass, or Cladosporium herbarum.
Statistical analyses were done using Stata version 8 (Stata Corporation, College Station, TX). Associations between the frequency of all individual cleaning exposures from the questionnaire and incidence rates of current asthma and wheeze were evaluated using binomial regression analyses with a log link. Associations with the incidence of physician-diagnosed asthma were determined by using Cox proportional hazards regression, with the onset of disease defined as the date of reported first attack of asthma. The exposure reference category in all analyses consisted of participants who used the cleaning product under study either never or less than once a week. All regression models were adjusted for sex, age, smoking, employment in a cleaning job during follow-up, and study center. Relative risks (RRs) and hazard ratios (HRs) for selected variables were estimated separately for each country. Potential heterogeneity between countries in the association between household exposure and asthma was examined by using standardized methods for random-effects meta-analysis (16).
RESULTS
The length of follow-up was on average 9 years (Table 1), with a twofold variation across individuals that was largely explained by study center. Two-thirds of the study population doing the cleaning and/or washing at home were women, ranging from 57 to 87% across countries. Only a small proportion (9%) were full-time homemakers at follow-up. About 6% had current asthma symptoms at the end of follow-up, whereas the incidence rate of physician-diagnosed asthma was 2.3 per 1,000 person-years. Depending on the definition, between 28 and 35% of the participants with asthma had BHR.
TABLE 1.
DEMOGRAPHIC AND RESPIRATORY HEALTH CHARACTERISTICS OF ECRHS II PARTICIPANTS DOING THE CLEANING AND/OR WASHING IN THEIR HOMES AND WHO HAD NO ASTHMA AT BASELINE (n = 3,503)
| ECRHS I (Baseline) | ECRHS II (Follow-up) | ||
|---|---|---|---|
| Length of follow-up, yr, mean (range) | 8.9 (5.8−11.7) | ||
| Age, yr, mean (range) | 33.7 (20−48) | 42.6 (28−57) | |
| Women, n (%) | 2,407 (68.7) | ||
| Current smokers, n (%) | 1,036 (29.6) | 951 (27.1) | |
| Ex-smokers, n (%) | 773 (22.1) | 909 (26.0) | |
| Full-time housewife or househusband, n (%) | N/A | 305 (8.7) | |
| Employment in cleaning job at any time, n (%) | 240 (6.9) | ||
| Current asthma symptoms or medication*, n (%) | 0 | 199 (5.7) | |
| Current wheeze without a cold†, n (%) | 0 | 226 (6.5) | |
| Physician-diagnosed asthma‡, n (%) | 0 | 71 (2.1) | |
| Bronchial hyperresponsiveness§, n (%) | 231 (8.8) | 247 (10.5) | |
| Atopy‖, n (%) | 710 (24.3) | 716 (24.0) | |
Definition of abbreviation: ECRHS = European Community Respiratory Health Survey; N/A = not available.
Attack of asthma and/or nocturnal attack of shortness of breath in the last 12 months and/or current asthma medication (n = 3,483).
Wheezing or whistling in the chest when not having a cold in the last 12 months (n = 3,480).
Diagnosis of asthma with recorded year of onset (n = 3,446).
Methacholine dose of 1 mg or less causing a fall of 20% in FEV1; n = 2,628 and 2,358 for ECRHS I and II, respectively.
Specific IgE to at least one out of four common aeroallergens; n = 2,924 and 2,978 for ECRHS I and II, respectively.
Frequency of use varied largely among the different cleaning products (Table 2). The frequencies of specific products were not strongly correlated. The correlation matrix showed that 95% of the Spearman's correlation coefficients were below 0.3 (results not presented). The highest correlation coefficients (0.41) were found between liquid multiuse cleaning products and perfumed or scented products, and between polishes and furniture sprays. Although for current asthma and wheeze most relative risk estimates were above unity, the majority of products were not significantly associated with asthma incidence. Consistently positive associations for most asthma definitions were observed for cleaning sprays in general (RR, 1.3–1.5), and glass-cleaning, furniture, and air-refreshing sprays in particular (Table 2). For all products, there were no apparent differences in asthma incidence between the exposure categories “never” and “less than 1 day per week” (data not shown).
TABLE 2.
ASSOCIATIONS BETWEEN THE USE OF CLEANING PRODUCTS AT LEAST WEEKLY AND THE INCIDENCE OF ASTHMA (n = 3,503)
| Cleaning Product | Use ≥ 1 d/wk Among All Participants (%) | Current Asthma* RR (95% CI) | Current Wheeze† RR (95% CI) | Physician-diagnosed Asthma‡ HR (95% CI) |
|---|---|---|---|---|
| Washing powders | 78.6 | 1.10 (0.75−1.63) | 1.28 (0.91−1.81) | 0.82 (0.43−1.54) |
| Liquid multiuse cleaning products | 83.1 | 0.94 (0.64−1.38) | 0.97 (0.70−1.35) | 0.98 (0.52−1.86) |
| Polishes, waxes | 8.7 | 1.12 (0.71−1.76) | 1.19 (0.77−1.85) | 1.42 (0.68−2.97) |
| Bleach | 28.0 | 1.22 (0.83−1.80) | 1.30 (0.90−1.87) | 1.10 (0.56−2.17) |
| Ammonia | 7.2 | 1.40 (0.87−2.23) | 1.31 (0.81−2.13) | 0.92 (0.33−2.59) |
| Decalcifiers, acids | 11.1 | 1.06 (0.70−1.61) | 1.18 (0.77−1.80) | 0.25 (0.06−1.04) |
| Solvents, stain removers | 5.5 | 1.54 (0.94−2.53) | 2.00 (1.30−3.07) | 0.48 (0.12−1.97) |
| Furniture sprays | 11.6 | 1.49 (0.99−2.23) | 1.46 (0.98−2.19) | 2.46 (1.26−4.80) |
| Glass-cleaning sprays | 22.1 | 1.35 (0.98−1.85) | 1.49 (1.12−2.00) | 1.43 (0.84−2.44) |
| Sprays for carpets, rugs, curtains | 1.3 | 1.24 (0.47−3.21) | 0.80 (0.26−2.41) | 0.80 (0.11−5.93) |
| Sprays for mopping the floor§ | 6.1 | 1.05 (0.59−1.85) | 1.03 (0.59−1.79) | 0.93 (0.30−2.85) |
| Oven sprays | 2.0 | 0.87 (0.33−2.28) | 1.24 (0.57−2.69) | 0.63 (0.09−4.64) |
| Ironing sprays | 3.0 | 1.66 (0.92−3.00) | 1.05 (0.48−2.30) | 1.51 (0.46−4.96) |
| Air-refreshing sprays | 16.2 | 1.71 (1.22−2.39) | 1.36 (0.98−1.88) | 1.46 (0.78−2.70) |
| Any spray | 42.1 | 1.49 (1.12−1.99) | 1.39 (1.06−1.80) | 1.28 (0.78−2.09) |
| Any perfumed or scented product | 67.8 | 1.09 (0.78−1.50) | 1.11 (0.83−1.49) | 1.29 (0.74−2.26) |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio; RR = relative risk.
RRs*† or HRs‡ with 95% CIs from log-binomial*† or Cox proportional hazards‡ regression models, adjusted for sex, age, smoking status, cleaning job, and study center. The reference category consisted of participants that used the cleaning product under study never or less than once a week. Each association was derived from a separate regression model.
Attack of asthma and/or nocturnal attack of shortness of breath in the last 12 months and/or current asthma medication (n = 3,483).
Wheezing or whistling in the chest when not having a cold in the last 12 months (n = 3,480).
Diagnosis of asthma with recorded year of onset (n = 3,446).
Information was not obtained in three study centers (Germany and Switzerland).
The association between use of any product in spray form and the incidence of asthma was studied in more detail. First, the risk of using sprays at least weekly was evaluated after stratification for sex, smoking status, and atopy (Table 3). The observed associations were similar for all groups (P for multiplicative interaction > 0.15). Only the risk of physician-diagnosed asthma in men was below 1, but confidence intervals were wide due to small numbers of exposed cases.
TABLE 3.
ASSOCIATIONS BETWEEN THE USE OF HOUSEHOLD CLEANING SPRAYS AT LEAST WEEKLY AND THE INCIDENCE OF ASTHMA, STRATIFIED BY SEX, CURRENT SMOKING, AND ATOPY AT FOLLOW-UP
| No. | Spray Use (%) | Current Asthma* RR (95% CI) | Current Wheeze† RR (95% CI) | Physician-diagnosed Asthma‡ HR (95% CI) | |
|---|---|---|---|---|---|
| Women | 2,407 | 48 | 1.45 (1.04−2.02) | 1.35 (0.97−1.88) | 1.51 (0.87−2.64) |
| Men | 1,096 | 30 | 1.76 (0.99−3.15) | 1.38 (0.89−2.14) | 0.61 (0.16−2.25) |
| Ever-smokers | 1,860 | 43 | 1.35 (0.91−1.99) | 1.32 (0.97−1.81) | 1.29 (0.67−2.50) |
| Never-smokers | 1,608 | 41 | 1.61 (1.05−2.47) | 1.51 (0.93−2.46) | 1.42 (0.68−2.97) |
| Atopics§ | 716 | 42 | 1.30 (0.80−2.13) | 1.39 (0.88−2.20) | 1.33 (0.56−3.12) |
| Nonatopics | 2,262 | 43 | 1.33 (0.90−1.95) | 1.36 (0.96−1.94) | 1.12 (0.58−2.16) |
For definition of abbreviations, see Table 2.
RRs*† or HRs‡ with 95% CIs from log-binomial*† or Cox proportional hazards‡ regression models, adjusted for sex, age, smoking status, cleaning job, and study center. The reference category consisted of participants that used sprays never or less than once a week.
Attack of asthma and/or nocturnal attack of shortness of breath in the last 12 months and/or current asthma medication.
Wheezing or whistling in the chest when not having a cold in the last 12 months.
Diagnosis of asthma with recorded year of onset.
Specific IgE to at least one of four common aeroallergens.
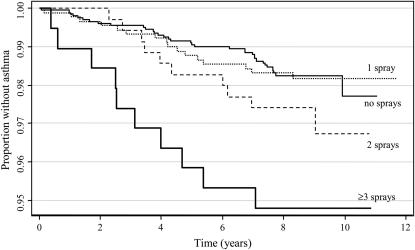
Second, the use of sprays was classified quantitatively according to the frequency of use, and according to the number of different types used at least weekly. A dose–response relationship was found between the frequency of use of any spray and the incidence of current asthma (Table 4). This trend was not observed for wheeze, whereas for physician-diagnosed asthma an increased risk was exclusively seen for the use of sprays at least 4 days a week. Dose–response relationships were apparent for all three outcomes when evaluating the number of different types of sprays used at least weekly (Table 4). The Kaplan-Meier plot illustrating the survival analysis for number of sprays and incidence of physician-diagnosed asthma is shown in Figure 1.
TABLE 4.
DOSE–RESPONSE RELATIONSHIPS BETWEEN THE USE OF HOUSEHOLD CLEANING SPRAYS AND THE INCIDENCE OF ASTHMA (n = 3,484)*
| Category | Frequency, n (%) | Current Asthma† RR (95% CI) | Current Wheeze‡ RR (95% CI) | Physician-diagnosed Asthma§ HR (95% CI) |
|---|---|---|---|---|
| Use of sprays < 1 d/wk | 2,016 (57.9) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Use of spray(s) 1 to 3 d/wk | 933 (26.8) | 1.36 (0.99−1.89) | 1.55 (1.17−2.06) | 0.93 (0.51−1.67) |
| Use of spray(s) 4 to 7 d/wk | 535 (15.4) | 1.75 (1.21−2.54) | 1.08 (0.73−1.59) | 2.11 (1.15−3.89) |
| P for linear trend | 0.002 | 0.204 | 0.041 | |
| One type of spray used ≥ 1 d/wk | 913 (26.2) | 1.37 (0.99−1.90) | 1.25 (0.92−1.69) | 0.97 (0.53−1.77) |
| Two types of spray used ≥ 1 d/wk | 355 (10.2) | 1.45 (0.92−2.27) | 1.63 (1.10−2.41) | 1.47 (0.70−3.06) |
| Three or more types of spray used ≥ 1 d/wk | 200 (5.7) | 2.40 (1.47−3.91) | 1.80 (1.11−2.94) | 2.96 (1.33−6.56) |
| P for linear trend | 0.001 | 0.003 | 0.022 |
For definition of abbreviations, see Table 2.
RRs†‡ or HRs§ with 95% CIs from log-binomial†‡ or Cox proportional hazards§ regression models, adjusted for sex, age, smoking status, cleaning job, and study center.
Nineteen participants did not provide complete quantitative information for all types of sprays.
Attack of asthma and/or nocturnal attack of shortness of breath in the last 12 months and/or current asthma medication.
Wheezing or whistling in the chest when not having a cold in the last 12 months.
Diagnosis of asthma with recorded year of onset (n = 3,446 with complete data).
Figure 1.
Kaplan-Meier survival curve for physician-diagnosed asthma according to the number of sprays used at least weekly. Onset of disease was defined as date of first attack of asthma.
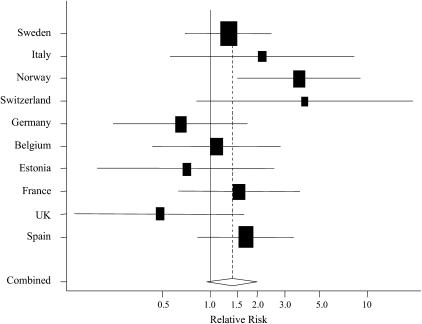
Third, the use of sprays was evaluated separately for each country. There was a more than twofold range in total frequency of use across countries (Table 5). The distribution of the most common sprays also differed qualitatively between countries. For instance, the use of furniture sprays was common in the United Kingdom and not in Germany, whereas for glass-cleaning sprays, this was the other way round. Meta-analysis of the country-specific associations between spray use at least weekly and asthma incidence showed that the risk was elevated in most countries (Figure 2). Differences in relative risk seemed apparent, although the test for heterogeneity did not reach conventional levels of statistical significance (P = 0.15). Similar results were found for wheeze (P for heterogeneity, 0.18) and physician-diagnosed asthma (P = 0.25; data not shown).
TABLE 5.
FREQUENCY OF USE AT LEAST WEEKLY OF THE MOST COMMON HOUSEHOLD CLEANING SPRAYS BY COUNTRY
| Country | No. | Any Spray (%) | Furniture (%) | Glass Cleaning (%) | Air Fresheners (%) |
|---|---|---|---|---|---|
| Sweden | 885 | 26.4 | 0.5 | 16.3 | 3.4 |
| Italy | 122 | 31.4 | 14.9 | 8.3 | 6.6 |
| Norway | 374 | 36.7 | 1.4 | 27.0 | 7.0 |
| Switzerland | 178 | 37.6 | 3.1 | 26.1 | 9.7 |
| Germany | 303 | 40.6 | 3.3 | 26.1 | 8.6 |
| Belgium | 298 | 41.8 | 8.4 | 21.2 | 20.5 |
| Estonia | 191 | 41.9 | 0.0 | 0.0 | 38.7 |
| France | 390 | 48.2 | 18.2 | 14.6 | 30.5 |
| United Kingdom | 182 | 55.5 | 33.3 | 10.1 | 31.3 |
| Spain | 580 | 66.0 | 35.6 | 43.9 | 25.6 |
Figure 2.
Association between the use of cleaning sprays at least once a week and the incidence of asthma symptoms or medication usage by country. Relative risk (RR) and 95% confidence interval (CI), adjusted within countries for study center, sex, age, smoking status, and employment in a cleaning job, are shown. The size of each box is proportional to the reciprocal of the variance of the estimate for the country. The diamond indicates 95% CI of the combined RR from the model, with country as the random effect (P = 0.15, test for heterogeneity). Countries are ranked from low to high frequency of spray use (see Table 5).
Excluding full-time homemakers or individuals who had (had) any employment in cleaning yielded very similar results. Adjustment of the presented analyses for occupational exposures to asthmagens or for socioeconomic status (either educational level defined using age of completing full-time education or social class based on longest held occupation) did not alter the results. Point estimates for the associations between any spray use and asthma varied only slightly (<5%), and RRs for current asthma and wheeze remained statistically significant.
Finally, associations of spray use with BHR were explored. Weekly use of sprays was not associated with BHR at follow-up (RR, 1.0). The same was true for asthma symptoms plus BHR (RR, 1.0, for current asthma plus BHR, and RR, 1.2, for wheeze plus BHR); however, an association seemed apparent for asthma diagnosis plus BHR (RR, 1.6; 95% CI, 0.7–3.6).
DISCUSSION
This is the first epidemiologic study that evaluated the risk of adult asthma related to nonoccupational use of common household cleaning products. We found an association between the use of products in spray form and the incidence of asthma according to either more sensitive or more specific definitions. This association was linked predominantly to the most commonly used air fresheners, glass cleaners, and furniture cleaning sprays; was consistent for various subgroups and not dependent on atopic status; and the risk increased when frequency of use or number of different sprays increased. A relevant number of adult asthma cases may be related to the use of household cleaning sprays, indicating an important public health issue.
The use of sprays during the 1990s was very common in all countries of our study. Market trends from household cleaners' manufacturers show a general increase of aerosolized applications in Europe (17). Sprays and more conventional liquid cleaners contain similar active ingredients, including alcohols, ammonia, chlorine-releasing agents, glycols and glycol ethers, sodium hydroxide, acryl polymers, and terpenes (18). The application through spraying is likely to facilitate respiratory exposure to these components, explaining why we have observed associations with the use of sprays but not liquid cleaners. The latter will give off volatile components but relevant inhalatory exposure will depend on the dilution used, the surface to which they are applied, and the ambient temperature, among other factors. We may have missed an association of asthma with liquid cleaner use by not being able to account for these exposure-modifying factors. It is likely that the application of a spray typically leads to some degree of relevant inhalatory exposure, and this may have resulted in less exposure misclassification than for liquid cleaners. However, there are few data available to describe the exposure patterns associated with use of different cleaning products, and there are few experimental studies on emissions and exposures and they have mainly focused on volatile components after application of cleaning products (18–20). Thus, although correlation between the use of sprays and other cleaning products was in general low, it is not unlikely that our findings reflect a risk of broader use of home cleaning products.
Not many studies have evaluated adverse respiratory effects of cleaning products. Our findings are consistent with occupational epidemiologic studies in which an increased asthma risk was related to professional use of sprays among both domestic (5, 14) and nondomestic (6) cleaning women. The observed associations may be (partly) due to chance, to confounding by a third variable, or may reflect a true adverse effect on new-onset asthma. Although chance can never be excluded in observational studies, this is highly unlikely here given the robust associations that were consistent for various subgroups based on host factors and country of residence, and the observed dose–response relationships. Confounding is possible if the use of sprays was associated with host or environmental risk factors of asthma. We controlled for potential host confounders, such as sex, age, and smoking status. In addition, we evaluated potential confounding effects of occupational exposures, and of socioeconomic status according to two definitions that can be regarded as reflecting a variety of housing and lifestyle factors (21). It is difficult to hypothesize other possible host or environmental factors that could have confounded the observed association between spray use and asthma.
Our study design precludes strong conclusions regarding the responsible effect mechanisms. Given the fact that asthma was related to several types of sprays with different chemical composition, and that the risk was not dependent on atopic status, we speculate that asthma could have been at least partly irritant induced. Cleaning sprays may contain sensitizers such as disinfectants, amines, pinene, or limonene (18, 19), and therefore a role of specific sensitization resulting in asthma is also plausible. From occupational settings, asthma can follow one-time intense irritant exposure, and there is increasing acceptance of the possibility that recurrent low-grade exposures to respiratory tract irritants can result in asthma as well (22). The underlying mechanisms are largely unknown, but a localized airway inflammatory response is likely involved. A similar phenomenon for repeated household exposures to irritants seems plausible, despite the fact that frequency of exposure in nonprofessional home cleaning is generally lower than in professional domestic or nondomestic cleaning.
There are a number of limitations in our study that need to be considered. First, data on both product use and health outcome were based on questionnaire information at follow-up, introducing the possibility of differential misclassification and a bias away from the null. This would be the case if participants with new-onset asthma reported more use and/or recalled better their use of cleaning sprays. However, this is unlikely to be a major explanation given the fact that during the 1990s there was not much public awareness of adverse respiratory effects of domestic cleaning activities. Data in this study were collected before 2003, the year in which an article was published on associations between domestic cleaning work and asthma (4), which received much media attention worldwide and likely initiated public awareness.
Second, scented products are widely reported by individuals with asthma to trigger symptoms (23). Although it is possible that the asthma in this study is due to the scented component of cleaning agents, it is more likely that those with asthma avoided such products and therefore could have biased associations toward the null. Analysis of the specific question covering all types of perfumed and scented cleaning products showed that the frequency of use was not associated with asthma (Table 2).
Third, results using the objective outcome BHR were not consistent with the main findings using the three a priori definitions of asthma. Only a minority of the participants with asthma showed BHR, and the vast majority (>80%) of participants with BHR did not have asthma. Thus, despite using a definition with relatively high specificity (a 20% fall in FEV1 using 1 mg methacholine as a cutoff), BHR was not particularly specific for asthma. With the combination of temporal variability and a generally moderate reproducibility of methacholine challenge testing, it is difficult to judge to which extent the lack of association of spray use with BHR contradicts the overall positive findings for asthma. An additional limitation was that current asthma and wheeze were defined as the occurrence in the previous year as reported at the follow-up interview. Although we used a prospective study design, it is possible that in the analyses of cumulative incidence (symptoms in the last year), the time order of exposure and effect was confused. In other words, individuals who developed asthma during follow-up could tend to clean their homes more thoroughly. Albeit with less statistical power, findings for the more specific asthma definition based on diagnosis using conventional survival analysis of incidence were consistent, and they therefore do not support this possibility.
Finally, although not statistically significant, there appeared to be a certain degree of heterogeneity in the association between spray use and asthma among countries. Unrecognized confounding could have been different for different countries, creating false-positive associations in some countries and/or hiding true positive associations in others. The qualitative differences in the use of sprays as outlined in Table 5 did not provide a clear hypothesis for the observed differences in the risk of any spray use among countries. Nevertheless, associations between the use of specific sprays and asthma incidence were more homogeneous across countries (results not presented). Whether chemical composition of cleaning sprays differs among countries, possibly related to the predominant brands sold on the local markets, remains unclear, and justifies more specific investigation.
Findings of our study may have significant implications for public health. Relative risks of 1.3 to 1.5 in combination with an overall proportion of 42% of weekly spray users suggest a population attributable fraction of about 15%. In other words, one in seven adult asthma cases could be attributed to common spray use. This indicates a relevant contribution of spray use to the burden of asthma in adults who do the cleaning in their homes. In addition, passive exposure might be relevant for individuals present in environments where sprays are being or have just been applied. One study even suggested that the use of cleaning and other household chemicals by the mother during pregnancy was related to wheeze in young children (24).
We conclude that frequent use of household cleaning sprays may be an important risk factor for adult asthma. This finding needs to be confirmed in future studies, with a particular emphasis on chemical composition and other exposure determinants, and on the effect mechanisms involved, including sensitization and inflammatory reactions.
Supported by the U.S. National Institutes of Health (NIH grant 1R01HL062633) and the Carlos III Health Institute of the Spanish Ministry of Health and Consumption (FIS grant 01/3058). The coordination of the European Community Respiratory Health Survey (ECRHS) II was supported by the European Commission, as part of their Quality of Life program. Further support for the local studies in ECRHS II included in this article is listed before the References.
Originally Published in Press as DOI: 10.1164/rccm.200612-1793OC on June 21, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Coordinating Center of ECRHS II: Project Leader: P. Burney; Statistician: S. Chinn; Principal Investigator: D. Jarvis; Project Coordinator: J. Knox; Principal Investigator: C. Luczynska; Assistant Statistician: J. Potts; Data Manager: S. Arinze; Laboratory Staff: M. Burrows, S. Hughes, K. Cheema.
Steering Committee for ECRHS II: Professor Josep M. Antó, Municipal Institute of Medical Research (IMIM), Pompeu Fabra University (UPF); Professor Peter Burney, Imperial College London (Project Leader); Dr. Isa Cerveri, University of Pavia; Professor Susan Chinn, King's College London; Professor Roberto de Marco, University of Verona; Dr. Thorarinn Gislason, Iceland University Hospital; Dr. Joachim Heinrich, GSF–Institute of Epidemiology; Assoc. Professor Christer Janson, Uppsala University; Dr. Deborah Jarvis, Imperial College London; Dr. Nino Künzli, Municipal Institute of Medical Research (IMIM), Institució Catalana de Recerca i Estudis Avançats (ICREA); Dr. Bénédicte Leynaert, Institut National de la Santé et de la Recherche Médicale (INSERM); Dr. Christina Luczynska, King's College London; Dr. Françoise Neukirch, Institut National de la Santé et de la Recherche Médicale (INSERM); Dr. J. Schouten, University of Groningen; Dr. Jordi Sunyer, Municipal Institute of Medical Research (IMIM), Pompeu Fabra University (UPF); Dr. Cecilie Svanes, University of Bergen; Professor Paul Vermeire, University of Antwerp; Dr. Matthias Wjst, GSF–Institute of Epidemiology.
Principal Investigators and Senior Scientific Team: Belgium: South Antwerp and Antwerp City (P. Vermeire, J. Weyler, M. Van Sprundel, V. Nelen); Estonia: Tartu (R. Jogi, A. Soon); France: Paris (F. Neukirch, B. Leynaert, R. Liard, M. Zureik), Grenoble (I. Pin, J. Ferran-Quentin); Germany: Erfurt (J. Heinrich, M. Wjst, C. Frye, I. Meyer); Italy: Turin (M. Bugiani, P. Piccioni, A. Carosso, W. Arossa, E. Caria, G. Castiglioni, E. Migliore, C. Romano, D. Fabbro, G. Ciccone, C. Magnani, P. Dalmasso, R. Bono, G. Gigli, A. Giraudo, M.C. Brussino, C. Bucca, G. Rolla), Verona (R. de Marco, G. Verlato, E. Zanolin, S. Accordini, A. Poli, V. Lo Cascio, M. Ferrari), Pavia (A. Marinoni, S. Villani, M. Ponzio, F. Frigerio, M. Comelli, M. Grassi, I. Cerveri, A. Corsico); Norway: Bergen (A. Gulsvik, E. Omenaas, C. Svanes, B. Laerum). Spain: Barcelona (J.M. Antó, J. Sunyer, M. Kogevinas, J.P. Zock, X. Basagana, A. Jaen, F. Burgos), Huelva (J. Maldonado, A. Pereira, J.L. Sanchez), Albacete (J. Martinez-Moratalla Rovira, E. Almar), Galdakao (N. Muniozguren, I. Urritia), Oviedo (F. Payo); Sweden: Uppsala (C. Janson, G. Boman, D. Norback, M. Gunnbjornsdottir), Goteborg (K. Toren, L. Lillienberg, A. Dahlman-Höglund, R. Sundberg), Umeå (E. Norrman, M. Soderberg, K. Franklin, B. Lundback, B. Forsberg, L. Nystrom); Switzerland: Basel (N. Künzli, B. Dibbert, M. Hazenkamp, M. Brutsche, U. Ackermann-Liebrich); United Kingdom: Norwich (D. Jarvis, B. Harrison), Ipswich (D. Jarvis, R. Hall, D. Seaton).
The following bodies funded the local studies in ECRHS II included in this paper: Albacete: Fondo de Investigaciones Santarias (FIS) (grant codes: 97/0035-01, 99/0034-01, and 99/0034-02), Hospital Universitario de Albacete, Consejeria de Sanidad; Antwerp: FWO (Fund for Scientific Research)–Flanders Belgium (grant code: G.0402.00), University of Antwerp, Flemish Health Ministry; Barcelona: SEPAR, Public Health Service (grant code: R01 HL62633-01), FIS (grant codes: 97/0035-01, 99/0034-01, and 99/0034-02) CIRIT (grant code: 1999SGR 00241) Red Respira ISCII; Basel: Swiss National Science Foundation, Swiss Federal Office for Education and Science, Swiss National Accident Insurance Fund (SUVA), USC NIEHS Center grant 5P30 ES07048; Bergen: Norwegian Research Council, Norwegian Asthma and Allergy Association (NAAF), Glaxo Wellcome AS, Norway Research Fund; Erfurt: GSF–National Research Centre for Environment and Health, Deutsche Forschungsgemeinschaft (DFG) (grant code: FR 1526/1-1); Galdakao: Basque Health Department; Goteborg: Swedish Heart Lung Foundation, Swedish Foundation for Health Care Sciences and Allergy Research, Swedish Asthma and Allergy Foundation, Swedish Cancer and Allergy Foundation; Grenoble: Program Hospitalier de Recherche Clinique–DRC de Grenoble 2000 no. 2610, Ministry of Health, Direction de la Recherche Clinique, Ministere de l'Emploi et de la Solidarite, Direction Generale de la Sante, CHU de Grenoble, Comite des Maladies Respiratoires de l'Isere; Hamburg: GSF–National Research Centre for Environment and Health, DFG (grant code: MA 711/4-1); Ipswich and Norwich: Asthma UK (formerly known as National Asthma Campaign); Huelva: FIS (grant codes: 97/0035-01, 99/0034-01, and 99/0034-02); Oviedo: FIS (grant codes: 97/0035-01, 99/0034-01, and 99/0034-02); Paris: Ministere de l'Emploi et de la Solidarite, Direction Generale de la Sante, UCB-Pharma (France), Aventis (France), Glaxo France, Program Hospitalier de Recherche Clinique–DRC de Grenoble 2000 no. 2610, Ministry of Health, Direction de la Recherche Clinique, CHU de Grenoble; Pavia: GlaxoSmithKline Italy, Italian Ministry of University and Scientific and Technological Research (MURST), local university funding for research 1998 and 1999 (Pavia, Italy); Tartu: Estonian Science Foundation; Turin: ASL 4 Regione Piemonte (Italy), AO CTO/ICORMA Regione Piemonte (Italy), MURST; GlaxoSmithKline Italy; Umeå: Swedish Heart Lung Foundation, Swedish Foundation for Health Care Sciences and Allergy Research, Swedish Asthma and Allergy Foundation, Swedish Cancer and Allergy Foundation; Uppsala: Swedish Heart Lung Foundation, Swedish Foundation for Health Care Sciences and Allergy Research, Swedish Asthma and Allergy Foundation, Swedish Cancer and Allergy Foundation; Verona: University of Verona; MURST; GlaxoSmithKline Italy.
References
- 1.Kogevinas M, Antó JM, Sunyer J, Tobias A, Kromhout H, Burney P. Occupational asthma in Europe and other industrialised areas: a population based study. Lancet 1999;353:1750–1754. [DOI] [PubMed] [Google Scholar]
- 2.Karjalainen A, Martikainen R, Karjalainen J, Klaukka T, Kurppa K. Excess incidence of asthma among Finnish cleaners employed in different industries. Eur Respir J 2002;19:90–95. [DOI] [PubMed] [Google Scholar]
- 3.Rosenman KD, Reilly MJ, Schill DP, Valiante D, Flattery J, Harrison R, Reinisch F, Pechter E, Davis L, Tumpowsky CM, et al. Cleaning products and work-related asthma. J Occup Environ Med 2003;45:556–563. [DOI] [PubMed] [Google Scholar]
- 4.Medina-Ramón M, Zock JP, Kogevinas M, Sunyer J, Antó JM. Asthma symptoms in women employed in domestic cleaning: a community-based study. Thorax 2003;58:950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medina-Ramón M, Zock JP, Kogevinas M, Sunyer J, Torralba Y, Borrell A, Burgos F, Antó JM. Asthma, chronic bronchitis and exposure to irritant agents in occupational domestic cleaning: a nested case-control study. Occup Environ Med 2005;62:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen J, Bach E. Work-related eye symptoms and respiratory symptoms in female cleaners. Occup Med 1999;49:291–297. [DOI] [PubMed] [Google Scholar]
- 7.Gorguner M, Aslan S, Inandi T, Cakir Z. Reactive airways dysfunction syndrome in housewives due to a bleach-hydrochloric acid mixture. Inhal Toxicol 2004;16:87–91. [DOI] [PubMed] [Google Scholar]
- 8.Mapp CE, Pozzato V, Pavoni V, Gritti G. Severe asthma and ARDS triggered by acute short-term exposure to commonly used cleaning detergents. Eur Respir J 2000;16:570–572. [DOI] [PubMed] [Google Scholar]
- 9.Tanen DA, Graeme KA, Raschke R. Severe lung injury after exposure to chloramine gas from household cleaners. N Engl J Med 1999;341:848–849. [DOI] [PubMed] [Google Scholar]
- 10.Deschamps D, Soler P, Rosenberg N, Baud F, Gervais P. Persistent asthma after inhalation of a mixture of sodium hypochlorite and hydrochloric acid. Chest 1994;105:1895–1896. [DOI] [PubMed] [Google Scholar]
- 11.Zock JP, Plana E, Dahlman-Hoglund A, Olivieri M, Kogevinas M; ECRHS Occupational Working Group. Use of household cleaning sprays and new-onset asthma: a community-based study in 10 European countries. Eur Respir J 2005;26:207s. [Google Scholar]
- 12.The European Community Respiratory Health Survey II Steering Committee. The European Community Respiratory Health Survey II. Eur Respir J 2002;20:1071–1079. [DOI] [PubMed] [Google Scholar]
- 13.Pekkanen J, Sunyer J, Chinn S. Nondifferential disease misclassification may bias incidence risk ratios away from the null. J Clin Epidemiol 2006;59:281–289. [DOI] [PubMed] [Google Scholar]
- 14.Zock JP, Kogevinas M, Sunyer J, Almar E, Muniozguren N, Payo F, Sánchez JL, Antó JM. Asthma risk, cleaning activities and use of specific cleaning products in Spanish indoor cleaners. Scand J Work Environ Health 2001;27:76–81. [DOI] [PubMed] [Google Scholar]
- 15.Medina M, Zock JP, Kogevinas M, Sunyer J, Antó JM. Validity and reproducibility of a modular questionnaire to determine asthma-related cleaning exposures in Spanish housewives. Eur Respir J 2000;16:520s. [Google Scholar]
- 16.Der Simonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 17.European Aerosol Federation. European aerosol production 2005. [Internet]. Brussels: Fédération Européenne des Aérosols (FEA); 2006. Available from: http://www.aerosol.org
- 18.Wolkoff P, Schneider T, Kildeso J, Degerth R, Jaroszewski M, Schunk H. Risk in cleaning: chemical and physical exposure. Sci Total Environ 1998;215:135–156. [DOI] [PubMed] [Google Scholar]
- 19.Nazaroff WW, Weschler CJ. Cleaning products and air fresheners: Exposure to primary and secondary air pollutants. Atmos Environ 2004;38:2841–2865. [Google Scholar]
- 20.Fedoruk MJ, Bronstein R, Kerger BD. Ammonia exposure and hazard assessment for selected household cleaning product uses. J Expo Anal Environ Epidemiol 2005;15:534–544. [DOI] [PubMed] [Google Scholar]
- 21.Basagaña X, Sunyer J, Kogevinas M, Zock JP, Duran-Tauleria E, Jarvis D, Burney P, Antó JM. Socioeconomic status and asthma prevalence in young adults. The European Community Respiratory Health Survey. Am J Epidemiol 2004;160:178–188. [DOI] [PubMed] [Google Scholar]
- 22.Balmes JR. Occupational airways diseases from chronic low-level exposures to irritants. Clin Chest Med 2002;23:727–735. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin CM, Bell IR, O'Rourke MK. Odor sensitivity and respiratory complaint profiles in a community-based sample with asthma, hay fever, and chemical odor intolerance. Toxicol Ind Health 1999;15:403–409. [DOI] [PubMed] [Google Scholar]
- 24.Sherriff A, Farrow A, Golding J, Henderson J. Frequent use of chemical household products is associated with persistent wheezing in pre-school age children. Thorax 2005;60:45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]