Abstract
Contrast sensitivity is strongly associated with daily functioning among older adults, but the genetic and environmental contributions to this ability are unknown. Using the classical twin method, we addressed this issue by examining contrast sensitivity at five spatial frequencies (1.5–18 cycles per degree) in 718 middle-aged male twins from the Vietnam Era Twin Study of Aging (VETSA). Heritability estimates were modest (14%–38%), whereas individual-specific environmental influences accounted for 62%–86% of the variance. Identifying the types of individual-specific events that impact contrast sensitivity may suggest interventions to modulate this ability and thereby improve overall quality of life as adults age.
Keywords: spatial, contrast, aging
Introduction
Basic visual abilities such as acuity, contrast sensitivity, motion detection, color discrimination, and depth perception are all affected by aging, but the sources of variability in visual status across older individuals are as yet incompletely understood. Increasingly, it is recognized that deficits in basic visual abilities contribute significantly to impairments in higher cognitive processes and activities of daily living. This relation between vision, cognition, and daily function has been reported in healthy older adults (Ball & Sekuler, 1986; Gilmore, Thomas, Klitz, Persanyi, & Tomsak, 1996; Gilmore, Spinks, & Thomas, 2006; Owsley, Sekuler, & Boldt, 1981) as well as in individuals with age-related neurodegenerative disorders, such as Alzheimer’s disease (Cronin-Golomb, Corkin, & Growdon, 1995; Dunne, Neargarder, Cipolloni, & Cronin-Golomb, 2004; Gilmore et al., 1996; Gilmore, Cronin-Golomb, Neargarder, & Morrison, 2005; Gilmore et al., 2006) and Parkinson’s disease (Amick, Cronin-Golomb, & Gilmore, 2003; Davidsdottir, Cronin-Golomb, & Lee, 2005). As the population ages it becomes more important to determine the sources of variation in visual abilities in order to permit the development of visual interventions that may improve cognitive and daily function. In particular, determination of the relative contributions of genetic and environmental influences on visual ability may be especially enlightening.
Spatial frequency contrast sensitivity is one of the most studied visual abilities, in part because of its association with deficits in daily function in older adults (e.g., Cormack, Tovee, & Ballard, 2000; Dargent-Molina, Hays, & Bréart, 1996; Dunne et al., 2004; Elliot, Bullimore, Patla, & Whitaker, 1996; Elliot, Hurst, & Weatherill, 1990; Lord, Clark, & Webster, 1991a & 1991b). It has been reported that up to 57% of the variance in performance of activities of daily living (ADL) in older adults is attributable to variability in acuity and contrast sensitivity (West, Rubin, Broman, Munoz, Bandeen-Roche, & Turano, 2002). One study indicated that a twofold reduction in contrast sensitivity resulted in a three- to five-fold increase of difficulty with ADLs (Rubin, Bandeen-Roche, Huang, Munoz, Schein, Fried, & West, 2001). Deficient contrast sensitivity may arise from dysfunction at multiple points along the visual pathways, from the lens and retina to primary visual cortex and higher cortical areas (reviewed in Cronin-Golomb & Gilmore, 2003; Matjucha & Katz, 1994; Spear, 1993).
In normal aging, changes in contrast sensitivity are well established for higher spatial frequencies. High-frequency loss is common in normal aging owing in part to changes in the lens and other anterior structures but mainly to neural factors, such as changes in the retina or central visual pathways (Matjucha & Katz, 1994; Owsley, Sekuler, & Siemsen, 1983; Owsley, Gardner, Sekuler, & Lieberman, 1985; Spear, 1993). In a study stratifying by age (Owsley et al., 1983), it was found that age had no effect on static contrast sensitivity at the lower frequencies of 0.5 and 1.0 cpd, but sensitivity decreased at higher frequencies (2.0 to 16.0 cpd) beginning at about 40 to 50 years of age. Given the evidence for genetic influence on other factors that show age-related change (see Bergeman, 1997; Finkel, Pedersen, Berg, & Johansson, 2000; Pedersen, 1996, for reviews), it is possible that genetic factors may also play a role in contrast sensitivity. The finding that age-related declines in contrast sensitivity are not seen at all spatial frequencies suggests that there may be different mechanisms involved, and that the importance of genetic and environmental factors may vary for different frequencies.
To our knowledge, there are no published data that address the relative influence of genetic and environmental influence on variation in contrast sensitivity. However, a number of disorders with a strong genetic component are also associated with contrast sensitivity loss at some or most spatial frequencies, including Alzheimer’s disease, especially at the lower frequencies (Cronin-Golomb, Corkin, Rizzo, Cohen, Growdon, & Banks, 1991; Cronin-Golomb, Cronin-Golomb, Dunne, Brown, Jain, Cipolloni, & Auerbach, 2000; Mendola, Cronin-Golomb, Corkin, & Growdon, 1995) and optic neuritis of various etiologies, especially for the middle range of frequencies (Ashworth, Aspinall, & Mitchell, 1989; Wright, Drasdo, & Harding, 1987). If there is evidence of genetic influence on contrast sensitivity, this could indicate that contrast sensitivity may be considered a possible endophenotype for these disorders (Gottesman & Gould, 2003). Alternatively, genes causing these disorders might have a direct influence on contrast sensitivity (e.g., as a result of visual cortex atrophy). The mechanisms through which the same or different genes may affect such disorders as well as contrast sensitivity can be determined by multivariate genetic analyses, but such analyses must await basic studies of genetic and environmental influences on contrast sensitivity itself.
In the present study, we used the twin method to examine the heritability of visual spatial frequency contrast sensitivity in a large cohort of middle-aged men from the first wave of the longitudinal Vietnam Era Twin Study of Aging (VETSA). The twin method allows the estimation of the relative influences of genes and environment on a particular trait or ability, such as contrast sensitivity. Because monozygotic (MZ) twins share all of their genes whereas dizygotic (DZ) twins, like other siblings, share on average 50% of their genes, the greater the difference is in the degree of similarity within MZ twin pairs compared to DZ pairs, the stronger the genetic influence is on those abilities. By examining the importance of genetic and environmental influences on contrast sensitivity in a middle-aged sample that we are following over time, we begin the first step in understanding the mechanisms that are responsible for both age-related change in contrast sensitivity and its subsequent effect on the overall quality of life among older adults. In order to place the results from our middle-aged sample in the context of aging, we also show the mean contrast sensitivity at each frequency from data that we have collected in another study of younger and older adults.
Methods
Description of the Vietnam Era Twin Study of Aging (VETSA)
Data collection began in 2003 for the longitudinal Vietnam Era Twin Study of Aging (VETSA). Study participants are from the Vietnam Era Twin (VET) Registry, a population-based sample of male-male twin pairs living throughout the United States. Registry members were born between 1939 and 1957, served in the military from 1965 to 1975, and are representative of all veterans from the Vietnam War era on a variety of socio-demographic variables (Goldberg, True, Eisen, Henderson, & Robinette, 1987; Eisen, True, Goldberg, Henderson, & Robinette, 1987). In the early 1990s, 3,322 VET Registry twin pairs participated in the Harvard Twin Study of Drug Abuse and Dependence, a telephone survey of lifetime substance use and psychopathology (Tsuang, Bar, Harley, & Lyons, 2001). Zygosity was determined by a combination of questionnaire and blood group typing, an approach that has been demonstrated to be 95% accurate (Eisen, Neuman, Goldberg, Rice, & True, 1989). Participants in the present study were randomly selected from those twin pairs who had participated in the Harvard Twin Study of Drug Abuse and Dependence.
Sample
VETSA Twin Sample
This report is based on the first 746 individuals who participated in VETSA. Twins were given the option of traveling to Boston University or the University of California, San Diego, for a day-long series of physical and cognitive assessments. Approximately equal numbers were studied at each site and 26 participants were tested off-site, with examiners traveling to their home towns. The present analyses include data from 718 participants for whom standard equipment and chart illumination were available: 185 complete MZ pairs and 155 complete DZ pairs, as well as data from 15 unpaired MZ twins and 23 unpaired DZ twins. We did not administer the contrast sensitivity test to 28 of the 746 participants; only two on-site participants did not take the test, but the test was not available for those who participated off-site. The study was undertaken with the understanding and written consent of each participant. All participants were in their 50s at the time of recruitment, two of whom were 60 by the time of the assessment. The mean level of education was 13.9 ± 2.1 years (range 4 – 20). The groups were matched for age. The overall mean age at assessment was 55.3 years (range 52 – 60), with an MZ mean of 55.1 (S.D. = 2.3), and a DZ mean of 55.4 (S.D. = 2.2).
Comparison Sample
We also have data from 14 younger men (ages 18 to 25 years, mean of 19.4, SD 1.8) and 16 older men (ages 61 to 82 years, mean of 68.4, SD 4.1) from a separate study in the Boston laboratory. Data for this study were collected using the same measures and procedures described below. For the healthy young comparison group, mean education was 13.4 years (range of 12–18, SD = 1.6) and median acuity was 20/16 (−0.10 LogMAR) (none worse than 20/20; 0.00 LogMAR). The healthy elderly comparison group had a mean education of 15.7 years (range of 9–21, SD = 3.6) and median acuity of 20/20 (none worse than 20/40; .301 LogMAR). The elderly group was screened for dementia, with all showing normal cognitive function on multiple neuropsychological measures. The comparison sample data are shown together with the VETSA data for descriptive purposes only.
Procedure and Measures
Acuity
Binocular central acuity was measured at 10 feet using the HOTV wall chart (Good-Lite Co., Forest Park, IL). Participants used their own refractive correction when necessary. All participants had corrected acuity equal to or better than 20/40 (.301 LogMAR). The median acuity score for both the MZ and DZ groups was 20/16 (−0.10 LogMAR). Comparison of the frequency of acuities for the MZ and DZ groups revealed no differences in the distribution of acuities (χ2 = 4.629, df = 5, p = .463).
Contrast Sensitivity
The Functional Acuity Contrast Test (FACT) was administered to assess static contrast sensitivity (Ginsburg, 1996). Whereas other chart tests of contrast sensitivity assess this function at a single spatial frequency, the FACT provides information on multiple spatial frequencies. This chart test is used in clinical and research settings and enables one to demonstrate the comparability of our sample to others described in the literature. Lighting for the chart was within the recommended luminance of 68–240 cd/m2. Participants viewed the chart binocularly from a distance of 10 feet. The chart consists of 5 rows, each with 9 circles, the diameter of each circle subtending 1.7 degrees of visual angle. Contrast decreases monotonically in nine steps from left to right with a range of .602 to 2.255 (.59% to 25% Michelson contrast), and a log step increment range of 0.109 to 0.176 (SD = .014). Moving down a column, the gratings increase in spatial frequency, including 1.5, 3, 6, 12, and 18 cycles per degree (cpd). In each circle, the gratings are oriented either vertically, tilted 15° to the left or 15° to the right. The participant’s task was to indicate verbally or by hand posture the direction in which the lines were oriented, beginning from leftmost circle to right across each row. A contrast sensitivity level was determined for each spatial frequency by finding the minimal perceptible contrast level needed to correctly identify the orientation of the grating for a given row. The participants were instructed to provide a response for each circle in the row until an incorrect response was obtained. The first incorrect response was recorded as the participant’s threshold for that row. In rare cases of uncertainty, we repeated the row to ensure reliability of response.
Health indicators
Presence of chronic medical conditions was based on self-report in a medical history interview. Participants indicated if a physician had ever told them they had any of a list of 49 conditions, including (among others) diabetes, glaucoma, or hypertension.
Data Analysis
The purpose of behavioral genetic research is to estimate the degree to which genetic and environmental factors influence individual differences (i.e., variation) in a measured behavior or trait. Within the twin design, the variance of any behavior or trait can be accounted for by four possible latent factors: additive (effects of different alleles “add up”) genetic influences (A); non-additive (a single gene of major influence and/or gene-gene interaction) genetic influences (D); common or shared environmental influences (C); and nonshared or individual-specific environmental influences (E) (Neale & Cardon, 1992). Because monozygotic twins share 100% of their genes, they correlate perfectly in terms of both additive (A) and non-additive (D) genetic influences. Dizygotic twins, on the other hand, share on average 50% of their genes (similar to full siblings), resulting in correlations of .50 for additive genetic influences and .25 for dominant genetic influences because fraternal twins stand only a 25 percent chance of sharing both paternal and maternal alleles. Shared environmental factors (C) are defined as environmental characteristics that influence both members of a twin pair equally, and are therefore correlated 1.0 across twin pairs, regardless of zygosity. Shared environmental influences include such factors as family socioeconomic status, shared peer groups, and neighborhood effects. By contrast, nonshared environmental factors (E) are environmental influences to which siblings are differentially exposed. By definition, nonshared environmental factors make siblings in the same family different from one another, and are therefore uncorrelated across twins. Possible nonshared environmental influences include differences in pre- and perinatal environments, accidents, and differences in social experiences (e.g., differential treatment by parents or dissimilar peer groups). Because measurement error is assumed to be random and therefore uncorrelated across twins, it is also included in the nonshared environmental variance.
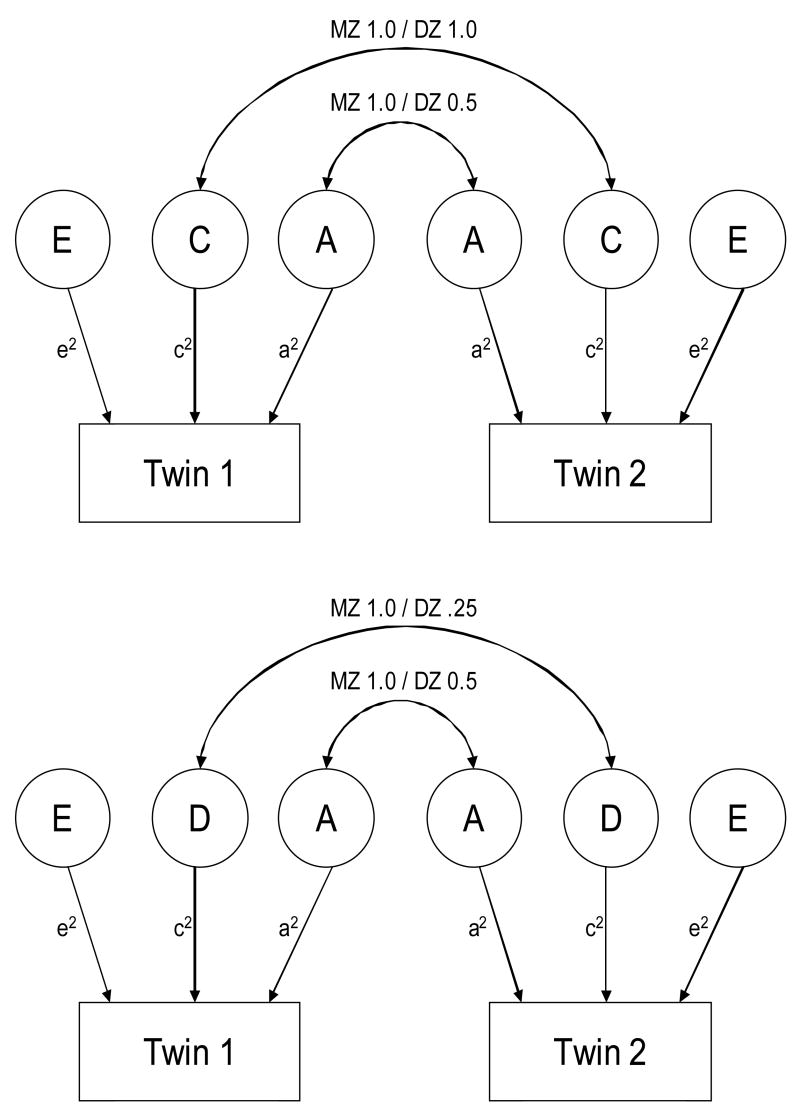
In order to estimate the genetic and environmental influences on visual contrast sensitivity, we used the maximum-likelihood based structural equation modeling package Mx (Neale, Boker, Xie, & Maes, 2002). Mx allows for biometrical models to be fit to data in order to differentiate the genetic and environmental influences on variation in a given trait or characteristic (phenotype). One of the limitations of the standard twin design is that it cannot model the effects of both non-additive genetic (D- dominance) and shared environmental (C- common) influences simultaneously. For this reason, twin studies often test the “ACE” and “ADE” models separately (see Figure 1). When MZ correlations are greater than DZ correlations, this implies the presence of genetic influence (A- additive). In cases where the MZ correlation is more than twice the DZ correlation, this implies that some of the genetic influence may operate non-additively, which translates into both A (additive) and D (dominance) effects. By contrast, if the MZ correlation is greater than the DZ correlation, but the DZ correlation is more than one-half the MZ correlation, this implies the presence of both additive genetic (A) and shared environmental (C- common) effects. Accordingly, both non-additive genetic influences and shared environmental influences cannot be estimated simultaneously, as they make opposite predictions about the relative difference between MZ and DZ correlations (Neale & Cardon, 1992).
Figure 1.
Univariate ACE (upper) and ADE (lower) path diagrams. Monozygotic twins share 100% of their genes; therefore, they correlate perfectly in terms of the additive (‘ A ’) and non-additive (‘D’; a single gene of major influence and/or gene-gene interaction) genetic influence. Dizygotic twins share on average 50% of their genes, resulting in correlations of roughly .50 for additive genetic influence and .25 for non-additive genetic influence. The familial environment (‘C’) for both types of twins is assumed to be equal and not affected by the observed similarity of the twins (Neale & Cardon, 1992). ‘ A ’= additive genetic influences; ‘C’= common or shared environmental influences; ‘D’= non-additive (a single gene of major influence and/or gene-gene interaction) genetic influences; ‘E’ = nonshared or individual-specific environmental influences (Neale & Cardon, 1992).
The overall fit of each model was determined by comparing its negative two log-likelihood (−2LL) to that of a saturated model which simply provides the observed means, variances, and covariance of the data without imposing model constraints. Therefore, a saturated model provides a representation of the data against which theoretical models can be tested. By comparing the saturated model to any of the “full” models (i.e., ACE, ADE) we can evaluate the degree to which the observed data adhere to fundamental assumptions of the twin design (i.e., equality of means and variances across groups). For a model to be considered a good fit to the data it must represent a non-significant reduction in fit relative to the saturated model. In other words, the probability value (p), based on the difference in −2LL (distributed as χ2 with degrees of freedom equal to the difference in degrees of freedom between the saturated model and theoretical model) must be greater than .05.
One of the advantages of using structural equation models is that they also allow us to compare the relative fit of alternative theoretical models that are nested within one another. For example, the E-only model is a nested submodel of the AE model, which itself is a nested submodel of both the ADE and ACE models. The difference in −2LL between nested submodels is also distributed as a chi-square (called the Likelihood Ratio Test statistic, LRT), and the significance of the LRT can be evaluated. If a nested submodel shows a significant LRT, this indicates that the reduced model does not fit as well as the comparison model. The Akaike’s Information Criterion (AIC) (Akaike, 1987) can also be used to compare competing models. The AIC is calculated by the difference in the −2LL minus two times the difference in degrees of freedom between two models (AIC=Δχ2−2*Δdf). More negative AIC values represent a superior balance between goodness-of-fit and parsimony. Because the ACE and ADE models are not nested within one another, the AIC must be used to determine which model provides a better fit to the observed covariance among twins.
Results
Preliminary analyses
A mixed design analysis of variance (ANOVA) with one between-subjects variable (zygosity) and one within-subjects variable (spatial frequency) was performed to compare MZ and DZ twins’ performances (contrast sensitivity at each spatial frequency) on the FACT assessment. Because a violation of the sphericity assumption was observed, the Greenhouse-Geisser correction was applied to the data (ε = .535). As expected, results revealed no main effect of zygosity (F [1, 708] = 0.05; p = .82), a main effect of spatial frequency (F [2.14, 1514.02] = 2718.02; p <.001), and a non-significant interaction between zygosity and spatial frequency (F [2.14, 1514.02] = 0.78; p = .46). We concluded that the variability of contrast sensitivity across spatial frequencies did not differ based on the zygosity of our participants. The MZ and DZ groups also showed similar rates of diabetes, hypertension, and other medical conditions that may have affected vision (see Table 1). Removing the few participants with glaucoma from analyses (3 MZ, 9 DZ) did not result in a significant change in univariate parameter estimates.
Table 1.
Lifetime prevalence of health-related conditions in the MZ and DZ samples
| MZ (N = 385) | DZ (N = 333) | χ2 | p value | |
|---|---|---|---|---|
| Diabetes | 13.8% | 9.6% | 3.0 | .083 |
| Glaucoma | 0.8% | 2.7% | 4.02 | .045 |
| Hypertension | 36.9% | 35.7% | 0.102 | .750 |
| Stroke | 1.3% | 1.2% | 0.01 | .943 |
| Lifetime Smoking | 67.3% | 67.6% | 0.007 | .933 |
| Current Smoking | 31.3% | 34.7% | 0.628 | .428 |
df = 1
Age differences in contrast sensitivity
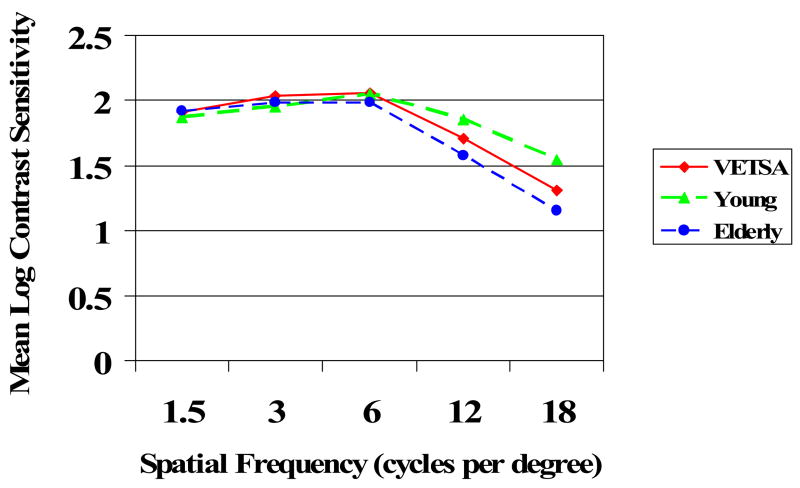
Figure 2 depicts the VETSA sample data (combined MZ and DZ twins) with reference to the contrast sensitivity curves of young comparison adults and elderly comparison adults who were tested on the contrast sensitivity chart in the Boston lab. We observed the normal age-related decline in contrast sensitivity at high spatial frequencies with relative preservation of sensitivity to low frequencies. As expected for middle-aged adults, the VETSA sample falls between the young and elderly groups for sensitivity at higher frequencies.
Figure 2.
Mean performance of young comparison adults (n = 14, ages 18 to 25), the VETSA sample (n = 714, ages 52 to 60), and elderly comparison adults (n = 16, ages 61 to 82) at each spatial frequency as a function of the mean log contrast sensitivity. Standard deviations at 1.5, 3, 6, 12, and 18 cpd respectively are as follows for each group: Young .12, .28, .27, .20, .27; VETSA .11, .14, .16, .24, .29; Elderly .08, .16, .14, .25, .23.
Genetic and environmental influences on variation in visual contrast sensitivity
Table 2 presents the cross-twin correlations, stratified by zygosity, for each spatial frequency. Without exception the correlation between MZ twins was greater than the respective DZ twin correlation, indicating the presence of genetic influence. Moreover, the MZ correlations were more than twice the DZ correlations, suggesting the possible presence of non-additive genetic effects, and lessening the likelihood that shared environmental factors play a significant role in variation in contrast sensitivity. Nevertheless, the 95% confidence intervals around the correlations were relatively large, and in many cases overlapped across MZ and DZ twins, indicating that shared environmental influences could not be completely ruled out. For this reason, both ADE and ACE models were fit to the data from each spatial frequency category, and the AIC statistic was used to determine which model fit the observed data better. We also fit the nested AE and E models, and used both the LRT and AIC statistics to determine which model provided the best fit overall.
Table 2.
Cross-twin correlations stratified by zygosity (95% confidence intervals)
| MZ | DZ | |
|---|---|---|
| 1.5 cpd | .203 (.132, .273) | −.040 (−.086, .006) |
| 3 cpd | .160 (.018, .295) | .020 (−.139, .178) |
| 6 cpd | .370 (.241, .486) | .141 (−.021, .296) |
| 12 cpd | .219 (.080, .351) | .027 (−.137, .190) |
| 18 cpd | .250 (.113, .377) | .077 (−.079, .229) |
At each spatial frequency, both the full ADE and ACE models provided a good fit to the data, with p values ranging from .218 to .609 (Table 3). Because the ADE model was the preferred model at all five spatial frequencies based on a comparison of AIC values, this was used as the comparison model for our two nested models (the AE and E-only models). For three of the five spatial frequencies examined (6, 12, & 18 cpd), we were able to reject an E model outright, based both on the significant reduction in fit relative to the saturated model and the significant LRT values. By contrast, the AE model did not show a significant reduction in fit relative to the saturated model, nor were the LRT values significant for the comparisons of the AE model with the full ADE model. This finding suggests that while the presence of non-additive genetic effects could not be completely ruled out at these frequencies, the magnitude of the non-additive effects was not significantly different from zero. Moreover, the AE model provided the lowest AIC value, indicating that it was the best model in all cases. At the two remaining spatial frequencies (1.5 and 3 cpd), we were unable to reject the E-only model based solely on a comparison with the saturated model, but there were other indications that the model without genetic influence was not the best-fitting model. First, as above, a comparison of the AE model with the full ADE model revealed a non-significant LRT value, indicating that non-additive genetic influence was not significantly different from zero. There was a significant LRT value from the comparison of the AE and E models at both frequencies, indicating that the model without genetic influence (E) fit significantly more poorly than the model that also included additive genetic influence (AE). As above, the AE model also yielded the lowest AIC values, indicating that it did provide the best fit to the data at all spatial frequencies. As shown in Table 4, the estimates of genetic influence obtained from these best-fitting AE models ranged from 14% to 38% of the total variance in contrast sensitivity, with the greatest effect occurring at 6 cpd.
Table 3.
Univariate model fitting results
| Model | −2LL | DF | Δ−2LL | ΔDF | p value | AIC | LRT | df | p value |
|---|---|---|---|---|---|---|---|---|---|
| 1.5 cpd | |||||||||
| Saturated | −1148.220 | 708 | - | - | - | - | - | - | - |
| ACE | −1138.337 | 714 | 9.883 | 6 | .130 | −2.117 | - | - | - |
| ADE | −1139.938 | 714 | 8.282 | 6 | .218 | −3.718 | - | - | - |
| AE | −1138.337 | 715 | 9.883 | 7 | .195 | −4.117 | 1.601 | 1 | .206 |
| E | −1133.745 | 716 | 14.475 | 8 | .070 | −1.525 | 4.592 | 1 | .032 |
| 3.0 cpd | |||||||||
| Saturated | −826.918 | 708 | - | - | - | - | - | - | - |
| ACE | −821.906 | 714 | 5.012 | 6 | .542 | −6.988 | - | - | - |
| ADE | −822.417 | 714 | 4.501 | 6 | .609 | −7.499 | - | - | - |
| AE | −821.906 | 715 | 5.012 | 7 | .658 | −8.988 | 0.511 | 1 | .475 |
| E | −817.540 | 716 | 9.378 | 8 | .311 | −6.622 | 4.366 | 1 | .037 |
| 6.0 cpd | |||||||||
| Saturated | −507.506 | 708 | - | - | - | - | - | - | - |
| ACE | −498.991 | 714 | 8.515 | 6 | .203 | −3.485 | - | - | - |
| ADE | −499.926 | 714 | 7.580 | 6 | .271 | −4.420 | - | - | - |
| AE | −498.991 | 715 | 8.515 | 7 | .289 | −5.485 | 0.935 | 1 | .334 |
| E | −469.054 | 716 | 38.452 | 8 | <.001 | 22.452 | 29.937 | 1 | < .001 |
| 12.0 cpd | |||||||||
| Saturated | 7.447 | 707 | - | - | - | - | - | - | - |
| ACE | 14.812 | 713 | 7.365 | 6 | .288 | −4.635 | - | - | - |
| ADE | 14.123 | 713 | 6.676 | 6 | .352 | −5.324 | - | - | - |
| AE | 14.812 | 714 | 7.365 | 7 | .392 | −6.635 | 0.689 | 1 | .407 |
| E | 23.200 | 715 | 15.753 | 8 | .046 | −0.247 | 8.388 | 1 | .004 |
| 18.0 cpd | |||||||||
| Saturated | 359.488 | 700 | - | - | - | - | - | - | - |
| ACE | 364.969 | 706 | 5.481 | 6 | .484 | −6.519 | - | - | - |
| ADE | 364.561 | 706 | 5.073 | 6 | .534 | −6.927 | - | - | - |
| AE | 364.969 | 707 | 5.481 | 7 | .602 | −8.519 | 0.408 | 1 | .523 |
| E | 377.522 | 708 | 18.034 | 8 | .021 | 2.034 | 12.553 | 1 | < .001 |
Note. Δ−2LL based on comparison of each model with the saturated model. The LRT is based on a comparison of nested models; the E model is a nested submodel of the AE model, and the AE model is a nested submodel of the ADE model.
Table 4.
Parameter estimates for AE models (95% confidence intervals)
| Additive Genetic (a2) | Non-additive Genetic (d2) | Unique Environment (e2) | |
|---|---|---|---|
| 1.5 cpd | .15 (.01, .29) | - | .85 (.71, .99) |
| 3 cpd | .14 (.01, .27) | - | .86 (.73, .99) |
| 6 cpd | .38 (.25, .49) | - | .62 (.51, .75) |
| 12 cpd | .19 (.06, .31) | - | .81 (.69, .94) |
| 18 cpd | .23 (.11, .35) | - | .77 (.65, .89) |
Discussion
The results of the present study demonstrate that the majority of the variance in visual contrast sensitivity, at all spatial frequencies, was accounted for by nonshared environmental influences. There was a modest genetic component to contrast sensitivity during midlife across all tested spatial frequencies, with different heritability estimates for the various individual frequencies. The strongest effect based on the best-fitting model (Table 4) was at the middle spatial frequency (6 cpd), for which the genetic contribution was 38%. Although the additive genetic effects were significantly different from zero, nonshared environmental influences explained the largest proportion of variation in contrast sensitivity at all frequencies, ranging from 62%–86%. We were unable to detect any influence of the environment shared by twins on contrast sensitivity during middle-age.
These findings may seem somewhat surprising; because contrast sensitivity is a basic biologically-based function, and one might accordingly expect more of the variance to be accounted for by genes. This is not necessarily the case, however; all things genetic must be biological, but all things biological do not have to be genetic. In this context, it is worth emphasizing the fact that heritability, which is the proportion of phenotypic variance that is attributable to genetic variance, is a statistical and a population construct that is not informative about specific genes or about particular individuals. The modest heritability of contrast sensitivity indicates little genetic variation, even though the basis of contrast sensitivity is likely to be genetic. Indeed, for some characteristics, little variation may be adaptive. For example, having two eyes is a characteristic that is determined by genes, but it must have extremely low heritability because—almost certainly for adaptive reasons—it manifests virtually no variation in the population.
It may be argued that poor reliability of the test, and hence measurement error, could have accounted for the results. The test estimated the contrast sensitivity threshold, with true threshold falling between the contrast level for which the participant accurately responded and the next contrast level for that same spatial frequency. Despite this design limitation, however, the reliability of the test is reasonable (.73 across spatial frequencies in patients with Alzheimer’s disease assessed with the Vistech, an earlier version of the FACT test; Cronin-Golomb et al., 1995) with greater reliability at higher than lower frequencies. Others have likewise reported variable reliability with the Vistech, with the same pattern of better reliability at higher than lower frequencies (reviewed in Pesudovs, Hazel, Doran, & Elliott, 2004). There is less information available regarding the FACT specifically, but Pesudovs and colleagues (2004) compared the FACT and Vistech and found similar reliability for the two tests as well as reporting the usual pattern of better reliability at higher than lower frequencies for both tests. Bühren, Terzi, Bach, Wesemann, & Kohnen (2006) reported that repeatability results with the FACT were consistent with those of earlier studies, but also acknowledged that in their study the luminance provided was lower than that recommended for use of the test. Further test-retest reliability assessment of the FACT is to be encouraged for future studies. In a separate study, we found that performance on the test correlated significantly with performance on a true threshold measure of contrast sensitivity in which participants (young adults, older adults, and individuals with Alzheimer’s disease) identified letters presented at varying levels of contrast (Cronin-Golomb, Gilmore, Neargarder, Morrison, & Laudate, in press). This finding offers some support for the reliability and usefulness of the FACT, which has the further advantage over most other computerized or chart tests in that it assesses contrast sensitivity at multiple spatial frequencies. In light of these findings, the large amount of variance accounted for by nonshared (individual-specific) environmental influences in the results of the present study is probably not attributable primarily to measurement error, though such error may make some contribution especially at the lower spatial frequencies. The results can be taken to mean that individual-specific environmental events must play a role in modulating the genetic/biological phenomenon of contrast sensitivity. Determining what type of events—whether external or biological—have an impact on contrast sensitivity will be important for generating potential approaches toward improving it.
The age range of 52 to 60 at assessment describes individuals whose contrast sensitivity presumably has undergone a degree of normal age-related change, some of which could reflect incipient disease effects (Owsley et al., 1983). As these individuals age, they are likely to develop age-related visual pathology (cataract, glaucoma, macular degeneration) as well as pathology associated with neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease. Common age-related disorders that have a significant genetic component include glaucoma (Gottfredsdottir et al., 1999), macular degeneration (Klein, Mauldin, & Stoumbos, 1994), and general thinning of the retinal nerve fiber layer (Hougaard, Kessel, Sander, Kyvik, Sorensen, & Larsen, 2003). In regard to healthy older adults, twin studies have revealed genetic contributions to aspects of vision that do not arise from disease processes, including the spherical equivalent of refractive error and axial length of the eyeball (Teikari & O’Donnell, 1989; Valluri, Minkovitz, Budak, Essary, Walker, Chansue, Carbera, Koch, & Pepose, 1999). As noted above, individual-specific environmental events—such as injuries, illness, or poor access to vision care, to name a few—may affect contrast sensitivity with age. That is, several possible genetic and environmental factors may alter the heritability of contrast sensitivity across the lifespan.
The all-male, relatively homogenous composition of our sample limits our ability to generalize these results to other populations. A further limitation of our study is that we used the FACT contrast sensitivity test as our sole measure of visual function besides corrected acuity. Because the contrast sensitivity threshold is not based on a continuous measurement, the threshold must be considered an estimate. An advantage of the FACT, however, is its ability to estimate thresholds at several spatial frequencies, which most tests of contrast sensitivity are not designed to do (Neargarder, Stone, Cronin-Golomb, & Oross, 2003).
Contrast sensitivity is an important visual function because of its role in predicting cognitive and functional decline in normal aging and in common age-related disorders. The low heritability and the relatively strong influence of individual-specific environmental events that we observed for contrast sensitivity suggests that a focus of future research should be on identifying the types of individual-specific environmental experiences that influence this ability. Altering those experiences by mid-adulthood may conceivably result in the delay of decline in cognitive and functional abilities that is associated with visual impairment. Longitudinal examination of the respective influences of genes and environment on contrast sensitivity may further address the probable efficacy of vision-based interventions to improve cognition and daily function in adults from middle age to the later years of the lifespan.
Acknowledgments
We are grateful to Melissa Amick, Ph.D., and Sandy Neargarder, Ph.D., for consultation on the vision measures; Uraina Clark, M.A., and Sigurros Davidsdottir, Ph.D., for collection of the young and elderly comparison data, and Grover C. Gilmore, Ph.D., Denise Valenti, O.D., and Arthur Wingfield, Ph.D., for helpful discussions. The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance, including: VA Cooperative Studies Program; Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Institutes of Health; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University; Schulman, Ronca, and Bucuvalas, Inc. This VETSA project is supported by grants from NIH/NIA (R01 AG018384, R01 AG018386, R01 AG022381, and R01 AG022982). Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Amick MM, Cronin-Golomb A, Gilmore GC. Visual processing of rapidly presented stimuli is normalized in Parkinson’s disease when proximal stimulus strength is enhanced. Vision Research. 2003;43:2827–2835. doi: 10.1016/s0042-6989(03)00476-0. [DOI] [PubMed] [Google Scholar]
- Ashworth B, Aspinall PA, Mitchell JD. Visual function in multiple sclerosis. Documenta Ophthalmologica. 1989;73:209–224. doi: 10.1007/BF00155090. [DOI] [PubMed] [Google Scholar]
- Ball K, Sekuler R. Improving visual perception in older observers. Journal of Gerontology. 1986;41:176–182. doi: 10.1093/geronj/41.2.176. [DOI] [PubMed] [Google Scholar]
- Bergeman CS. Aging: Genetic and environmental influences. Sage; Thousand Oaks, CA: 1997. [Google Scholar]
- Bühren J, Terzi E, Back M, Wesemann W, Kohnen T. Measuring contrast sensitivity under different lighting conditions: Comparison of three tests. Optometry and Vision Science. 2006;83:290–298. doi: 10.1097/01.opx.0000216100.93302.2d. [DOI] [PubMed] [Google Scholar]
- Cormack FK, Tovee M, Ballard C. Contrast sensitivity and visual acuity in patients with Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2000;15:614–620. doi: 10.1002/1099-1166(200007)15:7<614::aid-gps153>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A, Corkin S, Growdon JH. Visual dysfunction predicts cognitive deficits in Alzheimer’s disease. Optometry and Vision Science. 1995;72:168–176. doi: 10.1097/00006324-199503000-00004. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A, Corkin S, Rizzo JF, Cohen J, Growdon JH, Banks KS. Visual dysfunction in Alzheimer’s disease: Relation to normal aging. Annals of Neurology. 1991;29:41–52. doi: 10.1002/ana.410290110. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A, Cronin-Golomb M, Dunne T, Brown A, Jain K, Cipolloni PB, Auerbach SH. Facial frequency manipulation normalizes face discrimination in Alzheimer’s disease. Neurology. 2000;54:2316–2318. doi: 10.1212/wnl.54.12.2316. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A, Gilmore GC. Visual factors in cognitive dysfunction and enhancement in Alzheimer’s disease. In: Soraci SA, Murata-Soraci K, editors. Visual Information Processing. Westport CT: Praeger; 2003. pp. 3–34. [Google Scholar]
- Cronin-Golomb A, Gilmore GC, Neargarder S, Morrison SR, Laudate TM. Enhanced stimulus strength improves visual cognition in aging and Alzheimer’s disease. Cortex. doi: 10.1016/s0010-9452(08)70693-2. in press. [DOI] [PubMed] [Google Scholar]
- Dargent-Molina P, Hayes M, Bréart G. Sensory impairment and physical disability in elderly women living at home. International Journal of Epidemiology. 1996;25:621–629. doi: 10.1093/ije/25.3.621. [DOI] [PubMed] [Google Scholar]
- Davidsdottir S, Cronin-Golomb A, Lee AC. Visual and spatial symptoms in Parkinson’s disease. Vision Research. 2005;45:1285–1296. doi: 10.1016/j.visres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Dunne TE, Neargarder SA, Cipolloni PB, Cronin-Golomb A. Visual contrast enhances food and liquid intake in advanced Alzheimer’s disease. Clinical Nutrition. 2004;23:533–538. doi: 10.1016/j.clnu.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin (VET) Registry: An approach using questionnaires. Clinical Genetics. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Eisen S, True W, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: Method of construction. Acta Geneticae Medicae Gemellologiae (Roma) 1987;36:67–78. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- Elliot DB, Bullimore MA, Patla AE, Whitaker D. Effect of cataract simulation on clinical and real world vision. British Journal of Ophthalmology. 1996;80:799–804. doi: 10.1136/bjo.80.9.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott DB, Hurst MA, Weatherill J. Comparing clinical tests of visual function in cataract with the patient’s perceived visual disability. Eye. 1990;4:712–717. doi: 10.1038/eye.1990.100. [DOI] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL, Berg S, Johansson B. Quantitative genetic analysis of biobehavioral markers of aging in Swedish studies of adult twins. Journal of Aging and Health. 2000;12:47–68. doi: 10.1177/089826430001200103. [DOI] [PubMed] [Google Scholar]
- Gilmore GC, Cronin-Golomb A, Neargarder SA, Morrison SR. Enhanced stimulus contrast normalizes visual processing of rapidly presented letters in Alzheimer’s disease. Vision Research. 2005;45:1013–1020. doi: 10.1016/j.visres.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Gilmore GC, Spinks RA, Thomas CW. Age effects in coding tasks: Componential analysis and test of the sensory deficit hypothesis. Psychology and Aging. 2006;21:7–18. doi: 10.1037/0882-7974.21.1.7. [DOI] [PubMed] [Google Scholar]
- Gilmore GC, Thomas CW, Klitz T, Persanyi MW, Tomsak R. Contrast enhancement eliminates letter identification speed deficits in Alzheimer’s disease. Journal of Clinical Geropsychology. 1996;2:307–320. [Google Scholar]
- Ginsburg AP. Next generation contrast sensitivity testing. In: Rosenthal BP, Cole RG, editors. Functional assessment of low vision. St. Louis: Mosby Year Book Inc; 1996. pp. 77–88. [Google Scholar]
- Goldberg J, True W, Eisen S, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: Ascertainment bias. Acta Geneticae Medicae Gemellologiae (Roma) 1987;36:67–78. doi: 10.1017/s0001566000004608. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gottfredsdottir MS, Sverrisson T, Musch DC, Stefansson E. Chronic open-angle glaucoma and associated ophthalmic findings in monozygotic twins and their spouses in Iceland. Journal of Glaucoma. 1999;8:134–139. [PubMed] [Google Scholar]
- Hougaard JL, Kessel L, Sander B, Kyvik KO, Sorensen TIA, Larsen M. Evaluation of heredity as a determinant of retinal nerve fiber layer thickness as measured by optical coherence tomography. Investigative Ophthalmology and Visual Science. 2003;44:3011–3016. doi: 10.1167/iovs.02-1090. [DOI] [PubMed] [Google Scholar]
- Klein ML, Mauldin WM, Stoumbos VD. Heredity and age-related macular degeneration: Observations in monozygotic twins. Archives of Ophthalmology. 1994;112:932–937. doi: 10.1001/archopht.1994.01090190080025. [DOI] [PubMed] [Google Scholar]
- Lord SR, Clark RD, Webster IW. Visual acuity and contrast sensitivity in relation to falls in an elderly population. Age Ageing. 1991a;20:175–181. doi: 10.1093/ageing/20.3.175. [DOI] [PubMed] [Google Scholar]
- Lord SR, Clark RD, Webster IW. Physiological factors associated with falls in the elderly population. Journal of the American Geriatric Society. 1991b;39:1194–200. doi: 10.1111/j.1532-5415.1991.tb03574.x. [DOI] [PubMed] [Google Scholar]
- Matjucha ICA, Katz B. Neuro-ophthalmology of aging. In: Albert ML, Knoefel JE, editors. Clinical neurology of aging. 2. New York: Oxford University Press; 1994. pp. 421–447. [Google Scholar]
- Mendola JD, Cronin-Golomb A, Corkin S, Growdon JH. Prevalence of visual deficits in Alzheimer’s disease. Optometry and Vision Science. 1995;72:155–167. doi: 10.1097/00006324-199503000-00003. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6. Department of Psychiatry, Virginia Commonwealth University; Box 980126, Richmond, VA 23298–0126, USA: 2002. [Google Scholar]
- Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Kluwer Academic Publishers BV; Dordrecht, the Netherlands: 1992. [Google Scholar]
- Neargarder SA, Stone ER, Cronin-Golomb A, Oross S. The impact of acuity on performance of four clinical measures of contrast sensitivity in Alzheimer’s disease. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2003;58:54–62. doi: 10.1093/geronb/58.1.p54. [DOI] [PubMed] [Google Scholar]
- Owsley C, Gardner T, Sekuler R, Lieberman H. Role of the crystalline lens in the spatial vision loss of the elderly. Investigative Ophthalmology and Visual Science. 1985;26:1165–1170. [PubMed] [Google Scholar]
- Owsley C, Sekuler R, Boldt C. Aging and low-contrast vision: Face perception. Investigative Ophthalmology and Vision Science. 1981;21:362–365. [PubMed] [Google Scholar]
- Owsley C, Sekuler R, Siemsen D. Contrast sensitivity throughout adulthood. Vision Research. 1983;23:689–699. doi: 10.1016/0042-6989(83)90210-9. [DOI] [PubMed] [Google Scholar]
- Pedersen NL. Gerontological behavior genetics. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. Vol. 4. Academic Press; San Diego, CA: 1996. pp. 59–77. [Google Scholar]
- Pesudovs K, Hazel CA, Doran RML, Elliott DB. The usefulness of Vistech and FACT contrast sensitivity charts for cataract and refractive surgery outcomes research. British Journal of Ophthalmology. 2004;88:11–16. doi: 10.1136/bjo.88.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin GS, Bandeen-Roche K, Huang GH, Munoz B, Schein OD, Fried LP, West SK. The association of multiple visual impairments with self-reported visual disability: SEE project. Investigative Ophthalmology and Vision Science. 2001;42:64–72. [PubMed] [Google Scholar]
- Spear PD. Neural bases of visual deficits during aging. Vision Research. 1993;33:2589–2609. doi: 10.1016/0042-6989(93)90218-l. [DOI] [PubMed] [Google Scholar]
- Teikari JM, O’Donnell JJ. Astigmatism in 72 twin pairs. Cornea. 1989;8:263–266. [PubMed] [Google Scholar]
- Tsuang MT, Bar JL, Harley RM, Lyons MJ. The Harvard twin study of substance abuse: What we have learned. Harvard Review of Psychiatry. 2001;9:267–279. [PubMed] [Google Scholar]
- Valluri S, Minkovitz JB, Budak K, Essary LR, Walker RS, Chansue E, Carbera GM, Koch DD, Pepose JS. Comparative corneal topography and refractive variables in monozygotic and dizygotic twins. American Journal of Ophthalmology. 1999;127:158–163. doi: 10.1016/s0002-9394(98)00319-5. [DOI] [PubMed] [Google Scholar]
- West SK, Rubin GS, Broman AT, Munoz B, Bandeen-Roche K, Turano K. How does visual impairment affect performance on tasks of everyday life? The SEE Project. Arch Ophthalmol. 2002;120:774–780. doi: 10.1001/archopht.120.6.774. [DOI] [PubMed] [Google Scholar]
- Wright CR, Drasdo N, Harding GFA. Pathology of the optic nerve and visual association areas. Brain. 1987;110:107–120. doi: 10.1093/brain/110.1.107. [DOI] [PubMed] [Google Scholar]